Abstract
Cited2 (CBP/p300 interacting transactivator with ED-rich tail 2) is required for embryonic development, coactivation of transcription factor AP-2, and inhibition of hypoxia-inducible factor 1 transactivation. Cited2 is induced by multiple growth factors and cytokines and oncogenically transforms cells. Here, we show that the proliferation of Cited2−/− mouse embryonic fibroblasts ceases prematurely. This is associated with a reduction in growth fraction, senescent cellular morphology, and increased expression of the cell proliferation inhibitors p16INK4a, p19ARF, and p15INK4b. Deletion of INK4a/ARF (encoding p16INK4a and p19ARF) completely rescued the defective proliferation of Cited2−/− fibroblasts. However, the deletion of INK4a/ARF did not rescue the embryonic malformations observed in Cited2−/− mice, indicating that INK4a/ARF-independent pathways are likely to be involved here. We found that Cited2−/− fibroblasts had reduced expression of the polycomb-group genes Bmi1 and Mel18, which function as INK4a/ARF and Hox repressors. Complementation with CITED2-expressing retrovirus enhanced proliferation, induced Bmi1/Mel18 expression, and decreased INK4a/ARF expression. Bmi1- and Mel18-expressing retroviruses enhanced the proliferation of Cited2−/− fibroblasts, indicating that they function downstream of Cited2. Our results provide genetic evidence that Cited2 controls the expression of INK4a/ARF and fibroblast proliferation, at least in part via the polycomb-group genes Bmi1 and Mel18.
p300 and its paralog, CBP (CREB-binding protein), are ubiquitously expressed nuclear proteins that function as transcriptional coactivators and histone acetyl transferases, connecting DNA-bound transcription factors to the core transcriptional machinery (reviewed in references 19 and 50). p300 and CBP are essential for normal cardiovascular, neural, and hematopoietic development (31, 39, 60). p300 and CBP also play a fundamental role in cellular growth control (reviewed in reference 19). Genetic evidence indicates that CBP is a tumor suppressor. Patients with CBP mutations (Rubinstein-Taybi syndrome) (41) have a high incidence of neural and developmental tumors (38), and mice lacking a single CBP allele develop hematological malignancies (31). Consistent with this finding, p300 and CBP function as coactivators of the tumor suppressor p53 (8, 34). Paradoxically, p300 and CBP are also required for cell proliferation. Embryonic fibroblasts lacking p300 proliferate poorly in culture (60), and neutralization of p300/CBP by antibody injection inhibits progression through the G1/S transition (1). In keeping with this finding, many oncogenic transcription factors require either p300 or CBP for transactivation (reviewed in reference 19).
p300 and CBP also interact with members of the CBP/p300 interacting transactivator with ED-rich tail (CITED) family. These include CITED1/MSG1 (58); CITED2, splice isoforms of which are known as p35srj/Mrg1 (12, 33); and CITED4 (15, 59). Loss of Cited2 in mice results in embryonic lethality as a consequence of cardiac malformations, neural tube defects, and adrenal gland agenesis (9, 10, 43, 57, 61). At a biochemical level, CITED2 physically interacts with and coactivates all transcription factor AP-2 (TFAP2) isoforms and is necessary for TFAP2 function (9, 14). CITED2 also inhibits hypoxia-inducible factor 1 alpha (HIF-1α) transactivation by disrupting the HIF-1α-p300 interaction (12, 61). These molecular mechanisms are thought to underlie the embryonic malformations observed in mice lacking Cited2. CITED2 is induced by multiple growth factors and cytokines (e.g., interleukin-1α [IL-1α], IL-2, IL-4, IL-6, IL-9, IL-11, granulocyte-macrophage colony-stimulating factor, platelet-derived growth factor, and insulin), and overexpression of CITED2 results in oncogenic cell transformation (51). The response of CITED2 to mitogenic stimuli and its ability to transform cells suggest that it may function in cell growth control (51). To understand the genetic pathways by which CITED2 may control cell proliferation, we studied mouse embryonic fibroblasts lacking Cited2.
MATERIALS AND METHODS
Mice.
Cited2−/− and Cited2+/+ embryos were on a 129Ola/C57BL/6J mixed background or a C57BL/6J background as indicated and were generated as previously described (9). Cited2−/−:INK4a/ARF−/− (INK4, inhibitor of cyclin-dependent kinase; ARF, alternative reading frame) embryos and the relevant controls (see Fig. 5 and 6) were on an FVB/129Ola/C57BL/6J mixed background and were generated by intercrossing Cited2+/−:INK4a/ARF+/− mice. INK4a/ARF+/− mice (45) were kind gifts from Ronald DePinho (DFCI, Boston, Mass.). Mice, embryos, and fibroblasts were genotyped by using PCR with allele-specific primers (9).
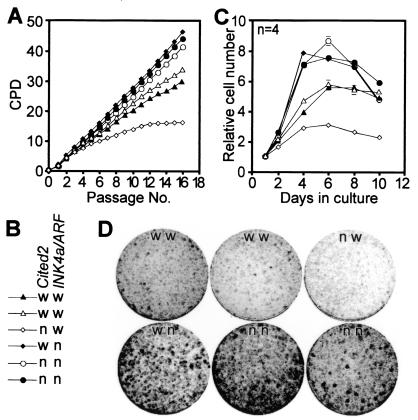
FIG. 5.
Proliferative properties of fibroblasts lacking both Cited2 and INK4a/ARF. (A and B) Fibroblasts were harvested simultaneously from independent embryos at 13.5 dpc. Cumulative fibroblast growth is shown as a plot of CPD versus passage number. Each line represents fibroblasts isolated from an independent embryo. Fibroblast genotypes (w, wild-type; n, null) for Cited2 and INK4a/ARF alleles are indicated in panel B. (C) Proliferative capacity of fibroblasts at passage 4. Fibroblasts were plated in quadruplicate into 12-well plates (2.5 × 104 cells per well) and fixed at the indicated time points, and relative cell numbers were determined by using crystal violet as previously described (25). Data (means ± SEMs) were normalized for cell numbers at day 1. The genotypes are indicated in panel B. (D) Colony formation assay. Fibroblasts (passage 1) were plated at a density of 4,000 cells per 9-cm plate, and colonies were visualized with Giemsa stain after 7 days. Fibroblast genotypes are indicated as wild-type (w) or null (n) for Cited2 and INK4a/ARF alleles, as indicated in panel B.
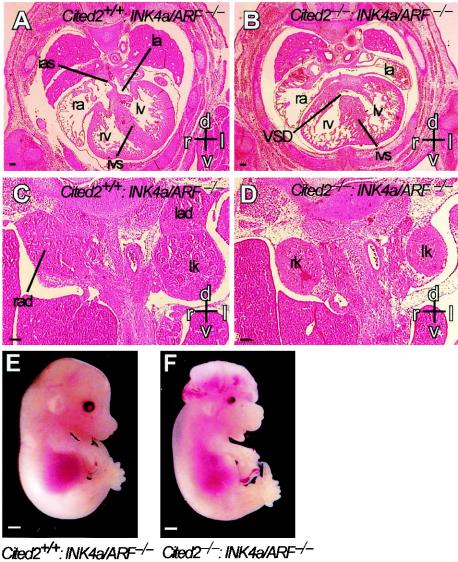
FIG. 6.
Embryonic malformations in embryos lacking both Cited2 and INK4a/ARF. (A through D) Hematoxylin- and eosin-stained transverse sections from Cited2+/+:INK4a/ARF−/− and Cited2−/−:INK4a/ARF−/− embryos at 14.5 dpc. Scale bars, 0.1 mm. Axes: d, dorsal; v, ventral; r, right; l, left. (A) Transverse section through the thorax of a Cited2+/+:INK4a/ARF−/− embryo shows normal cardiac anatomy. The interatrial septum (ias) separates the right (ra) and left (la) atria. The right (rv) and left (lv) ventricles are separated by a normal interventricular septum (ivs) (B) Corresponding section of a Cited2−/−:INK4a/ARF−/− embryo shows a ventricular septal defect (VSD). (C) Transverse section through the abdomen of a Cited2+/+:INK4a/ARF−/− embryo showing normal right (rad) and left (lad) adrenal glands and left kidney (lk). (D) Corresponding section of a Cited2−/−:INK4a/ARF−/− embryo shows lack of right and left adrenal glands. (E and F) Cranial development in Cited2+/+:INK4a/ARF−/− and Cited2−/−:INK4a/ARF−/− embryos at 14.5 dpc. Scale bar, 1 mm. (E) Cited2+/+:INK4a/ARF−/− embryo showing normal cranial development. (F) Cited2−/−:INK4a/ARF−/− embryo showing exencephaly.
Fibroblast isolation, passage, growth curves, and colony formation assays.
Murine embryonic fibroblasts were prepared from littermate embryos at 13.5 or 15.5 days postcoitum (dpc) as previously described (36). Adherent fibroblasts were harvested the following day and plated at a density of 1.5 × 104 cells per cm2 (passage 0) and passaged every 3 days thereafter at the same density. Population doubling per passage was calculated as log(nf/no)/log2, where no is the initial and nf the final number of cells at each passage (13). When no was greater than nf, the population doubling was defined as 0. Cumulative population doubling (CPD) at each passage was calculated by adding population doubling per passage (13). Senescence-associated β-galactosidase was detected as previously described (17). For growth curves, the indicated number of cells per well of a 12-well plate were plated and harvested at the indicated time points and relative cell numbers were measured with crystal violet as previously described (46). Values were normalized to day 1 for the indicated culture, and each point was determined in quadruplicate. For colony formation, fibroblasts were plated at the densities indicated and the colonies were visualized with Giemsa stain as previously described (13).
Growth fraction.
Cells were plated at a density of 1.5 × 104/cm2 onto glass coverslips at the indicated passages, and after 48 h, 10 μM bromodeoxyuridine (BrdU) was added for 24 h. The coverslips were fixed, incubated in HCl, and then stained with anti-BrdU monoclonal antibody (Becton-Dickinson) followed by secondary sheep anti-mouse fluorescein isothiocyanate-conjugated antibody. Cells were stained with propidium iodide (PI) and mounted in Vectashield containing PI (Vector). Nuclear uptake of BrdU and PI was quantitated on a laser scanning cytometer (CompuCyte, Cambridge, Mass.) and analyzed with WinCyte software according to the manufacturer's instructions. Data from the results for 3,000 to 5,000 cells were acquired for each individual experiment. The growth fraction was calculated as the percentage of BrdU-positive cells in the culture.
Retroviruses.
CITED2, CITED2Δ, Mel18, Bmi1, and control retroviral supernatants were generated by using the bicistronic pLZRS-IRES-GFP plasmid and Phoenix producer cells (gifts from Garry Nolan, Stanford, Calif.). Fibroblasts were infected as previously described (25). The Bmi1 retrovirus has been described previously (25). The Mel18 retrovirus was generated from pSG5-Mel18, which contains a mouse Mel18 cDNA insert (gift from M. Kanno, Hiroshima, Japan) with a modified translation start site (GCCACCATGG) that changes the second amino acid from H to D. We used PCR to convert the Mel18 translation start site back to the wild-type form (GGCATCATGC) (GenBank accession no. D90085) and subcloned an EcoRI-SanDI fragment (containing the open reading frame) into pLZRS-IRES-GFP. Infection efficiencies typically exceeded 80%. Plasmid vectors were generated by using standard molecular cloning techniques (7).
Blotting.
Northern blotting was performed as previously described (7) by using 6 to 10 μg of total RNA (RNeasy Mini-kit, QIAGEN) transferred to Hybond N+ membranes (Amersham). 28S and 18S rRNA species were visualized by staining with 0.05% methylene blue. Murine p19ARF, p19INK4d, and p15INK4b cDNA plasmids were gifts from Charles Sherr (HHMI, Memphis, Tenn.) and Gordon Peters (Cancer Research UK, London, United Kingdom). p16INK4a- and p19ARF-specific probes (INK4a/ARF1α and INK4a/ARF1β) were generated from respective cDNA templates by PCR by using exon-specific primers. Northern blotting for Mel18, Bmi1, and Mph1 was performed from early passage, nonconfluent mouse embryonic fibroblasts by using the respective murine probes. The Mph1 probe (IMAGE:3512187) was obtained from MRC-HGMP, Cambridge, United Kingdom. The relative signal intensity was measured by using NIH Image software on scanned autoradiograms. Western blotting with anti-Bmi1 monoclonal antibody (229F6; Upstate Biotechnology, Lake Placid, N.Y.), p16INK4a (M156; Santa Cruz) and anti-p19ARF (Ab80; Abcam, Cambridge, United Kingdom) polyclonal antibodies, and antitubulin antibody (T-5293; Sigma) was performed according to the instructions of the manufacturer.
Histology.
Embryos were fixed in 4% paraformaldehyde, dehydrated in ethanol, and embedded in paraffin wax. Sections (7 μm thick) were stained with hematoxylin and eosin.
RESULTS
Premature proliferation arrest of Cited2−/− fibroblasts.
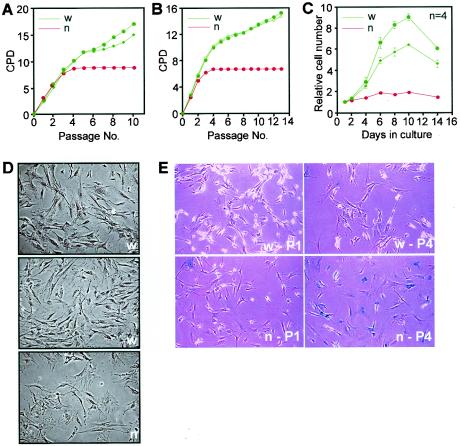
We investigated the proliferative capacity of Cited2+/+ and Cited2−/− primary mouse embryonic fibroblasts. Fibroblasts were derived from littermate embryos and passaged every 3 days in parallel and under identical conditions at a density of 1.5 × 104 cells/cm2, equivalent to a 3T3 protocol (36). Cited2+/+ fibroblasts proliferated rapidly and then slowed transiently at passages 5 and 6, following which proliferation was rapid and continuous (Fig. 1A). Cited2−/− fibroblasts proliferated normally in the first three passages but slowed dramatically and then arrested permanently. This premature proliferation arrest was confirmed in three independent experiments by using independently isolated fibroblasts (data not shown) as well as fibroblasts isolated from coisogenic mice generated by backcrossing the Cited2 mutation to the C57BL/6J background for more than nine generations (Fig. 1B). These results were also corroborated by plating fibroblasts at passage 3 and assaying cell growth over the next 14 days without replating (Fig. 1C). By passage 3, the Cited2−/− fibroblasts had a decreased ability to proliferate in comparison to that of the Cited2+/+ fibroblasts. Cultures of either genotype had indistinguishable spindle-shaped cells at initial plating. With passage, Cited2−/− fibroblast cultures rapidly accumulated cells that had a flattened appearance and cytoplasmic enlargement and expressed senescence-associated β-galactosidase (17) (Fig. 1D and E).
FIG. 1.
Proliferation properties and morphology of Cited2−/− mouse embryonic fibroblasts. (A) Cumulative growth of Cited2+/+ (w) and Cited2−/− (n) fibroblast cultures prepared from 15.5-dpc embryos on a 129Ola/C57BL/6J mixed background shown as a plot of CPD versus passage number. (B) These results are also reproduced in Cited2+/+ (w) and Cited2−/− (n) fibroblast cultures isolated from 13.5-dpc embryos on a coisogenic C57BL/6J background. (C) Proliferative capacity of fibroblasts at passage 3. Fibroblasts prepared from embryos on a 129Ola/C57BL/6J background were plated in quadruplicate into 12-well plates (6 × 103 cells per well) and fixed at the indicated time points, and relative cell numbers were determined by using crystal violet. Data (means ± standard errors of the means [SEMs]) were normalized for cell numbers at day 1. (D) Morphology of Cited2+/+ (w) and Cited2−/− (n) fibroblasts at passage 4. Cited2−/− cultures rapidly accumulate cells that appear flat with cytoplasmic enlargement. (E) Cited2+/+ (w) and Cited2−/− (n) fibroblasts were stained for senescence-associated β-galactosidase at passage 1 (P1) or passage 4 (P4).
Reduction in growth fraction.
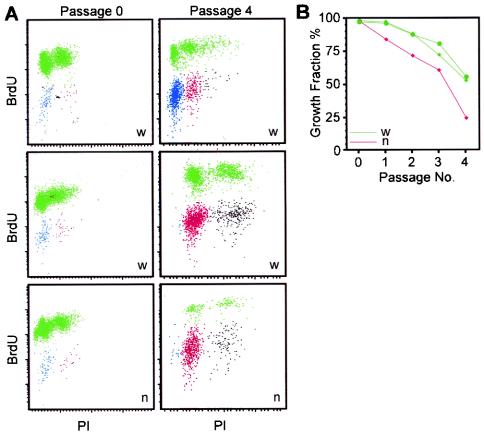
To determine the mechanism of impaired proliferation in Cited2−/− fibroblasts, the fraction of actively proliferating cells (11) was measured by labeling parallel cultures with BrdU for 24 h. Bivariate analysis for BrdU uptake and PI staining showed that the growth fraction of Cited2+/+ and Cited2−/− fibroblasts was close to 100% when initially placed in culture (Fig. 2A and B). With serial passage, the growth fraction of Cited2−/− fibroblasts declined more rapidly than that of Cited2+/+ fibroblasts. The fall in growth fraction in the Cited2−/− cultures was evident as early as the first passage. All cultures were initially predominantly diploid, as determined by PI staining (Fig. 2A). With serial passage, cultures became tetraploid (Fig. 2A, middle and lower panels). Although the wild-type culture shown in the top panel of Fig. 2A was still predominantly diploid at this passage, it became predominantly tetraploid at the next passage (data not shown).
FIG. 2.
Growth fraction of Cited2−/− mouse embryonic fibroblasts. (A) Scattergrams of nuclear DNA synthesis (BrdU) vs. DNA content (PI) of Cited2+/+ (w) and Cited2−/− (n) fibroblast cultures at passages 0 and 4. BrdU-labeled cells are indicated in green. Unlabeled cells are indicated in blue (2n), red (4n), or black (8n DNA content). (B) Growth fraction of Cited2−/− and Cited2+/+ fibroblast cultures plotted against passage number.
Cited2−/− fibroblasts have increased expression of INK4a/ARF.
The above results showed that Cited2−/− fibroblasts cease to proliferate prematurely when cultured, do not spontaneously immortalize, and express morphological features of cellular senescence. Senescence in mouse embryonic fibroblasts is associated with increased levels of the alternatively spliced products of the INK4a/ARF locus, p16INK4a, and p19ARF (Fig. 3A) (reviewed in references 48 and 49). Members of the INK4 family function to inhibit cyclin-dependent kinases 4 and 6, whereas p19ARF functions to inhibit MDM2, a repressor of p53. We examined the expression of INK4a/ARF in early passage Cited2+/+ and Cited2−/− fibroblasts (Fig. 3B through F). Northern blots were probed with a p19ARF cDNA, which detects both p16INK4a and p19ARF (Fig. 3A and B) as comigrating products. This probe showed that INK4a/ARF expression was clearly increased (2.7-fold) in Cited2−/− fibroblasts (Fig. 3B). Specific probes that discriminate between the alternatively spliced p16INK4a and p19ARF transcripts showed that the expression of both transcripts was increased (4.2- and 2.7-fold, respectively) in fibroblasts lacking Cited2 (Fig. 3C and D). The expression of p15INK4b, a member of the INK4 family (21) (Fig. 3E), was also increased (2.5-fold) in Cited2−/− fibroblasts (Fig. 3A). We observed no change in the expression of p19INK4d, another INK4 family member (23) (Fig. 3F). Consistent with these observations, the levels of p16INK4a and p19ARF proteins were also increased (2.5- and 2.7-fold, respectively) in Cited2−/− fibroblasts at passage 1 (Fig. 3G and H).
FIG. 3.
INK4a/ARF expression in Cited2−/− fibroblasts. (A) Representation of the INK4a/ARF and INK4b locus showing exon structure and alternative splicing (dashed lines) that generates the three different cell cycle inhibitors p15INK4b, p16INK4a, and p19ARF. (B through F) Northern blots of total RNA obtained from independent pairs of Cited2+/+ (w) and Cited2−/− (n) fibroblasts derived from littermate embryos. The passage number (P) is indicated in each panel. (B) A full-length p19ARF cDNA was used as a probe. This probe detects both p19ARF and p16INK4a isoforms, which comigrate. (C) p16INK4a was detected by using a specific exon 1α probe. (D) p19ARF was detected by using a specific exon 1β probe. (E and F) p15INK4b and p19INK4d transcripts were detected by using full-length cDNA probes. The bottom section in each panel shows 28S and 18S RNA species, confirming equal loading. (G and H) Western blots of total cell lysates from Cited2+/+ and Cited2−/− fibroblasts at passage 1 were probed with anti-p16INK4a and anti-p19ARF antibodies (top panels) and with an antitubulin monoclonal antibody (bottom panels) to demonstrate equal loading.
Complementation with CITED2 enhances proliferation.
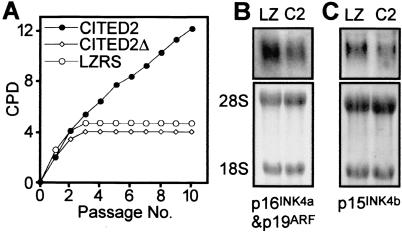
To determine if these changes were specific for loss of Cited2, we infected Cited2−/− fibroblasts with a bicistronic retrovirus expressing human CITED2 (which is 94% conserved with mouse Cited2) and GFP (green fluorescent protein) driven by a retroviral long terminal repeat (Fig. 4A). These fibroblasts showed a marked enhancement of proliferation that was maintained for the period studied (i.e., greater than 30 days) and retained their spindle shape (data not shown). In comparison, parallel infection with a control GFP-expressing retrovirus (LZRS) or with retrovirus expressing CITED2Δ, a mutant lacking the overlapping CBP/p300 and TFAP2 binding domains (9, 12), did not enhance proliferation. These results were confirmed in two further experiments using independently isolated Cited2−/− fibroblasts. Infection of Cited2−/− fibroblasts with the CITED2-expressing retrovirus also led to a modest reduction in INK4a/ARF and INK4b expression (1.5- and 1.6-fold, respectively) (Fig. 4B and C) in comparison to control retrovirus.
FIG. 4.
Complementation of Cited2−/− fibroblasts with CITED2. (A) Cited2−/− fibroblasts (derived from an embryo at 13.5 dpc) were infected with CITED2, CITED2Δ (lacking residues 215 to 270), or LZRS (control) retrovirus. Infected fibroblasts were replated on day 7 (passage 0) and passaged every 3 days. Cumulative growth of retrovirally complemented Cited2−/− fibroblasts is shown as a plot of CPD versus passage number. The results were reproduced in two further independent experiments by using independently isolated fibroblasts. (B and C) Northern blots of total RNA obtained from retrovirally complemented Cited2−/− fibroblasts. LZ indicates infection with LZRS (control) retrovirus, and C2 indicates infection with CITED2-expressing retrovirus. The transcripts detected are indicated in each panel. The bottom panels in each figure show 28S and 18S RNA species, confirming equal loading. (B) A full-length p19ARF cDNA was used as a probe. This probe detects both p19ARF and p16INK4a isoforms, which comigrate. (C) A p15INK4b transcript was detected by using a full-length cDNA probe.
An intact INK4a/ARF gene is essential for proliferation arrest in Cited2−/− fibroblasts.
The above results suggested that Cited2 enhances fibroblast proliferation by repressing INK4a/ARF and/or INK4b. To definitively establish the role of INK4a/ARF in mediating the premature proliferation arrest of Cited2−/− fibroblasts, we generated fibroblasts lacking both Cited2 and INK4a/ARF (Cited2−/−:INK4a/ARF −/−) and compared their proliferation with that of Cited2−/−:INK4a/ARF+/+, Cited2+/+:INK4a/ARF−/−, and wild-type fibroblasts (Fig. 5A and B). Consistent with previous observations (45), fibroblasts lacking INK4a/ARF proliferated more rapidly than wild-type fibroblasts during serial passage in culture, with no slowing of proliferation for the duration of the experiment (48 days). Consistent with the observations in Fig. 1, Cited2−/− fibroblasts arrested prematurely and permanently. Fibroblasts lacking both Cited2 and INK4a/ARF proliferated almost as rapidly as those lacking INK4a/ARF, with no slowing of proliferation. These results were further confirmed in independently isolated fibroblasts lacking both Cited2 and INK4a/ARF (data not shown). We then examined the proliferative capacity of these fibroblasts by plating them at passage 4 and assaying cell growth over the next 10 days without replating (Fig. 5C). In keeping with the above observations, Cited2−/− fibroblasts had markedly reduced proliferative ability, and INK4a/ARF−/− fibroblasts proliferated more rapidly than wild-type fibroblasts. Fibroblasts lacking both Cited2 and INK4a/ARF proliferated as rapidly as those lacking only INK4a/ARF. We also examined the ability of these fibroblasts to form colonies when plated at low density, an independent measure of the proliferative potential of primary cells (13). In this assay, wild-type and Cited2−/− fibroblasts formed small colonies with very low efficiency (Fig. 5D). INK4a/ARF−/− fibroblasts efficiently formed large colonies. Fibroblasts lacking both Cited2 and INK4a/ARF formed colonies almost as well as those lacking only INK4a/ARF. These results indicate that intact INK4a/ARF function is essential for the reduced proliferative capacity and premature proliferation arrest observed in Cited2−/− fibroblasts.
Elimination of INK4a/ARF does not rescue embryonic malformations in Cited2−/− mice.
To determine if INK4a/ARF plays a role in the genesis of embryonic malformations in mice lacking Cited2, we examined embryos lacking both Cited2 and INK4a/ARF. Like embryos lacking only Cited2 (9), those lacking both Cited2 and INK4a/ARF had cardiac malformations (Fig. 6B), adrenal agenesis (Fig. 6D), and exencephaly (Fig. 6F). In these experiments, exencephaly was observed in 4 of 8 embryos lacking Cited2 and 6 of 13 embryos lacking both Cited2 and INK4a/ARF. Control embryos that were wild type for Cited2 but lacked INK4a/ARF had normal heart, adrenal, and neural development (Fig. 6A, C, and E). These results indicate that Cited2 controls other pathways that are relevant for embryonic development.
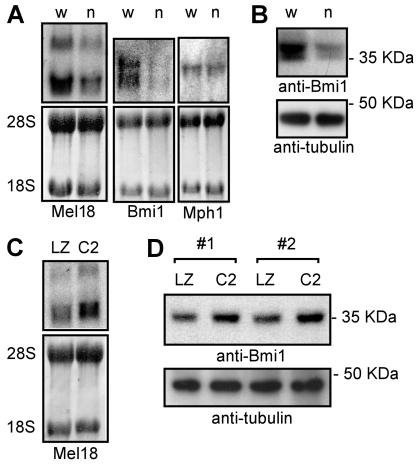
Cells lacking Cited2 have reduced Bmi1 and Mel18 expression.
The above data indicated that Cited2 enhances cell proliferation by repressing INK4a/ARF. Genetic evidence indicates that in primary mouse fibroblasts the polycomb-group gene Bmi1 represses p16INK4a and p19ARF and that Mel18 (a Bmi1 paralog) represses p16INK4a (25). We therefore examined the expression of these genes in early passage Cited2+/+ and Cited2−/− fibroblasts derived from littermate embryos. We found that both Mel18 and Bmi1 expression was reduced (2.3- and 2.2-fold, respectively) in Cited2−/− fibroblasts (Fig. 7A and B). There was no significant change in the expression of Mph1, another polycomb group gene. We also examined the fibroblasts for TBX2, another INK4a/ARF repressor (24), but expression of this gene was not detected in wild-type or Cited2−/− fibroblasts (data not shown). Infection of Cited2−/− fibroblasts with CITED2-expressing retrovirus resulted in a modest increase in expression of Mel18 (1.5-fold) and Bmi1 (1.4-fold) (Fig. 7C and D).
FIG. 7.
Expression of Mel18 and Bmi1 in fibroblasts lacking Cited2. (A) Northern blots of total RNA obtained from Cited2+/+ (w) and Cited2−/− (n) fibroblasts derived from littermate embryos. The top panels show blots probed for Mel18, Bmi1, or Mph1, as indicated. Two Mel18 isoforms are noted (52). The bottom panels show 28S and 18S RNA species to show equal loading. (B) Western blot of total cell lysate probed with an anti-Bmi1 monoclonal antibody (top) and reprobed with an antitubulin monoclonal antibody (bottom) to show equal loading. (C) Northern blot of total RNA obtained from Cited2−/− fibroblasts infected in parallel with retroviruses. LZ indicates infection with LZRS (control) retrovirus, and C2 indicates infection with CITED2-expressing retrovirus. The top panel shows a blot probed with a Mel18 cDNA. The bottom panel shows 28S and 18S RNA species to show equal loading. (D) Western blot of total cell lysate from Cited2−/− fibroblasts. LZ indicates infection with LZRS (control) retrovirus and C2 indicates infection with CITED2-expressing retrovirus. The experiment was performed in duplicate using independently isolated Cited2−/− fibroblast lines (nos. 1 and 2). The top panel shows a blot probed with an anti-Bmi1 antibody. The bottom panel shows a blot probed with an antitubulin antibody to show equal loading.
Bmi1 and Mel18 enhance proliferation of cells lacking Cited2.
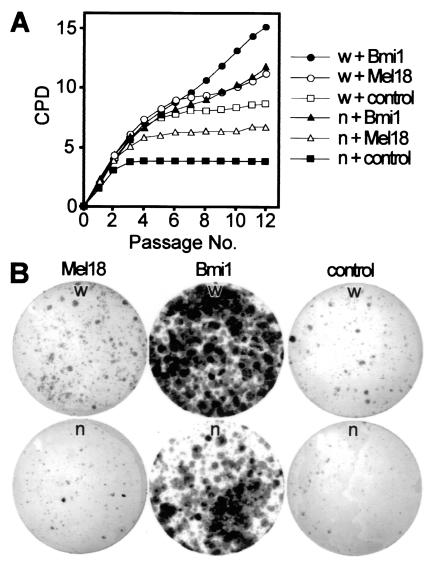
The above data indicated that Cited2 is required for normal Bmi1 and Mel18 expression. To determine if the observed deficiency of Bmi1 and Mel18 expression in Cited2−/− fibroblasts would explain their premature proliferation arrest, we infected Cited2−/− and Cited2+/+ fibroblasts with bicistronic retroviruses expressing either Bmi1 and GFP, Mel18 and GFP, or GFP alone (as the control) and performed cell proliferation and colony formation assays (Fig. 8A and B). Infection with Bmi1- or Mel18-expressing retroviruses led to enhanced proliferation compared to that for the control retrovirus, regardless of the Cited2 genotype (Fig. 8A). The Bmi1 retrovirus enhanced proliferation better than the Mel18 retrovirus, again regardless of Cited2 genotype. We next examined the ability of these fibroblasts to form colonies when plated at low density (Fig. 8B). Consistent with the above results, infection with Bmi1- or Mel18-expressing retroviruses led to enhanced colony formation (with Bmi1 being more efficient than Mel18) compared to that for the control retrovirus, regardless of the Cited2 genotype. This enhancement of proliferation by Bmi1 and by Mel18 was reproducibly observed in Cited2−/− fibroblasts obtained from independently harvested embryos. These results indicate that Bmi1 or Mel18 can enhance the proliferation of fibroblasts lacking Cited2 and that Cited2 function is not required for proliferation enhancement by these polycomb-group proteins.
FIG. 8.
Growth properties of Cited2−/− and Cited2+/+ embryonic fibroblasts infected with Bmi1- and Mel18-expressing retroviruses. (A) Proliferation of Cited2−/− (n) and Cited2+/+ (w) fibroblasts (derived from littermate embryos on a C57BL/6J background at 13.5 dpc) infected with Bmi1, Mel18, or control retroviruses. Fibroblasts were harvested, infected with retroviruses the following day, replated on day 4 (referred to as P0), and passaged in parallel every 3 days at identical conditions. The proliferation of retrovirally complemented fibroblasts is shown as plots of CPD versus passage number. (B) Colony formation assay. Cited2−/− and Cited2+/+ fibroblasts (at passage 6) infected with the indicated retroviruses were plated at a density of 4,000 cells per 9-cm plate, and colonies were visualized with Giemsa stain after 16 days.
DISCUSSION
Normal primary mouse embryonic fibroblasts proliferate rapidly when explanted but soon slow down in response to the stress of culture (reviewed in reference 48). This is associated with cell cycle exit, cytoplasmic enlargement, and expression of senescence-associated β-galactosidase, which are characteristics of cellular senescence (17). At a molecular level in murine cells, this senescent phenotype is associated with increased levels of the cell proliferation inhibitors p16INK4a and p19ARF and activation of the p19ARF target p53 and the p53 target gene p21CIP1 (reviewed in references 48 and 49). Genetic evidence indicates that intact p19ARF and p53 are necessary for the senescent phenotype of cultured mouse embryonic fibroblasts (45; reviewed in reference 48). Deletion of p19ARF alone abolishes the senescent phenotype, whereas deletion of p16INK4a alone does not have this effect, indicating that p19ARF plays a critical role (28, 30, 47). Notably, the spontaneous immortalization of wild-type fibroblasts observed when they are serially passaged in culture typically results from spontaneous mutations in p19ARF or p53 (28, 62). Premature senescence of primary fibroblasts is associated with mutations in genes such as Bmi1, JunD, Atm, and Lig4 that function upstream of p19ARF (18, 25, 27, 56). It is also associated with mutations in Mel18 (25) and Id1 (5), which repress p16INK4a expression. Overexpression of activated cellular oncogenes such as Ras (37, 46) and MEK (35) in primary cells results in senescence by activating the p19ARF-p53 pathway.
The results presented here provide genetic evidence that Cited2, a growth factor and cytokine-inducible gene with oncogenic potential (51), is necessary for fibroblast proliferation in culture. Fibroblasts lacking Cited2 stop dividing prematurely, display typical senescent morphology, and express senescence-associated β-galactosidase (Fig. 1 and 2). These results suggested that they are hypersensitive to culture-induced stress. The expression of p16INK4a, p19ARF, and p15INK4b but not p19INK4d was markedly increased in Cited2−/− fibroblasts (Fig. 3). These results indicated that Cited2 is required for the coordinated repression of the physically linked INK4a/ARF and INK4b genes. INK4a/ARF and INK4b were also repressed by complementation with CITED2, which also enhanced cellular proliferation, suggesting a causal mechanism (Fig. 4). Although the reduction of INK4a/ARF and INK4b by retrovirally transduced CITED2 was modest, it was reproducible and supports the idea that Cited2 represses these genes.
Embryos lacking Cited2 invariably have heart and adrenal gland defects, and ∼50% of embryos have exencephaly (9, 42-44). Premature fibroblast senescence occurred regardless of the presence or absence of exencephaly (e.g., the mutant embryos in Fig. 1A and B had normal neural development). Nevertheless, it was possible that the embryonic heart or adrenal malformation itself affects (through secondary changes) the growth of fibroblasts. To address this issue, we performed the complementation experiment with CITED2 and showed, reproducibly, that complementation with retroviral CITED2 markedly enhances proliferation of Cited2−/− fibroblasts (Fig. 4A). This effect was specific, as it was not seen with a CITED2 mutant lacking residues 215 to 270. Successful complementation indicates that no secondary change, e.g., one induced by the heart, adrenal, or neural defect, was responsible for the fibroblast growth defect. Thus, the premature senescence observed in fibroblasts lacking Cited2 is indeed specific and is unlikely to be due to the preexisting embryonic malformation. Notably, residues 215 to 270 of CITED2 contain the overlapping TFAP2 and CBP/p300 binding domains (9, 12), suggesting that binding of CITED2 to CBP/p300 and/or TFAP2 is required for enhancement of cell proliferation.
We also found that deletion of INK4a/ARF completely rescued the proliferation defect in fibroblasts lacking Cited2 (Fig. 5). This finding was observed reproducibly in fibroblasts obtained from independently isolated embryos. As the proliferation defect in Cited2-deficient fibroblasts is reproducibly observed on both mixed (Fig. 1A and Fig. 5A and C) and pure (Fig. 1B) genetic backgrounds, the reproducible rescue of senescence by deletion of INK4a/ARF in Cited2−/− fibroblasts indicates that random segregation of genetic modifiers in these experiments does not likely play an important role. Taken together with the increased levels of INK4a/ARF observed in Cited2−/− fibroblasts and the suppression of INK4a/ARF by complementation with CITED2, these experiments show that the elevated levels of INK4a/ARF observed in Cited2−/− fibroblasts play a major causal role in generating the premature senescence phenotype and that INK4a/ARF is a critical downstream target of Cited2 in fibroblasts. The complete rescue in cell proliferation that we observed also indicates that no other downstream mechanism (e.g., the activation of p53 or p27 by a different mechanism, such as HIF-1 activation in cells lacking Cited2) is likely to be involved. These results are also supported by experiments which show that fibroblasts lacking Cited2 are efficiently immortalized by overexpression of the p19ARF repressor TBX2 (24) and by antisense p19ARF retrovirus (16) (K. R. Kranc and S. Bhattacharya, unpublished observations).
The above data indicated that Cited2 enhances cell proliferation by repressing INK4a/ARF. However, deletion of the INK4a/ARF locus did not rescue the embryonic malformations (cardiac, adrenal, and neural) associated with mutation in Cited2 (Fig. 6). Thus, the Cited2-mediated repression of INK4a/ARF observed in fibroblasts does not appear to play a significant role in embryonic development. This finding indicates that Cited2 has two independent functions: first, a role in embryonic development, and second, a role in fibroblast proliferation under conditions of culture-induced stress. One possible mechanism is that Cited2 positively regulates genes that not only repress INK4a/ARF but also have independent roles in development. Members of the polycomb family (e.g., Bmi1 and Mel18) are known to play these dual roles (2, 25, 55).
Genetic evidence indicates that in primary mouse fibroblasts Bmi1 represses p16INK4a and p19ARF and Mel18 represses p16INK4a (25). Deletion of either Bmi1 or its paralog Mel18 leads to reduced lymphocyte precursor proliferation and premature proliferation arrest of primary mouse embryonic fibroblasts (3, 25). Bmi1 is also necessary for self-renewal of hematopoietic stem cells (32, 40). Deletion of INK4a/ARF in mice lacking Bmi1 rescues premature fibroblast senescence and postnatal cerebellar and lymphoid defects (25). However, Mel18 has more complex functions, as evidenced by the fact that it can also function as a cell proliferation inhibitor in other cell types (29, 53). Differences in Bmi1 and Mel18 function are also suggested by distinct phenotypes observed in mutant mice: for instance, cerebellar defects are observed in mice lacking Bmi1, and colonic smooth muscle defects are seen in mice lacking Mel18 (2, 55). Bmi1 and Mel18 function as transcriptional repressors that interact with a similar set of polycomb-group proteins (6, 20, 22, 26, 54). They function during development to repress Hox genes, and deletion of either gene leads to defects in anteroposterior axis formation (2, 55). Mel18 and Bmi1 act synergistically in a dose-dependent manner during development to maintain Hox gene expression and cell survival (4). Importantly, we have observed anteroposterior patterning defects in Cited2 mutant embryos. These include fusion of cranial ganglia (9) and of the cervical vertebrae (S. D. Bamforth and S. Bhattacharya, unpublished observations). These observations prompted us to examine Bmi1 and Mel18 expression in fibroblasts lacking Cited2.
We found that fibroblasts lacking Cited2 have a marked reduction in levels of Bmi1 and Mel18 transcripts (Fig. 7). Complementation of Cited2−/− fibroblasts with CITED2 led to a modest increase in the expression of Bmi1 and Mel18. We also found that both Bmi1 and Mel18 enhanced the proliferation of fibroblasts regardless of the Cited2 genotype (Fig. 8), indicating that Cited2 is not necessary for proliferation enhancement by these polycomb-group genes and supporting the idea that Bmi1 and Mel18 function downstream of Cited2. However, after infection with Bmi1- and Mel18-expressing retroviruses, Cited2+/+ fibroblasts proliferated faster than Cited2−/− fibroblasts, implying that Cited2 deficiency cannot be completely rescued by overexpression of Bmi1 or Mel18 individually. One possibility is that other Cited2 functions that are independent of Bmi1 or Mel18 may be important. Another possibility is that Cited2 is required for the coordinated induction of Bmi1 and Mel18, which is not mimicked by the forced expression of either Bmi1 or Mel18 alone.
In summary, these data indicate that Cited2 is required for normal Bmi1 and Mel18 expression in primary mouse embryonic fibroblasts and that Bmi1 and Mel18 function downstream of Cited2. The mechanism by which Cited2 induces Bmi1 and Mel18 is not understood at present. One possibility is that a coactivation function of Cited2 is required for Bmi1/Mel18 expression. Alternatively, Cited2 may function several steps away, even perhaps via nonautonomous cell mechanisms, to control Bmi1/Mel18 expression. These possibilities require further investigation. Taken together, our results provide genetic evidence that Cited2 controls the expression of INK4a/ARF and fibroblast proliferation at least in part via the polycomb-group genes Bmi1 and Mel18 and provide a mechanism by which Cited2 may function as an oncogene.
Acknowledgments
We thank Ronald DePinho for the generous gift of INK4a/ARF mutant mice, Charles Sherr and Gordon Peters for gifts of probes, Garry Nolan for LZRS plasmids and Phoenix cells, and Derek Davies (Cancer Research UK) for help with cytometry.
K.R.K. is a Wellcome Prize student and a Keith Murray senior scholar at Lincoln College. These studies were funded by a Wellcome Trust senior fellowship award to S.B.
REFERENCES
- 1.Ait-Si-Ali, S., A. Polesskaya, S. Filleur, R. Ferreira, A. Duquet, P. Robin, A. Vervish, D. Trouche, F. Cabon, and A. Harel-Bellan. 2000. CBP/p300 histone acetyl-transferase activity is important for the G1/S transition. Oncogene 19:2430-2437. [DOI] [PubMed] [Google Scholar]
- 2.Akasaka, T., M. Kanno, R. Balling, M. A. Mieza, M. Taniguchi, and H. Koseki. 1996. A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122:1513-1522. [DOI] [PubMed] [Google Scholar]
- 3.Akasaka, T., K. Tsuji, H. Kawahira, M. Kanno, K. Harigaya, L. Hu, Y. Ebihara, T. Nakahata, O. Tetsu, M. Taniguchi, and H. Koseki. 1997. The role of mel-18, a mammalian Polycomb group gene, during IL-7-dependent proliferation of lymphocyte precursors. Immunity 7:135-146. [DOI] [PubMed] [Google Scholar]
- 4.Akasaka, T., M. van Lohuizen, N. van der Lugt, Y. Mizutani-Koseki, M. Kanno, M. Taniguchi, M. Vidal, M. Alkema, A. Berns, and H. Koseki. 2001. Mice doubly deficient for the Polycomb Group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development 128:1587-1597. [DOI] [PubMed] [Google Scholar]
- 5.Alani, R. M., A. Z. Young, and C. B. Shifflett. 2001. Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc. Natl. Acad. Sci. USA 98:7812-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkema, M. J., M. Bronk, E. Verhoeven, A. Otte, L. J. van't Veer, A. Berns, and M. van Lohuizen. 1997. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian polycomb complex. Genes Dev. 11:226-240. [DOI] [PubMed] [Google Scholar]
- 7.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Short protocols in molecular biology, 3rd ed. John Wiley & Sons, Inc., New York, N.Y.
- 8.Avantaggiati, M. L., V. Ogryzko, K. Gardner, A. Giordano, A. S. Levine, and K. Kelly. 1997. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89:1175-1184. [DOI] [PubMed] [Google Scholar]
- 9.Bamforth, S. D., J. Braganca, J. J. Eloranta, J. N. Murdoch, F. I. Marques, K. R. Kranc, H. Farza, D. J. Henderson, H. C. Hurst, and S. Bhattacharya. 2001. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 29:469-474. [DOI] [PubMed] [Google Scholar]
- 10.Barbera, J. P., T. A. Rodriguez, N. D. Greene, W. J. Weninger, A. Simeone, A. J. Copp, R. S. Beddington, and S. Dunwoodie. 2002. Folic acid prevents exencephaly in Cited2 deficient mice. Hum. Mol. Genet. 11:283-293. [DOI] [PubMed] [Google Scholar]
- 11.Baserga, R. 1989. Measuring parameters of growth, p. 1-16. In R. Baserga (ed.), Cell growth and division: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 12.Bhattacharya, S., C. L. Michels, M. K. Leung, Z. P. Arany, A. L. Kung, and D. M. Livingston. 1999. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 13:64-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blasco, M. A., H. W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. [DOI] [PubMed] [Google Scholar]
- 14.Braganca, J., J. J. Eloranta, S. D. Bamforth, J. C. Ibbitt, H. C. Hurst, and S. Bhattacharya. 2003. Physical and functional interactions among AP-2 transcription factors, p300/CREB-binding protein, and CITED2. J. Biol. Chem. 278:16021-16029. [DOI] [PubMed] [Google Scholar]
- 15.Braganca, J., T. Swingler, F. I. Marques, T. Jones, J. J. Eloranta, H. C. Hurst, T. Shioda, and S. Bhattacharya. 2002. Human CREB-binding protein/p300-interacting transactivator with ED-rich tail (CITED) 4, a new member of the CITED family, functions as a co-activator for transcription factor AP-2. J. Biol. Chem. 277:8559-8565. [DOI] [PubMed] [Google Scholar]
- 16.Carnero, A., J. D. Hudson, C. M. Price, and D. H. Beach. 2000. p16INK4A and p19ARF act in overlapping pathways in cellular immortalization. Nat. Cell Biol. 2:148-155. [DOI] [PubMed] [Google Scholar]
- 17.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank, K. M., N. E. Sharpless, Y. Gao, J. M. Sekiguchi, D. O. Ferguson, C. Zhu, J. P. Manis, J. Horner, R. A. DePinho, and F. W. Alt. 2000. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol. Cell 5:993-1002. [DOI] [PubMed] [Google Scholar]
- 19.Goodman, R. H., and S. Smolik. 2000. CBP/p300 in cell growth, transformation, and development. Genes Dev. 14:1553-1577. [PubMed] [Google Scholar]
- 20.Gunster, M. J., D. P. Satijn, K. M. Hamer, J. L. den Blaauwen, D. de Bruijn, M. J. Alkema, M. van Lohuizen, R. van Driel, and A. P. Otte. 1997. Identification and characterization of interactions between the vertebrate polycomb-group protein BMI1 and human homologs of polyhomeotic. Mol. Cell. Biol. 17:2326-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannon, G. J., and D. Beach. 1994. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371:257-261. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto, N., H. W. Brock, M. Nomura, M. Kyba, J. Hodgson, Y. Fujita, Y. Takihara, K. Shimada, and T. Higashinakagawa. 1998. RAE28, BMI1, and M33 are members of heterogeneous multimeric mammalian Polycomb group complexes. Biochem. Biophys. Res. Commun. 245:356-365. [DOI] [PubMed] [Google Scholar]
- 23.Hirai, H., M. F. Roussel, J. Y. Kato, R. A. Ashmun, and C. J. Sherr. 1995. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell. Biol. 15:2672-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs, J. J., P. Keblusek, E. Robanus-Maandag, P. Kristel, M. Lingbeek, P. M. Nederlof, T. van Welsem, M. J. van De Vijver, E. Y. Koh, G. Q. Daley, and M. van Lohuizen. 2000. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19ARF) and is amplified in a subset of human breast cancers. Nat. Genet. 26:291-299. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, J. J., K. Kieboom, S. Marino, R. A. DePinho, and M. van Lohuizen. 1999. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397:164-168. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs, J. J., and M. van Lohuizen. 1999. Cellular memory of transcriptional states by Polycomb-group proteins. Semin. Cell Dev. Biol. 10:227-235. [DOI] [PubMed] [Google Scholar]
- 27.Kamijo, T., E. van de Kamp, M. J. Chong, F. Zindy, J. A. Diehl, C. J. Sherr, and P. J. McKinnon. 1999. Loss of the ARF tumor suppressor reverses premature replicative arrest but not radiation hypersensitivity arising from disabled Atm function. Cancer Res. 59:2464-2469. [PubMed] [Google Scholar]
- 28.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 29.Kanno, M., M. Hasegawa, A. Ishida, K. Isono, and M. Taniguchi. 1995. mel-18, a Polycomb group-related mammalian gene, encodes a transcriptional negative regulator with tumor suppressive activity. EMBO J. 14:5672-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krimpenfort, P., K. C. Quon, W. J. Mooi, A. Loonstra, and A. Berns. 2001. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413:83-86. [DOI] [PubMed] [Google Scholar]
- 31.Kung, A. L., V. I. Rebel, R. T. Bronson, L. E. Ch'ng, C. A. Sieff, D. M. Livingston, and T. P. Yao. 2000. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 14:272-277. [PMC free article] [PubMed] [Google Scholar]
- 32.Lessard, J., and G. Sauvageau. 2003. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423:255-260. [DOI] [PubMed] [Google Scholar]
- 33.Leung, M. K., T. Jones, C. L. Michels, D. M. Livingston, and S. Bhattacharya. 1999. Molecular cloning and chromosomal localization of the human CITED2 gene encoding p35srj/Mrg1. Genomics 61:307-313. [DOI] [PubMed] [Google Scholar]
- 34.Lill, N. L., S. R. Grossman, D. Ginsberg, J. DeCaprio, and D. M. Livingston. 1997. Binding and modulation of p53 by p300/CBP coactivators. Nature 387:823-827. [DOI] [PubMed] [Google Scholar]
- 35.Lin, A. W., M. Barradas, J. C. Stone, L. van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loo, D., C. Rawson, T. Ernst, S. Shirahata, and D. Barnes. 1989. Primary and multipassage culture of mouse embryo cells in serum-containing and serum-free media, p. 17-35. In R. Baserga (ed.), Cell growth and division: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 37.Malumbres, M., I. Perez De Castro, M. I. Hernandez, M. Jimenez, T. Corral, and A. Pellicer. 2000. Cellular response to oncogenic Ras involves induction of the Cdk4 and Cdk6 inhibitor p15INK4b. Mol. Cell. Biol. 20:2915-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, R. W., and J. H. Rubinstein. 1995. Tumors in Rubinstein-Taybi syndrome. Am. J. Med. Genet. 56:112-115. [DOI] [PubMed] [Google Scholar]
- 39.Oike, Y., N. Takakura, A. Hata, T. Kaname, M. Akizuki, Y. Yamaguchi, H. Yasue, K. Araki, K. Yamamura, and T. Suda. 1999. Mice homozygous for a truncated form of CREB-binding protein exhibit defects in hematopoiesis and vasculo-angiogenesis. Blood 93:2771-2779. [PubMed] [Google Scholar]
- 40.Park, I. K., D. Qian, M. Kiel, M. W. Becker, M. Pihalja, I. L. Weissman, S. J. Morrison, and M. F. Clarke. 2003. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423:302-305. [DOI] [PubMed] [Google Scholar]
- 41.Petrij, F., R. H. Giles, H. G. Dauwerse, J. J. Saris, R. C. M. Hennekam, M. Masuno, N. Tommerup, G. B. Ommen, R. H. Goodman, D. J. M. Peters, and M. H. Breuning. 1995. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376:348-351. [DOI] [PubMed] [Google Scholar]
- 42.Schneider, J. E., S. D. Bamforth, C. R. Farthing, K. Clarke, S. Neubauer, and S. Bhattacharya. 2003. High-resolution imaging of normal anatomy, and neural and adrenal malformations in mouse embryos using magnetic resonance microscopy. J. Anat. 202:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider, J. E., S. D. Bamforth, C. R. Farthing, K. Clarke, S. Neubauer, and S. Bhattacharya. 2003. Rapid identification and 3D reconstruction of complex cardiac malformations in transgenic mouse embryos using fast gradient echo sequence magnetic resonance imaging. J. Mol. Cell. Cardiol. 35:217-222. [DOI] [PubMed] [Google Scholar]
- 44.Schneider, J. E., S. D. Bamforth, S. M. Grieve, K. Clarke, S. Bhattacharya, and S. Neubauer. 2003. High-resolution, high-throughput magnetic resonance imaging of mouse embryonic anatomy using a fast gradient-echo sequence. MAGMA 16:43-51. [DOI] [PubMed] [Google Scholar]
- 45.Serrano, M., H. Lee, L. Chin, C. Cordon-Cardo, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27-37. [DOI] [PubMed] [Google Scholar]
- 46.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 47.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413:86-91. [DOI] [PubMed] [Google Scholar]
- 48.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102:407-410. [DOI] [PubMed] [Google Scholar]
- 49.Sherr, C. J., and J. D. Weber. 2000. The ARF/p53 pathway. Curr. Opin. Genet. Dev. 10:94-99. [DOI] [PubMed] [Google Scholar]
- 50.Shikama, N., J. Lyon, and N. B. La Thangue. 1997. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell. Biol. 7:230-236. [DOI] [PubMed] [Google Scholar]
- 51.Sun, H. B., Y. X. Zhu, T. Yin, G. Sledge, and Y. C. Yang. 1998. MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc. Natl. Acad. Sci. USA 95:13555-13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tagawa, M., T. Sakamoto, K. Shigemoto, H. Matsubara, Y. Tamura, T. Ito, I. Nakamura, A. Okitsu, K. Imai, and M. Taniguchi. 1990. Expression of novel DNA-binding protein with zinc finger structure in various tumor cells. J. Biol. Chem. 265:20021-20026. [PubMed] [Google Scholar]
- 53.Tetsu, O., H. Ishihara, R. Kanno, M. Kamiyasu, H. Inoue, T. Tokuhisa, M. Taniguchi, and M. Kanno. 1998. mel-18 negatively regulates cell cycle progression upon B cell antigen receptor stimulation through a cascade leading to c-myc/cdc25. Immunity 9:439-448. [DOI] [PubMed] [Google Scholar]
- 54.Trimarchi, J. M., B. Fairchild, J. Wen, and J. A. Lees. 2001. The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc. Natl. Acad. Sci. USA 98:1519-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Lugt, N. M., J. Domen, K. Linders, M. van Roon, E. Robanus-Maandag, H. te Riele, M. van der Valk, J. Deschamps, M. Sofroniew, M. van Lohuizen, et al. 1994. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8:757-769. [DOI] [PubMed] [Google Scholar]
- 56.Weitzman, J. B., L. Fiette, K. Matsuo, and M. Yaniv. 2000. JunD protects cells from p53-dependent senescence and apoptosis. Mol. Cell 6:1109-1119. [DOI] [PubMed] [Google Scholar]
- 57.Weninger, W. J., and T. Mohun. 2002. Phenotyping transgenic embryos: a rapid 3-D screening method based on episcopic fluorescence image capturing. Nat. Genet. 30:59-65. [DOI] [PubMed] [Google Scholar]
- 58.Yahata, T., M. P. de Caestecker, R. J. Lechleider, S. Andriole, A. B. Roberts, K. J. Isselbacher, and T. Shioda. 2000. The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors. J. Biol. Chem. 275:8825-8834. [DOI] [PubMed] [Google Scholar]
- 59.Yahata, T., H. Takedatsu, S. L. Dunwoodie, J. Braganca, T. Swingler, S. L. Withington, J. Hur, K. R. Coser, K. J. Isselbacher, S. Bhattacharya, and T. Shioda. 2002. Cloning of mouse Cited4, a member of the CITED family p300/CBP-binding transcriptional coactivators: induced expression in mammary epithelial cells. Genomics 80:601-613. [DOI] [PubMed] [Google Scholar]
- 60.Yao, T. P., S. P. Oh, M. Fuchs, N. D. Zhou, L. E. Ch'ng, D. Newsome, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361-372. [DOI] [PubMed] [Google Scholar]
- 61.Yin, Z., J. Haynie, X. Yang, B. Han, S. Kiatchoosakun, J. Restivo, S. Yuan, N. R. Prabhakar, K. Herrup, R. A. Conlon, B. D. Hoit, M. Watanabe, and Y. C. Yang. 2002. The essential role of Cited2, a negative regulator for HIF-1α, in heart development and neurulation. Proc. Natl. Acad. Sci. USA 99:10488-10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zindy, F., D. E. Quelle, M. F. Roussel, and C. J. Sherr. 1997. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 15:203-211. [DOI] [PubMed] [Google Scholar]