Abstract
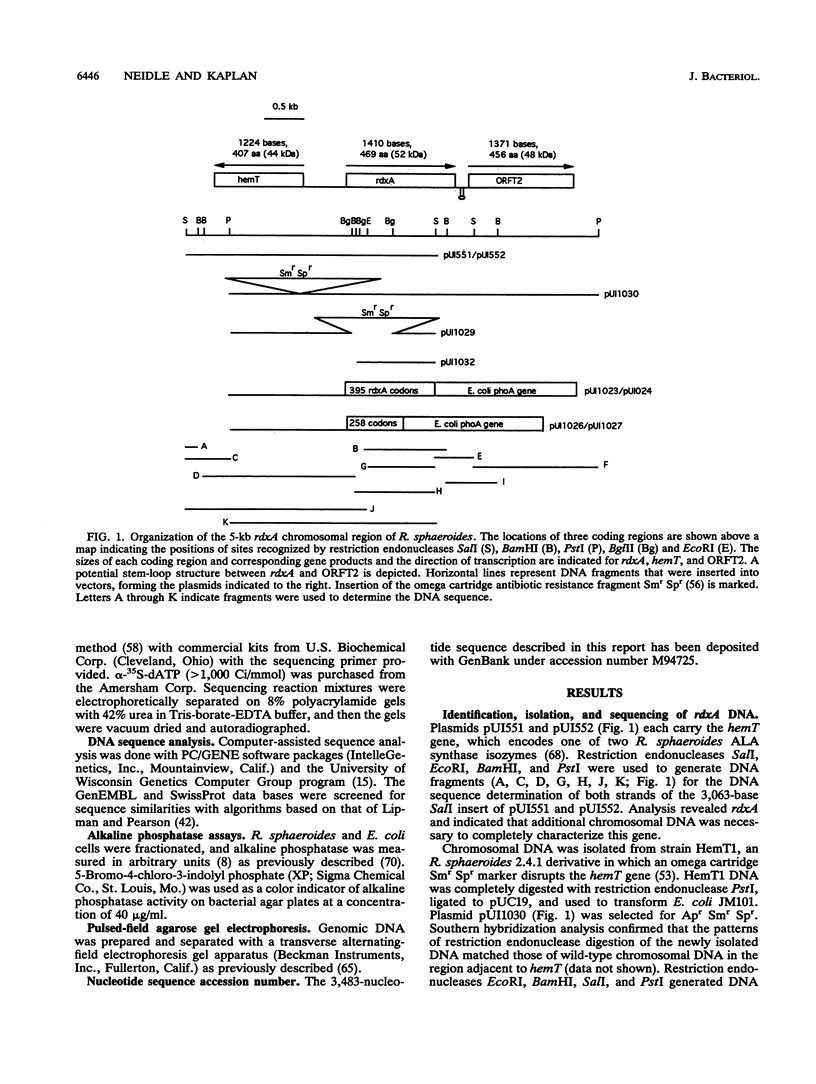
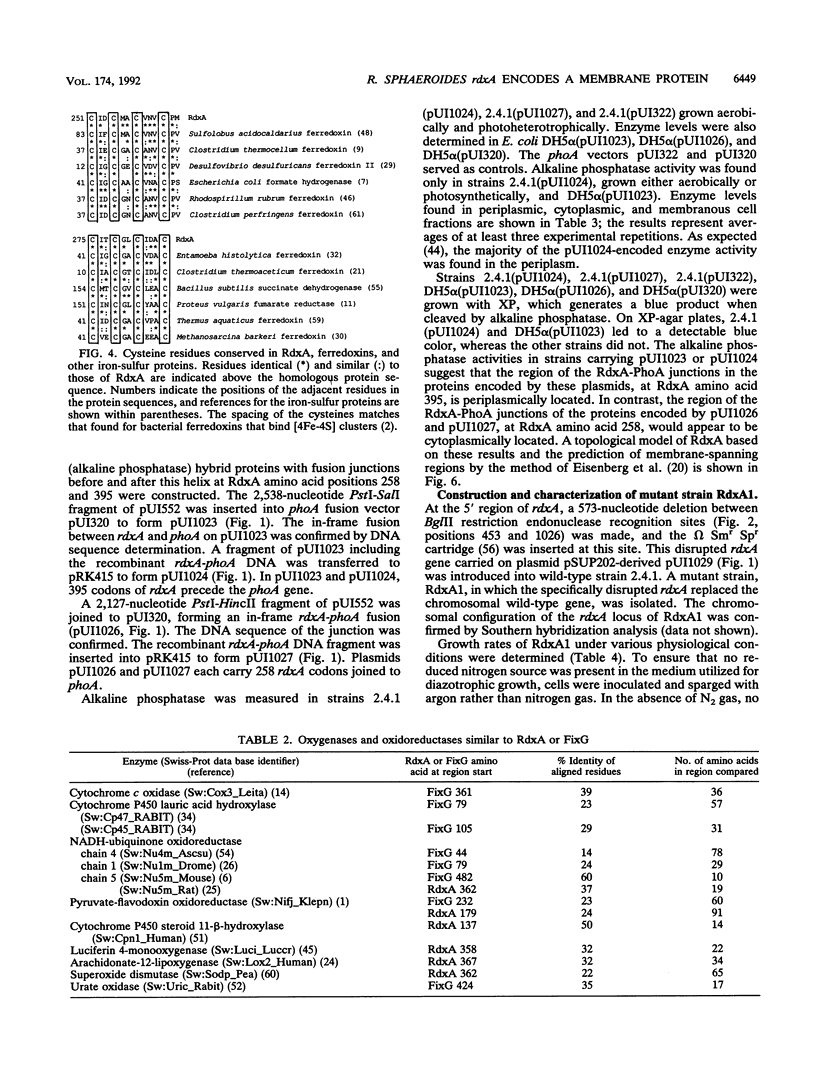
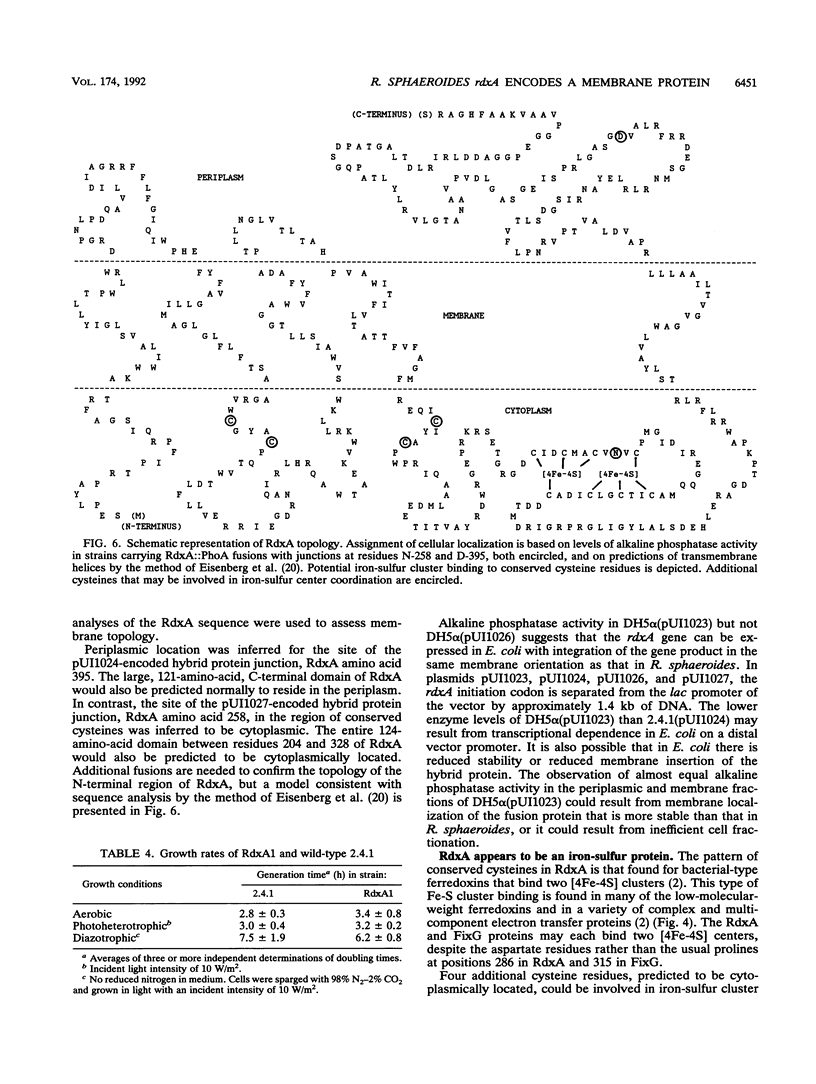
In the photosynthetic bacterium Rhodobacter sphaeroides, a chromosomal gene, rdxA, which encodes a 52-kDa protein, was found to be homologous to fixG, the first gene of a Rhizobium meliloti nitrogen fixation operon on the pSym plasmid (D. Kahn, M. David, O. Domergue, M.-L. Daveran, J. Ghai, P. R. Hirsch, and J. Batut, J. Bacteriol. 171:929-939, 1989). The deduced amino acid sequences of RdxA and FixG are 53% identical and 73% similar; sequence analyses suggested that each has five transmembrane helices and a central region resembling bacterial-type ferredoxins. Translational fusion proteins with an alkaline phosphatase reporter group were expressed in both R. sphaeroides and Escherichia coli and were used to assess the membrane topology of RdxA. Its ferredoxinlike sequence, which may bind two [4Fe-4S] centers, was found to be cytoplasmically located. Genetic disruptions showed that rdxA is not essential for nitrogen fixation in R. sphaeroides. Immediately downstream of rdxA, an open reading frame (ORFT2) that encoded a 48-kDa protein was found. This DNA sequence was not homologous to any region of the R. meliloti fixG operon. The N-terminal sequence of the ORFT2 gene product resembled amino acid sequences found in members of the GntR family of regulatory proteins (D. J. Haydon and J. R. Guest, FEMS Microbiol. Lett. 79:291-296, 1991). The rdxA gene was localized to the smaller of two R. sphaeroides chromosomes, upstream of and divergently transcribed from hemT, which encodes one of two 5-aminolevulinate synthase isozymes. The rdxA and hemT genes may share a transcriptional regulatory region. Southern hybridization analysis demonstrated the presence of an rdxA homolog on the R. sphaeroides large chromosome. The functions of this homolog, like those of rdxA, remain to be determined, but roles in oxidation-reduction processes are likely.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Rump A., Klipp W., Priefer U. B., Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988 Oct 5;203(3):715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990 May;4(8):2483–2491. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Buck D., Guest J. R. Overexpression and site-directed mutagenesis of the succinyl-CoA synthetase of Escherichia coli and nucleotide sequence of a gene (g30) that is adjacent to the suc operon. Biochem J. 1989 Jun 15;260(3):737–747. doi: 10.1042/bj2600737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm R., Sauter M., Böck A. Nucleotide sequence and expression of an operon in Escherichia coli coding for formate hydrogenlyase components. Mol Microbiol. 1990 Feb;4(2):231–243. doi: 10.1111/j.1365-2958.1990.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Cole S. T. Nucleotide sequence and comparative analysis of the frd operon encoding the fumarate reductase of Proteus vulgaris. Extensive sequence divergence of the membrane anchors and absence of an frd-linked ampC cephalosporinase gene. Eur J Biochem. 1987 Sep 15;167(3):481–488. doi: 10.1111/j.1432-1033.1987.tb13362.x. [DOI] [PubMed] [Google Scholar]
- Colonna-Romano S., Arnold W., Schlüter A., Boistard P., Pühler A., Priefer U. B. An Fnr-like protein encoded in Rhizobium leguminosarum biovar viciae shows structural and functional homology to Rhizobium meliloti FixK. Mol Gen Genet. 1990 Aug;223(1):138–147. doi: 10.1007/BF00315806. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Haran T. E., Nadeau J. G. Intrinsically bent DNA. J Biol Chem. 1990 May 5;265(13):7093–7096. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRusso C. C. Nucleotide sequence of the fadR gene, a multifunctional regulator of fatty acid metabolism in Escherichia coli. Nucleic Acids Res. 1988 Aug 25;16(16):7995–8009. doi: 10.1093/nar/16.16.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein R., Scheirer D. C., Fowle W. H., Ausubel F. M. Nodules elicited by Rhizobium meliloti heme mutants are arrested at an early stage of development. Mol Gen Genet. 1991 Dec;230(3):423–432. doi: 10.1007/BF00280299. [DOI] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Systematic method for the detection of potential lambda Cro-like DNA-binding regions in proteins. J Mol Biol. 1987 Apr 5;194(3):557–564. doi: 10.1016/0022-2836(87)90681-4. [DOI] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 1990 Dec 25;18(24):7267–7277. doi: 10.1093/nar/18.24.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Yang S. S., Ljungdahl L. G., Travis J., Reilly C. F. Complete amino acid sequence of the 4Fe-4S, thermostable ferredoxin from Clostridium thermoaceticum. Biochemistry. 1982 Jul 6;21(14):3294–3298. doi: 10.1021/bi00257a007. [DOI] [PubMed] [Google Scholar]
- Fickett J. W. Recognition of protein coding regions in DNA sequences. Nucleic Acids Res. 1982 Sep 11;10(17):5303–5318. doi: 10.1093/nar/10.17.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita T., Miwa Y., Nihashi J., Aratani Y. Organization and transcription of the gluconate operon, gnt, of Bacillus subtilis. J Biol Chem. 1986 Oct 15;261(29):13744–13753. [PubMed] [Google Scholar]
- Funk C. D., Furci L., FitzGerald G. A. Molecular cloning, primary structure, and expression of the human platelet/erythroleukemia cell 12-lipoxygenase. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5638–5642. doi: 10.1073/pnas.87.15.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989 Jun;28(6):497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Garesse R. Drosophila melanogaster mitochondrial DNA: gene organization and evolutionary considerations. Genetics. 1988 Apr;118(4):649–663. doi: 10.1093/genetics/118.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez M. A., Ditta G. S., Helinski D. R. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991 Mar 14;350(6314):170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- Guerlesquin F., Bruschi M., Bovier-Lapierre G., Bonicel J., Couchoud P. Primary structure of the two (4 Fe-4 S) clusters ferredoxin from Desulfovibrio desulfuricans (strain Norway 4). Biochimie. 1983 Jan;65(1):43–47. doi: 10.1016/s0300-9084(83)80027-3. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P., Moura I., Moura J. J., Xavier A. V., Santos M. H., LeGall J., Howard J. B. Amino acid sequence of a 3Fe:3S ferredoxin from the "archaebacterium" Methanosarcina barkeri (DSM 800). J Biol Chem. 1982 Dec 10;257(23):14192–14197. [PubMed] [Google Scholar]
- Haydon D. J., Guest J. R. A new family of bacterial regulatory proteins. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):291–295. doi: 10.1016/0378-1097(91)90101-f. [DOI] [PubMed] [Google Scholar]
- Irwin D. M., Kocher T. D., Wilson A. C. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991 Feb;32(2):128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- Johnson E. F., Walker D. L., Griffin K. J., Clark J. E., Okita R. T., Muerhoff A. S., Masters B. S. Cloning and expression of three rabbit kidney cDNAs encoding lauric acid omega-hydroxylases. Biochemistry. 1990 Jan 30;29(4):873–879. doi: 10.1021/bi00456a004. [DOI] [PubMed] [Google Scholar]
- Kahn D., David M., Domergue O., Daveran M. L., Ghai J., Hirsch P. R., Batut J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J Bacteriol. 1989 Feb;171(2):929–939. doi: 10.1128/jb.171.2.929-939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kendall K. J., Cohen S. N. Complete nucleotide sequence of the Streptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol. 1988 Oct;170(10):4634–4651. doi: 10.1128/jb.170.10.4634-4651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kolaskar A. S., Reddy B. V. A method to locate protein coding sequences in DNA of prokaryotic systems. Nucleic Acids Res. 1985 Jan 11;13(1):185–194. doi: 10.1093/nar/13.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Kaplan S. cis-acting regulatory elements involved in oxygen and light control of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992 Feb;174(4):1146–1157. doi: 10.1128/jb.174.4.1146-1157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Tatsumi H., Nakano E. Cloning and sequence analysis of cDNA for luciferase of a Japanese firefly, Luciola cruciata. Gene. 1989 Apr 30;77(2):265–270. doi: 10.1016/0378-1119(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Inoue K., Hase T., Hiura H., Kakuno T., Yamashita J., Horio T. Structure of the extracellular ferredoxin from Rhodospirillum rubrum: close similarity to clostridial ferredoxins. J Biochem. 1983 May;93(5):1385–1390. doi: 10.1093/oxfordjournals.jbchem.a134273. [DOI] [PubMed] [Google Scholar]
- Minami Y., Wakabayashi S., Wada K., Matsubara H., Kerscher L., Oesterhelt D. Amino acid sequence of a ferredoxin from thermoacidophilic archaebacterium, Sulfolobus acidocaldarius. Presence of an N6-monomethyllysine and phyletic consideration of archaebacteria. J Biochem. 1985 Mar;97(3):745–753. doi: 10.1093/oxfordjournals.jbchem.a135114. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Moore M. D., Kaplan S. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol. 1992 Mar;174(5):1505–1514. doi: 10.1128/jb.174.5.1505-1514.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet E., Dupont J., Vitek A., White P. C. Characterization of two genes encoding human steroid 11 beta-hydroxylase (P-450(11) beta). J Biol Chem. 1989 Dec 15;264(35):20961–20967. [PubMed] [Google Scholar]
- Motojima K., Goto S. Cloning of rabbit uricase cDNA reveals a conserved carboxy-terminal tripeptide in three species. Biochim Biophys Acta. 1989 Jun 1;1008(1):116–118. doi: 10.1016/0167-4781(89)90178-4. [DOI] [PubMed] [Google Scholar]
- Phillips M. K., Hederstedt L., Hasnain S., Rutberg L., Guest J. R. Nucleotide sequence encoding the flavoprotein and iron-sulfur protein subunits of the Bacillus subtilis PY79 succinate dehydrogenase complex. J Bacteriol. 1987 Feb;169(2):864–873. doi: 10.1128/jb.169.2.864-873.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Nakazawa K., Hon-Nami K., Oshima T. Purification, some properties and amino acid sequence of Thermus thermophilus HB8 ferredoxin. Biochim Biophys Acta. 1981 Apr 28;668(2):277–289. doi: 10.1016/0005-2795(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Scioli J. R., Zilinskas B. A. Cloning and characterization of a cDNA encoding the chloroplastic copper/zinc-superoxide dismutase from pea. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7661–7665. doi: 10.1073/pnas.85.20.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Darlison M. G., Lewis H. M., Guest J. R. The pyruvate dehydrogenase complex of Escherichia coli K12. Nucleotide sequence encoding the pyruvate dehydrogenase component. Eur J Biochem. 1983 Jun 1;133(1):155–162. doi: 10.1111/j.1432-1033.1983.tb07441.x. [DOI] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Chromosome transfer in Rhodobacter sphaeroides: Hfr formation and genetic evidence for two unique circular chromosomes. J Bacteriol. 1992 Feb;174(4):1135–1145. doi: 10.1128/jb.174.4.1135-1145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T. N., Moore M. D., Kaplan S. Cloning and characterization of the 5-aminolevulinate synthase gene(s) from Rhodobacter sphaeroides. Gene. 1988 Oct 15;70(1):139–151. doi: 10.1016/0378-1119(88)90112-6. [DOI] [PubMed] [Google Scholar]
- Varga A. R., Kaplan S. Construction, expression, and localization of a CycA::PhoA fusion protein in Rhodobacter sphaeroides and Escherichia coli. J Bacteriol. 1989 Nov;171(11):5830–5839. doi: 10.1128/jb.171.11.5830-5839.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Neckelmann N., Simpson L. Sequences of six genes and several open reading frames in the kinetoplast maxicircle DNA of Leishmania tarentolae. J Biol Chem. 1984 Dec 25;259(24):15136–15147. [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]