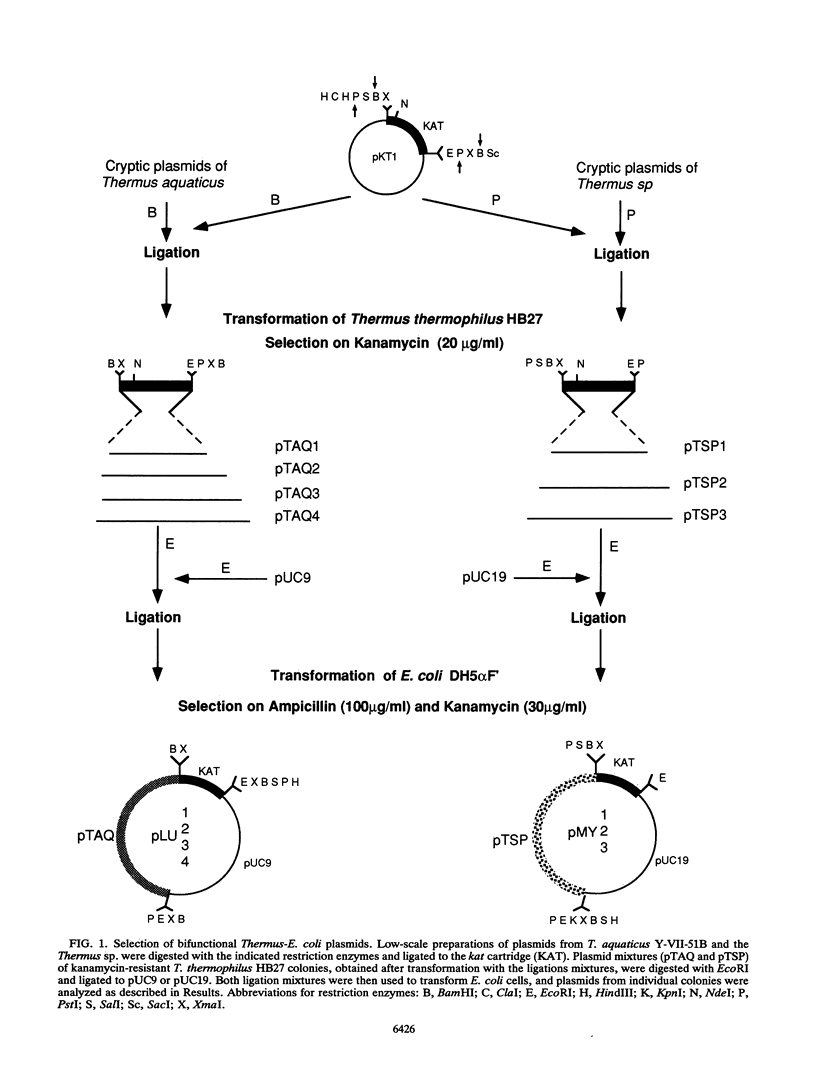
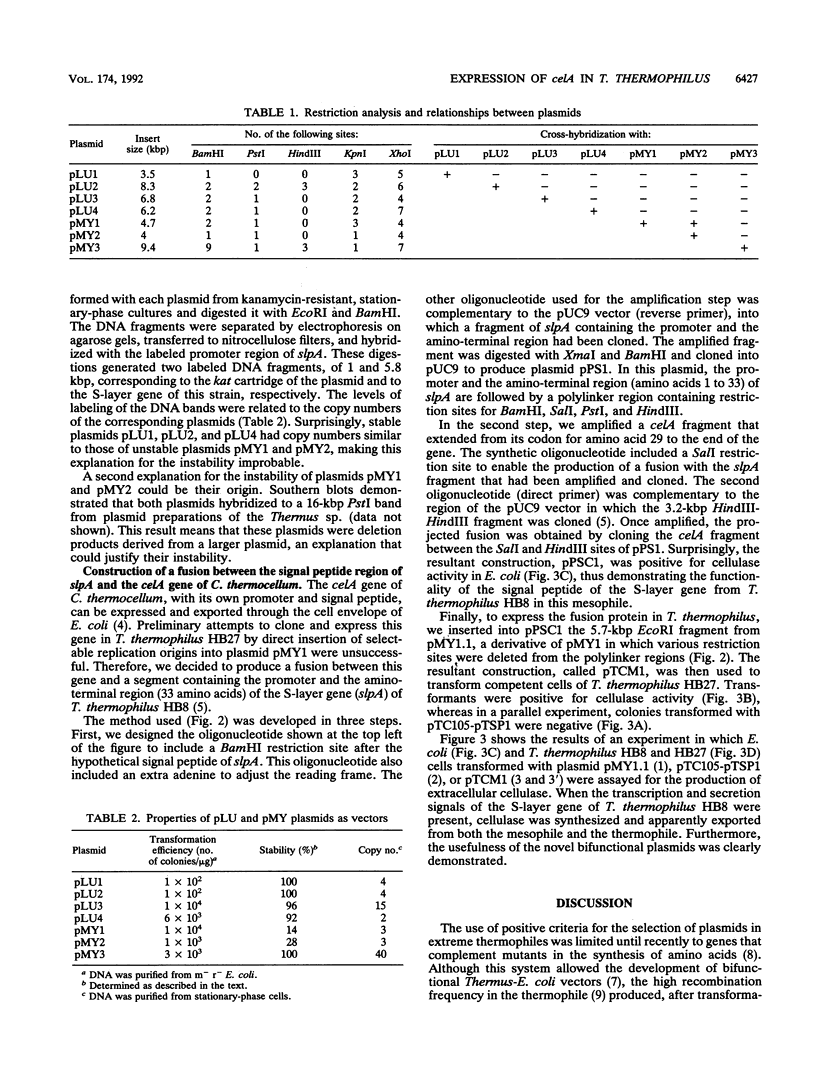
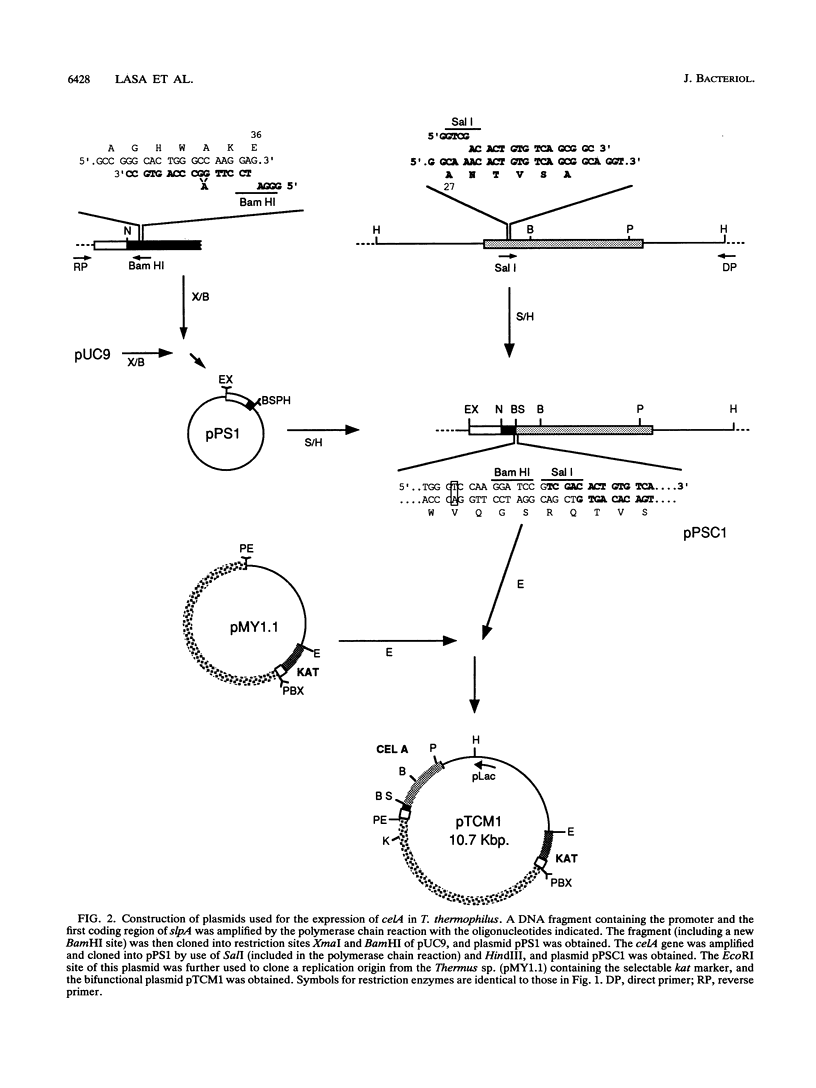
Abstract
We describe the self-selection of replication origins of undescribed cryptic plasmids from Thermus aquaticus Y-VII-51B (ATCC 25105) and a Thermus sp. strain (ATCC 27737) by random insertion of a thermostable kanamycin adenyltransferase cartridge. Once selected, these autonomous replication origins were cloned into the Escherichia coli vector pUC9 or pUC19. The bifunctional plasmids were analyzed for their sizes, relationships, and properties as shuttle vectors for Thermus-Escherichia cloning. Seven different vectors with diverse kanamycin resistance levels, stabilities, transformation efficiencies, and copy numbers were obtained. As a general rule, those from T. aquaticus (pLU1 to pLU4) were more stable than those from the Thermus sp. (pMY1 to pMY3). To probe their usefulness, we used one of the plasmids (pMY1) to clone in E. coli a modified form of the cellulase gene (celA) from Clostridium thermocellum in which the native signal peptide was replaced in vitro by that from the S-layer gene of T. thermophilus HB8. The hybrid product was expressed and exported by E. coli. When the gene was transferred by transformation into T. thermophilus, the cellulase protein was also expressed and secreted at 70 degrees C.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P., Cornet P., Aubert J. P. Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum. J Bacteriol. 1985 Apr;162(1):102–105. doi: 10.1128/jb.162.1.102-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraldo M. L., de Pedro M. A., Berenguer J. Cloning and expression in Escherichia coli of the structural gene coding for the monomeric protein of the S layer of Thermus thermophilus HB8. J Bacteriol. 1991 Sep;173(17):5346–5351. doi: 10.1128/jb.173.17.5346-5351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y., Arikawa Y., Furukawa K. A plasmid vector for an extreme thermophile, Thermus thermophilus. FEMS Microbiol Lett. 1990 Oct;60(1-2):97–101. doi: 10.1016/0378-1097(90)90352-q. [DOI] [PubMed] [Google Scholar]
- Koyama Y., Furukawa K. Cloning and sequence analysis of tryptophan synthetase genes of an extreme thermophile, Thermus thermophilus HB27: plasmid transfer from replica-plated Escherichia coli recombinant colonies to competent T. thermophilus cells. J Bacteriol. 1990 Jun;172(6):3490–3495. doi: 10.1128/jb.172.6.3490-3495.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y., Hoshino T., Tomizuka N., Furukawa K. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J Bacteriol. 1986 Apr;166(1):338–340. doi: 10.1128/jb.166.1.338-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lasa I., Castón J. R., Fernández-Herrero L. A., de Pedro M. A., Berenguer J. Insertional mutagenesis in the extreme thermophilic eubacteria Thermus thermophilus HB8. Mol Microbiol. 1992 Jun;6(11):1555–1564. doi: 10.1111/j.1365-2958.1992.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mather M. W., Fee J. A. Development of plasmid cloning vectors for Thermus thermophilus HB8: expression of a heterologous, plasmid-borne kanamycin nucleotidyltransferase gene. Appl Environ Microbiol. 1992 Jan;58(1):421–425. doi: 10.1128/aem.58.1.421-425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M., Yasumura S., Aiba S. Cumulative effect of intragenic amino-acid replacements on the thermostability of a protein. 1986 Sep 25-Oct 1Nature. 323(6086):356–358. doi: 10.1038/323356a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]