Abstract
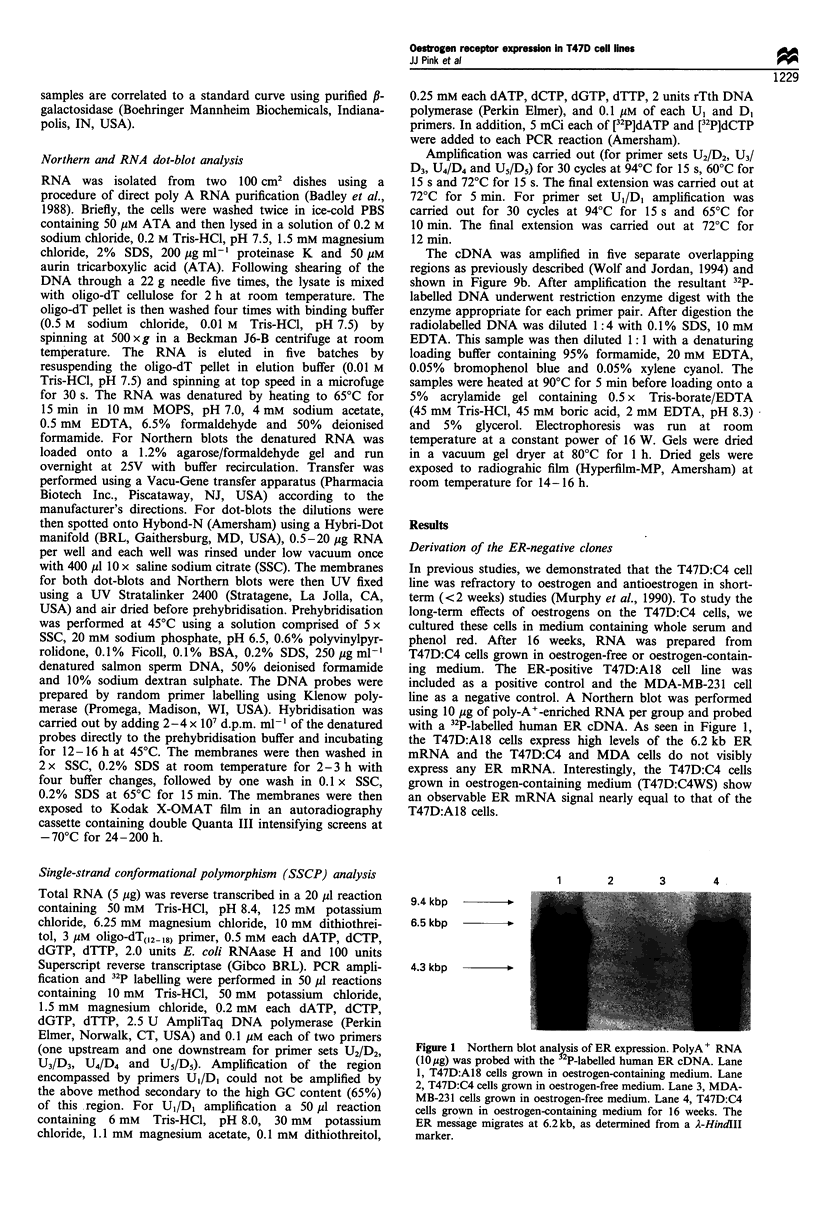
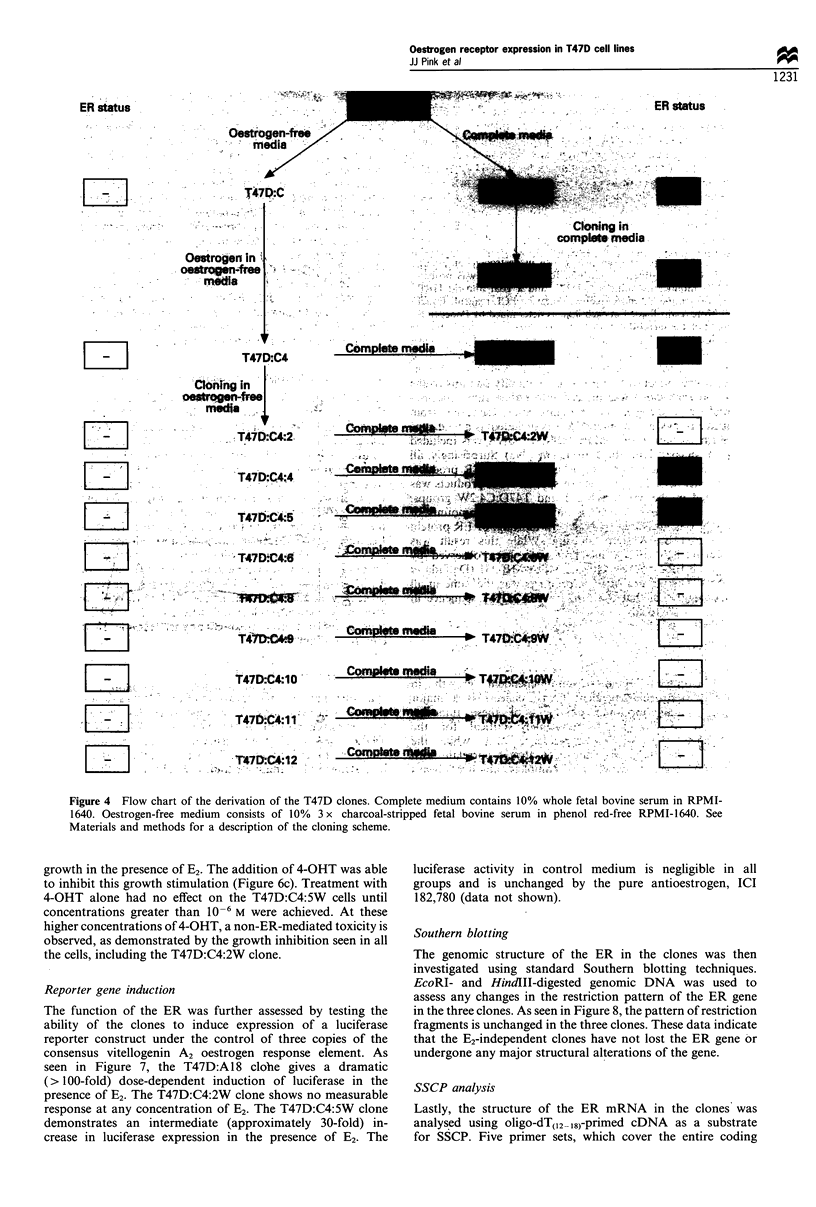
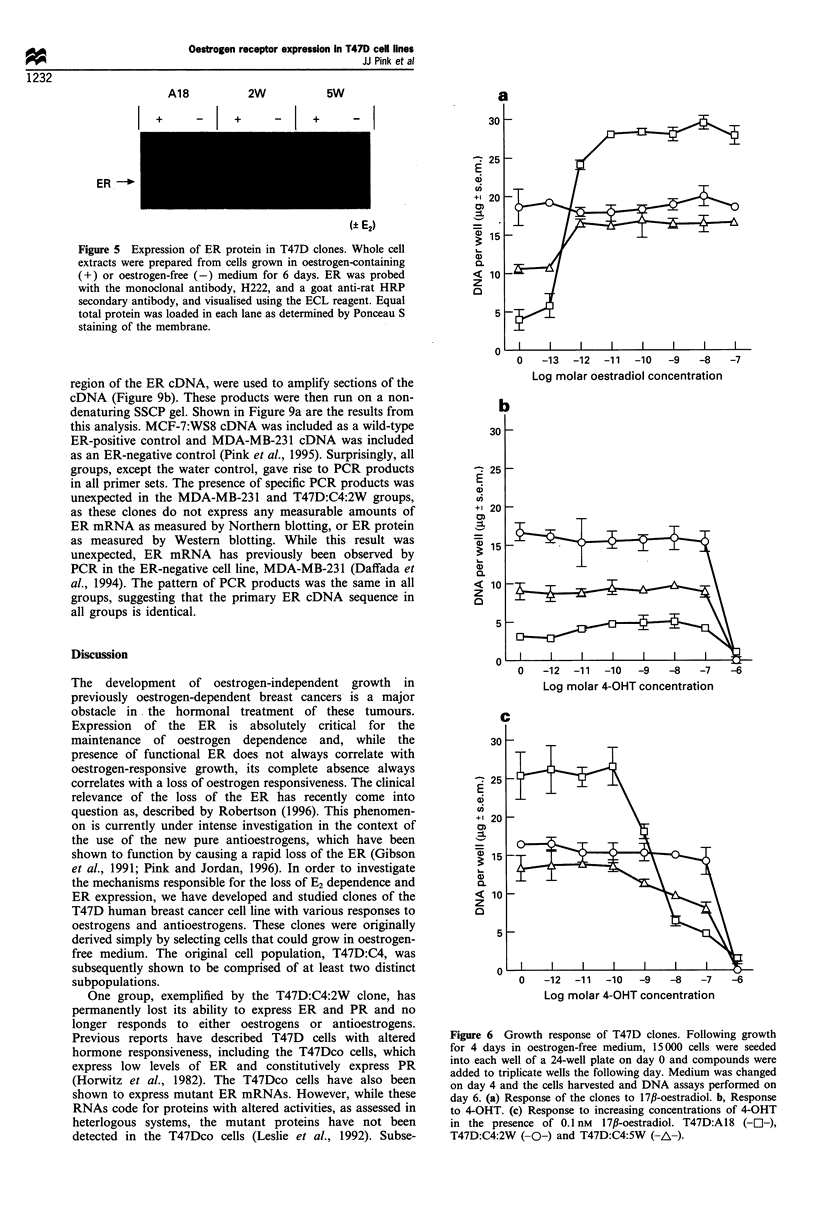
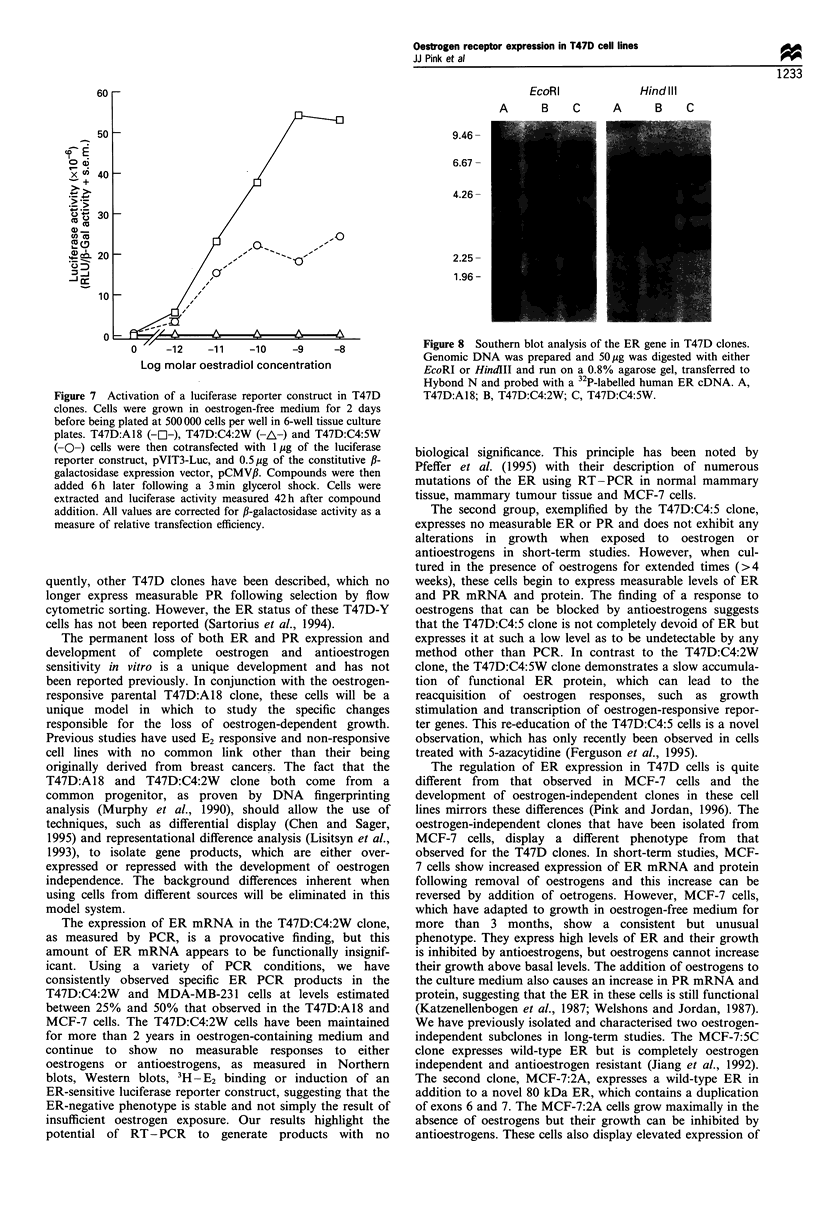
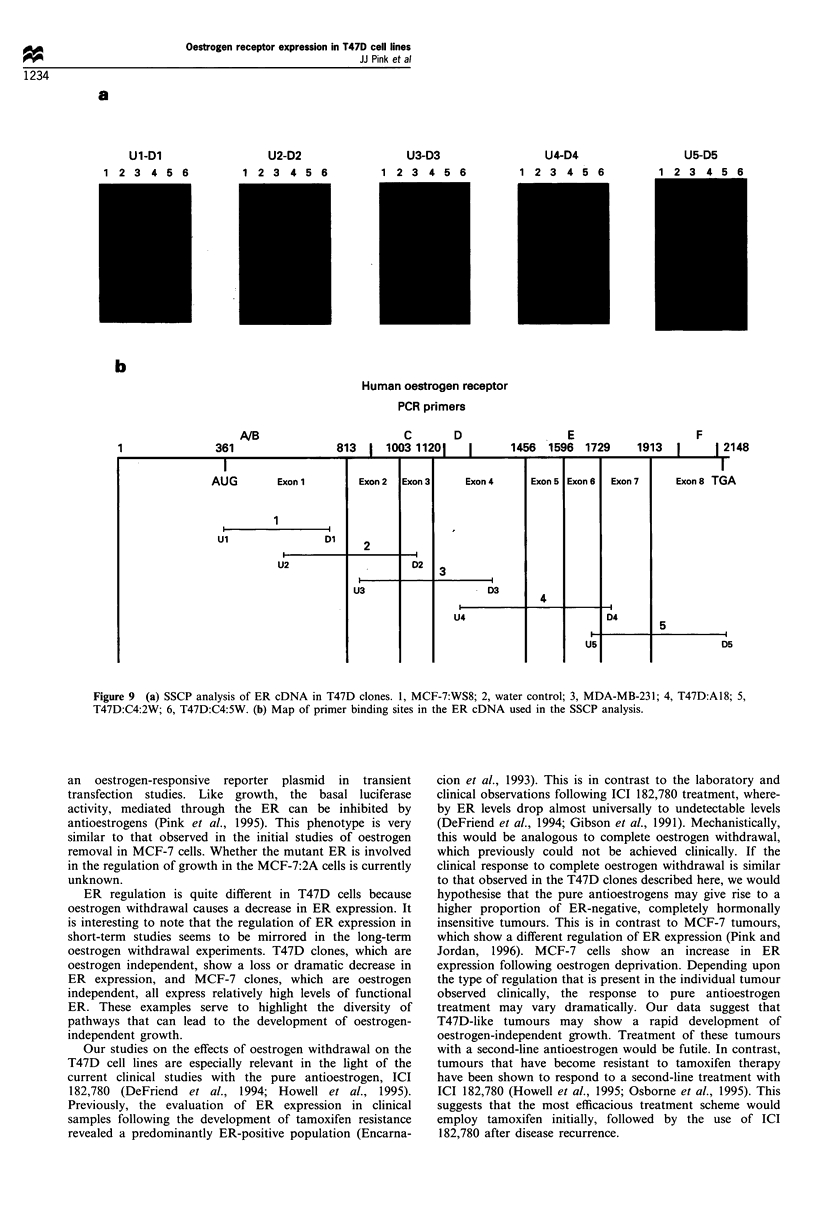
The development of antioestrogen resistance is a major clinical obstacle encountered in the treatment of breast cancer. By long-term growth in oestrogen-free medium, we have derived an oestrogen-independent, anti-oestrogen resistant cell line from the oestrogen receptor (ER)-positive, oestrogen-dependent T47D human breast cancer cell line. This cell line grows maximally in oestrogen-free medium and is resistant to all tested antioestrogens. This cell line does not express any measurable amounts of ER mRNA or protein and, in short-term studies, these cells show no response to either oestrogens or antioestrogens. However, return of these cells to oestrogen-containing medium for more than 8 weeks resulted in the re-expression of ER mRNA and protein. Subsequent limiting dilution subcloning of the T47D:C4 line revealed two phenotypically distinct clones, one which did not express measurable ER after long-term growth in oestrogen-containing medium and one which expressed ER mRNA and protein after a number of weeks in oestrogen-containing medium. In the absence of oestrogen, both types of cells are ER-negative as determined by Northern and Western blotting and lack of any oestrogen-dependent responses. The clone which re-expresses the ER (T47D:C4:5W) now responds to E2 with a 50% increase in growth and a 30-fold induction of an ER-responsive luciferase reporter construct. Long-term growth of the stably ER-negative clone (T47D:C4:2W) causes no measurable oestrogen-mediated responses, as assessed by ER expression, growth stimulation or luciferase induction. Interestingly, ER mRNA can be detected in both cell types by using reverse transcriptase-polymerase chain reaction (RT-PCR). This suggests that the ER mRNA present in the T47D:C4:2W clone is either inefficiently translated or is present at such a low level as to be functionally irrelevant. These novel clonal cell lines will prove to be invaluable in the study of the regulation of ER expression and regulatory pathways leading to oestrogen-independent growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. C., Locke E. R., Soule H. D. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973 Sep 10;248(17):6251–6253. [PubMed] [Google Scholar]
- Brünner N., Boulay V., Fojo A., Freter C. E., Lippman M. E., Clarke R. Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Res. 1993 Jan 15;53(2):283–290. [PubMed] [Google Scholar]
- Chalbos D., Vignon F., Keydar I., Rochefort H. Estrogens stimulate cell proliferation and induce secretory proteins in a human breast cancer cell line (T47D). J Clin Endocrinol Metab. 1982 Aug;55(2):276–283. doi: 10.1210/jcem-55-2-276. [DOI] [PubMed] [Google Scholar]
- Chen Z., Sager R. Differential expression of human tissue factor in normal mammary epithelial cells and in carcinomas. Mol Med. 1995 Jan;1(2):153–160. [PMC free article] [PubMed] [Google Scholar]
- Clark G. M., Osborne C. K., McGuire W. L. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984 Oct;2(10):1102–1109. doi: 10.1200/JCO.1984.2.10.1102. [DOI] [PubMed] [Google Scholar]
- Clarke R., Brünner N., Katzenellenbogen B. S., Thompson E. W., Norman M. J., Koppi C., Paik S., Lippman M. E., Dickson R. B. Progression of human breast cancer cells from hormone-dependent to hormone-independent growth both in vitro and in vivo. Proc Natl Acad Sci U S A. 1989 May;86(10):3649–3653. doi: 10.1073/pnas.86.10.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffada A. A., Johnston S. R., Nicholls J., Dowsett M. Detection of wild type and exon 5-deleted splice variant oestrogen receptor (ER) mRNA in ER-positive and -negative breast cancer cell lines by reverse transcription/polymerase chain reaction. J Mol Endocrinol. 1994 Dec;13(3):265–273. doi: 10.1677/jme.0.0130265. [DOI] [PubMed] [Google Scholar]
- DeFriend D. J., Howell A., Nicholson R. I., Anderson E., Dowsett M., Mansel R. E., Blamey R. W., Bundred N. J., Robertson J. F., Saunders C. Investigation of a new pure antiestrogen (ICI 182780) in women with primary breast cancer. Cancer Res. 1994 Jan 15;54(2):408–414. [PubMed] [Google Scholar]
- Dickson R. B., Lippman M. E. Hormonal control of human breast cancer cell lines. Cancer Surv. 1986;5(3):617–624. [PubMed] [Google Scholar]
- Encarnación C. A., Ciocca D. R., McGuire W. L., Clark G. M., Fuqua S. A., Osborne C. K. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat. 1993;26(3):237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- Ferguson A. T., Lapidus R. G., Baylin S. B., Davidson N. E. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 1995 Jun 1;55(11):2279–2283. [PubMed] [Google Scholar]
- Fernandez P., Burghardt R., Smith R., Nodland K., Safe S. High passage T47D human breast cancer cells: altered endocrine and 2,3,7,8-tetrachlorodibenzo-p-dioxin responsiveness. Eur J Pharmacol. 1994 Jan 3;270(1):53–65. doi: 10.1016/0926-6917(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Gibson M. K., Nemmers L. A., Beckman W. C., Jr, Davis V. L., Curtis S. W., Korach K. S. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991 Oct;129(4):2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- Graham M. L., 2nd, Krett N. L., Miller L. A., Leslie K. K., Gordon D. F., Wood W. M., Wei L. L., Horwitz K. B. T47DCO cells, genetically unstable and containing estrogen receptor mutations, are a model for the progression of breast cancers to hormone resistance. Cancer Res. 1990 Oct 1;50(19):6208–6217. [PubMed] [Google Scholar]
- Horwitz K. B., Freidenberg G. R. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985 Jan;45(1):167–173. [PubMed] [Google Scholar]
- Horwitz K. B., Mockus M. B., Lessey B. A. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell. 1982 Mar;28(3):633–642. doi: 10.1016/0092-8674(82)90218-5. [DOI] [PubMed] [Google Scholar]
- Howell A., DeFriend D., Robertson J., Blamey R., Walton P. Response to a specific antioestrogen (ICI 182780) in tamoxifen-resistant breast cancer. Lancet. 1995 Jan 7;345(8941):29–30. doi: 10.1016/s0140-6736(95)91156-1. [DOI] [PubMed] [Google Scholar]
- Jiang S. Y., Wolf D. M., Yingling J. M., Chang C., Jordan V. C. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol Cell Endocrinol. 1992 Dec;90(1):77–86. doi: 10.1016/0303-7207(92)90104-e. [DOI] [PubMed] [Google Scholar]
- Karey K. P., Sirbasku D. A. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 1988 Jul 15;48(14):4083–4092. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Kendra K. L., Norman M. J., Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987 Aug 15;47(16):4355–4360. [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Leslie K. K., Tasset D. M., Horwitz K. B. Functional analysis of a mutant estrogen receptor isolated from T47Dco breast cancer cells. Am J Obstet Gynecol. 1992 Apr;166(4):1053–1061. doi: 10.1016/s0002-9378(11)90590-0. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Lisitsyn N., Lisitsyn N., Wigler M. Cloning the differences between two complex genomes. Science. 1993 Feb 12;259(5097):946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- Luyten G. P., Hoogeveen A. T., Galjaard H. A fluorescence staining method for the demonstration and measurement of lysosomal enzyme activities in single cells. J Histochem Cytochem. 1985 Sep;33(9):965–968. doi: 10.1177/33.9.3926869. [DOI] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick A., Chambon P. Characterization of the estrogen receptor in two antiestrogen-resistant cell lines, LY2 and T47D. Cancer Res. 1990 Jan 15;50(2):333–338. [PubMed] [Google Scholar]
- Murphy C. S., Meisner L. F., Wu S. Q., Jordan V. C. Short- and long-term estrogen deprivation of T47D human breast cancer cells in culture. Eur J Cancer Clin Oncol. 1989 Dec;25(12):1777–1788. doi: 10.1016/0277-5379(89)90348-9. [DOI] [PubMed] [Google Scholar]
- Murphy C. S., Pink J. J., Jordan V. C. Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res. 1990 Nov 15;50(22):7285–7292. [PubMed] [Google Scholar]
- Nardulli A. M., Katzenellenbogen B. S. Progesterone receptor regulation in T47D human breast cancer cells: analysis by density labeling of progesterone receptor synthesis and degradation and their modulation by progestin. Endocrinology. 1988 Apr;122(4):1532–1540. doi: 10.1210/endo-122-4-1532. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Coronado-Heinsohn E. B., Hilsenbeck S. G., McCue B. L., Wakeling A. E., McClelland R. A., Manning D. L., Nicholson R. I. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995 May 17;87(10):746–750. doi: 10.1093/jnci/87.10.746. [DOI] [PubMed] [Google Scholar]
- Pfeffer U., Fecarotta E., Vidali G. Coexpression of multiple estrogen receptor variant messenger RNAs in normal and neoplastic breast tissues and in MCF-7 cells. Cancer Res. 1995 May 15;55(10):2158–2165. [PubMed] [Google Scholar]
- Pink J. J., Jiang S. Y., Fritsch M., Jordan V. C. An estrogen-independent MCF-7 breast cancer cell line which contains a novel 80-kilodalton estrogen receptor-related protein. Cancer Res. 1995 Jun 15;55(12):2583–2590. [PubMed] [Google Scholar]
- Pink J. J., Jordan V. C. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996 May 15;56(10):2321–2330. [PubMed] [Google Scholar]
- Pink J. J., Wu S. Q., Wolf D. M., Bilimoria M. M., Jordan V. C. A novel 80 kDa human estrogen receptor containing a duplication of exons 6 and 7. Nucleic Acids Res. 1996 Mar 1;24(5):962–969. doi: 10.1093/nar/24.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese C. C., Warshaw M. L., Murai J. T., Siiteri P. K. Alternative models for estrogen and androgen regulation of human breast cancer cell (T47D) growth. Ann N Y Acad Sci. 1988;538:112–121. doi: 10.1111/j.1749-6632.1988.tb48856.x. [DOI] [PubMed] [Google Scholar]
- Robinson S. P., Jordan V. C. The paracrine stimulation of MCF-7 cells by MDA-MB-231 cells: possible role in antiestrogen failure. Eur J Cancer Clin Oncol. 1989 Mar;25(3):493–497. doi: 10.1016/0277-5379(89)90262-9. [DOI] [PubMed] [Google Scholar]
- Sartorius C. A., Groshong S. D., Miller L. A., Powell R. L., Tung L., Takimoto G. S., Horwitz K. B. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994 Jul 15;54(14):3868–3877. [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Jordan V. C. Adaptation of estrogen-dependent MCF-7 cells to low estrogen (phenol red-free) culture. Eur J Cancer Clin Oncol. 1987 Dec;23(12):1935–1939. doi: 10.1016/0277-5379(87)90062-9. [DOI] [PubMed] [Google Scholar]
- Wilding G., Lippman M. E., Dickson R. B. The cellular response of human breast cancer to estrogen. Prog Clin Biol Res. 1988;262:181–196. [PubMed] [Google Scholar]
- Wolf D. M., Jordan V. C. The estrogen receptor from a tamoxifen stimulated MCF-7 tumor variant contains a point mutation in the ligand binding domain. Breast Cancer Res Treat. 1994;31(1):129–138. doi: 10.1007/BF00689683. [DOI] [PubMed] [Google Scholar]