Abstract
Tumour pH plays a significant role in cancer treatment. However, because of the limitations of the current measurement techniques, spatially and temporally resolved pH data, obtained non-invasively in solid tumours, are not available. Fluorescence ratio imaging microscopy (FRIM) has been used previously for noninvasive, dynamic evaluation of pH in neoplastic tissue in vivo (Martin GR, Jain RK 1994, Cancer Res., 54, 5670-5674). However, owing to problems associated with quantitative fluorescence in thick biological tissues, these studies were limited to thin (50 microns) tumours. We, therefore, adapted the FRIM technique for pH determination in thick (approximately 2 mm) solid tumours in vivo using a pinhole illumination-optical sectioning (PIOS) method. Results show that (1) steep interstitial pH gradients (5 microns resolution), with different spatial patterns, exist between tumour blood vessels; (2) pH decreased by an average of 0.10 pH units over a distance of 40 microns away from the blood vessel wall, and by 0.33 pH units over a 70 microns distance; (3) the maximum pH drop, defined as the pH difference between the intervessel midpoint and the vessel wall, was positively correlated with the intervessel distance; (4) 45 min following a systemic glucose injection (6 g kg-1 i.v), interstitial pH gradients were shifted to lower pH values by an average of 0.15 pH units, while the spatial gradient (slope) was maintained, when compared with preglucose values. This pH decrease was not accompanied by significant changes in local blood flow. pH gradients returned to near-baseline values 90 min after glucose injection; (5) interstitial tumour pH before hyperglycaemia and the glucose-induced pH drop strongly depended on the local vessel density; and (6) sodium bicarbonate treatment, either acute (1 M, 0.119 ml h-1 for 3 h i.v.) or chronic (1% in drinking water for 8 days), did not significantly change interstitial tumour pH. Modified FRIM may be combined with other optical methods (e.g. phosphorescence quenching) to evaluate non-invasively the spatial and temporal characteristics of extracellular pH, intracellular pH and pO2 in solid tumours. This will offer unique information about tumour metabolism and its modification by treatment modalities used in different cancer therapies.
Full text
PDF


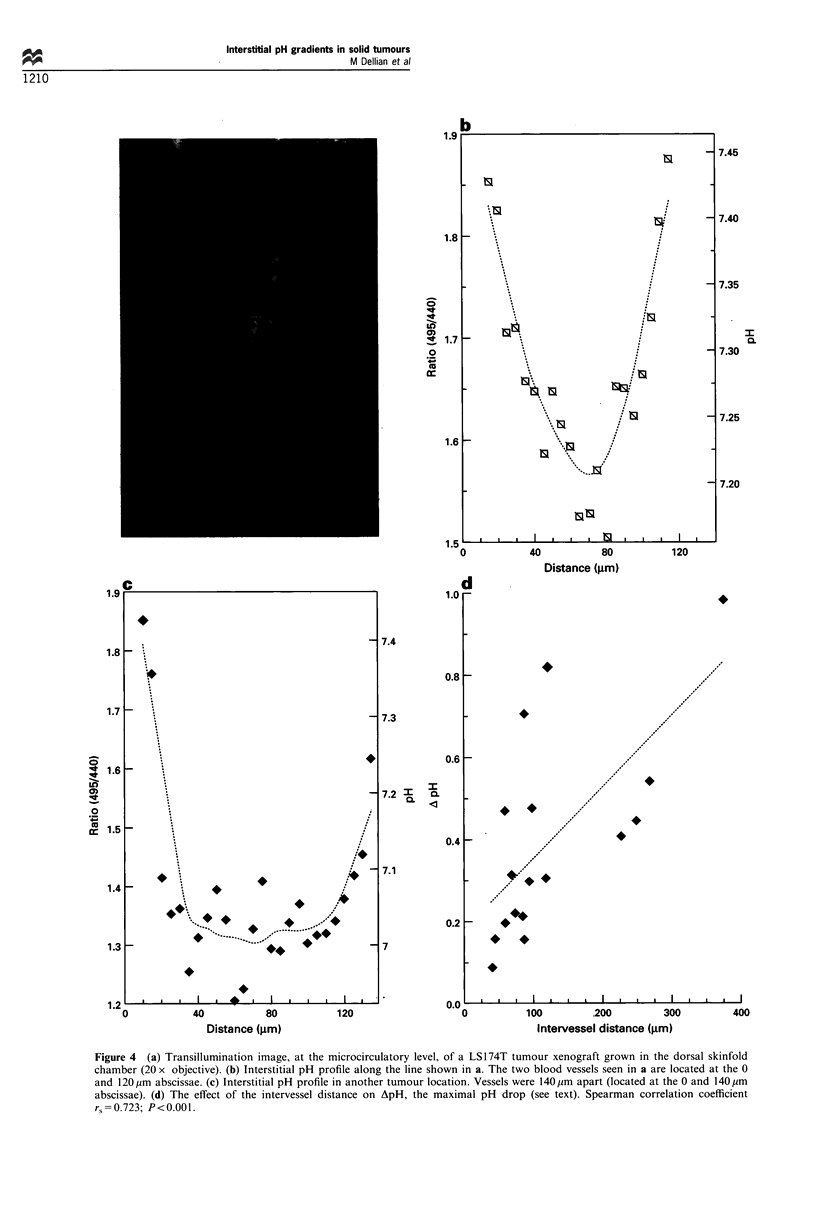




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby B. S. pH studies in human malignant tumours. Lancet. 1966 Aug 6;2(7458):312–315. doi: 10.1016/s0140-6736(66)92598-0. [DOI] [PubMed] [Google Scholar]
- Casciari J. J., Sotirchos S. V., Sutherland R. M. Glucose diffusivity in multicellular tumor spheroids. Cancer Res. 1988 Jul 15;48(14):3905–3909. [PubMed] [Google Scholar]
- Casciari J. J., Sotirchos S. V., Sutherland R. M. Mathematical modelling of microenvironment and growth in EMT6/Ro multicellular tumour spheroids. Cell Prolif. 1992 Jan;25(1):1–22. doi: 10.1111/j.1365-2184.1992.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Casciari J. J., Sotirchos S. V., Sutherland R. M. Variations in tumor cell growth rates and metabolism with oxygen concentration, glucose concentration, and extracellular pH. J Cell Physiol. 1992 May;151(2):386–394. doi: 10.1002/jcp.1041510220. [DOI] [PubMed] [Google Scholar]
- Daly P. F., Cohen J. S. Magnetic resonance spectroscopy of tumors and potential in vivo clinical applications: a review. Cancer Res. 1989 Feb 15;49(4):770–779. [PubMed] [Google Scholar]
- Dewhirst M. W., Secomb T. W., Ong E. T., Hsu R., Gross J. F. Determination of local oxygen consumption rates in tumors. Cancer Res. 1994 Jul 1;54(13):3333–3336. [PubMed] [Google Scholar]
- DiPette D. J., Ward-Hartley K. A., Jain R. K. Effect of glucose on systemic hemodynamics and blood flow rate in normal and tumor tissues in rats. Cancer Res. 1986 Dec;46(12 Pt 1):6299–6304. [PubMed] [Google Scholar]
- Engin K., Leeper D. B., Thistlethwaite A. J., Tupchong L., McFarlane J. D. Tumor extracellular pH as a prognostic factor in thermoradiotherapy. Int J Radiat Oncol Biol Phys. 1994 Apr 30;29(1):125–132. doi: 10.1016/0360-3016(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Gatenby R. A. The potential role of transformation-induced metabolic changes in tumor-host interaction. Cancer Res. 1995 Sep 15;55(18):4151–4156. [PubMed] [Google Scholar]
- Gerweck L. E., Rhee J. G., Koutcher J. A., Song C. W., Urano M. Regulation of pH in murine tumor and muscle. Radiat Res. 1991 May;126(2):206–209. [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Smith S. H., Haggerty A. C. Modifications of the acid-base status of the internal milieu of tumors. J Natl Cancer Inst. 1965 Jun;34(6):857–869. [PubMed] [Google Scholar]
- Hawkins R. A., Phelps M. E. PET in clinical oncology. Cancer Metastasis Rev. 1988 Jun;7(2):119–142. doi: 10.1007/BF00046482. [DOI] [PubMed] [Google Scholar]
- Hwang Y. Y., Kim S. G., Evelhoch J. L., Ackerman J. J. Nonglycolytic acidification of murine radiation-induced fibrosarcoma 1 tumor via 3-O-methyl-D-glucose monitored by 1H, 2H, 13C, and 31P nuclear magnetic resonance spectroscopy. Cancer Res. 1992 Mar 1;52(5):1259–1266. [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Jähde E., Rajewsky M. F. Tumor-selective modification of cellular microenvironment in vivo: effect of glucose infusion on the pH in normal and malignant rat tissues. Cancer Res. 1982 Apr;42(4):1505–1512. [PubMed] [Google Scholar]
- Kallinowski F., Vaupel P., Runkel S., Berg G., Fortmeyer H. P., Baessler K. H., Wagner K., Mueller-Klieser W., Walenta S. Glucose uptake, lactate release, ketone body turnover, metabolic micromilieu, and pH distributions in human breast cancer xenografts in nude rats. Cancer Res. 1988 Dec 15;48(24 Pt 1):7264–7272. [PubMed] [Google Scholar]
- Kallinowski F., Vaupel P. pH distributions in spontaneous and isotransplanted rat tumours. Br J Cancer. 1988 Sep;58(3):314–321. doi: 10.1038/bjc.1988.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K., Guth P. H., Kaunitz J. D. In vivo measurement of rat gastric surface cell intracellular pH. Am J Physiol. 1991 Sep;261(3 Pt 1):G548–G552. doi: 10.1152/ajpgi.1991.261.3.G548. [DOI] [PubMed] [Google Scholar]
- Karuri A. R., Dobrowsky E., Tannock I. F. Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br J Cancer. 1993 Dec;68(6):1080–1087. doi: 10.1038/bjc.1993.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunig M., Yuan F., Menger M. D., Boucher Y., Goetz A. E., Messmer K., Jain R. K. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992 Dec 1;52(23):6553–6560. [PubMed] [Google Scholar]
- Martin G. R., Jain R. K. Fluorescence ratio imaging measurement of pH gradients: calibration and application in normal and tumor tissues. Microvasc Res. 1993 Sep;46(2):216–230. doi: 10.1006/mvre.1993.1048. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Jain R. K. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994 Nov 1;54(21):5670–5674. [PubMed] [Google Scholar]
- Martin G. R., Jain R. K. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994 Nov 1;54(21):5670–5674. [PubMed] [Google Scholar]
- Mordon S., Devoisselle J. M., Maunoury V. In vivo pH measurement and imaging of tumor tissue using a pH-sensitive fluorescent probe (5,6-carboxyfluorescein): instrumental and experimental studies. Photochem Photobiol. 1994 Sep;60(3):274–279. doi: 10.1111/j.1751-1097.1994.tb05104.x. [DOI] [PubMed] [Google Scholar]
- Newell K., Franchi A., Pouysségur J., Tannock I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1127–1131. doi: 10.1073/pnas.90.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Salomon J. C., Silvestre P. Isolation of a Chinese hamster fibroblast mutant defective in hexose transport and aerobic glycolysis: its use to dissect the malignant phenotype. Proc Natl Acad Sci U S A. 1980 May;77(5):2698–2701. doi: 10.1073/pnas.77.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D., Robinson B., Tannock I. F. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer Res. 1986 Jun;46(6):2821–2826. [PubMed] [Google Scholar]
- Spencer T. L., Lehninger A. L. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976 Feb 15;154(2):405–414. doi: 10.1042/bj1540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanke H. J., van Oostveldt P., van Duijn P. A parameter for the distribution of fluorophores in cells derived from measurements of inner filter effect and reabsorption phenomenon. Cytometry. 1982 May;2(6):359–369. doi: 10.1002/cyto.990020602. [DOI] [PubMed] [Google Scholar]
- Torres Filho I. P., Leunig M., Yuan F., Intaglietta M., Jain R. K. Noninvasive measurement of microvascular and interstitial oxygen profiles in a human tumor in SCID mice. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2081–2085. doi: 10.1073/pnas.91.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P. W., Frinak S., Bicher H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 1981 May;41(5):2008–2013. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Volk T., Jähde E., Fortmeyer H. P., Glüsenkamp K. H., Rajewsky M. F. pH in human tumour xenografts: effect of intravenous administration of glucose. Br J Cancer. 1993 Sep;68(3):492–500. doi: 10.1038/bjc.1993.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward-Hartley K. A., Jain R. K. Effect of glucose and galactose on microcirculatory flow in normal and neoplastic tissues in rabbits. Cancer Res. 1987 Jan 15;47(2):371–377. [PubMed] [Google Scholar]
- Ward K. A., Jain R. K. Response of tumours to hyperglycaemia: characterization, significance and role in hyperthermia. Int J Hyperthermia. 1988 May-Jun;4(3):223–250. doi: 10.3109/02656738809051100. [DOI] [PubMed] [Google Scholar]
- von Ardenne M., von Ardenne A. Berechnung des pH-Profils im Interkapillarraum der Krebsgewebe für die F¿lle mit und ohne Langzeit-Glukose-Infusion. Res Exp Med (Berl) 1977 Sep 30;171(2):177–189. doi: 10.1007/BF01851365. [DOI] [PubMed] [Google Scholar]