Abstract
Tryptophan uptake appears to be the Achilles' heel in yeast physiology, since under a variety of seemingly diverse toxic conditions, it becomes the limiting factor for cell growth. When growing cells of Saccharomyces cerevisiae are subjected to high hydrostatic pressure, tryptophan uptake is down-regulated, leading to cell cycle arrest in the G1 phase. Here we present evidence that the two tryptophan permeases Tat1 and Tat2 are differentially regulated by Rsp5 ubiquitin ligase in response to high hydrostatic pressure. Analysis of high-pressure growth mutants revealed that the HPG1 gene was allelic to RSP5. The HPG1 mutation or the bul1Δ bul2Δ double mutation caused a marked increase in the steady-state level of Tat2 but not of Tat1, although both permeases were degraded at high pressure in an Rsp5-dependent manner. There were marked differences in subcellular localization. Tat1 localized predominantly in the plasma membrane, whereas Tat2 was abundant in the internal membranes. Moreover, Tat1 was associated with lipid rafts, whereas Tat2 localized in bulk lipids. Surprisingly, Tat2 became associated with lipid rafts upon the occurrence of a ubiquitination defect. These results suggest that ubiquitination is an important determinant of the localization and regulation of these tryptophan permeases. Determination of the activation volume (ΔV≠) for Tat1- and Tat2-mediated tryptophan uptake (89.3 and 50.8 ml/mol, respectively) revealed that both permeases are highly sensitive to membrane perturbation and that Tat1 rather than Tat2 is likely to undergo a dramatic conformational change during tryptophan import. We suggest that hydrostatic pressure is a unique tool for elucidating the dynamics of integral membrane protein functions as well as for probing lipid microenvironments where they localize.
Multiple lines of evidence indicate that tryptophan uptake in Saccharomyces cerevisiae is impaired under a variety of diverse toxic conditions and becomes the limiting factor for cell growth. Many cold-sensitive mutants are tryptophan auxotrophs and have mutations in tryptophan permease or biosynthetic genes (14, 61). erg6 mutants, which are defective in ergosterol biosynthesis, are also defective in tryptophan uptake (25). The TAT1 and TAT2 genes, which encode the low- and high-affinity tryptophan permeases, were originally identified as genes conferring growth resistance to the immunosuppressive drug FK506 (33, 55). Addition of excess tryptophan or overexpression of TAT2 or TRP1 confers resistance to this drug (33, 55) or the ability to grow at low temperatures on trp1 strains (3, 14). Similar phenotypes have been described in studies of sphingolipid toxicity (16, 17, 24, 62) and the target of rapamycin (TOR)-signaling pathway (11, 56). One possibility is that there may be a general endocytosis defect in many membrane permeases under stress conditions. The volatile anesthetic isoflurane and the antineoplastic agent 4-phenylbutyric acid are known to impair tryptophan uptake by yeast, and overexpression of either Tat1 or Tat2 confers resistance to the drug (28, 48). No single explanation has been offered to explain how tryptophan availability compensates under a variety of seemingly diverse stress conditions.
Microorganisms respond to changes in hydrostatic pressure, and high-pressure conditions result in subtle variations in certain regulatory systems in mesophiles as well as piezophiles (reviewed in references 1, 2, 4, and 10). It has been demonstrated that increasing hydrostatic pressure in the range of 15 to 25 MPa (approximately 150 to 250 atm; atmospheric pressure of ∼0.1 MPa equals 1 bar, 0.9869 atm, and 1.0197 kg of force/cm2) inhibits tryptophan uptake by cells and induces degradation of Tat2, leading to cell cycle arrest in the G1 phase (3). The pressure-induced G1 arrest was observed only for tryptophan auxotrophs such as trp1 strains. However, if tryptophan is readily available, cells are capable of growth at 15 to 25 MPa (3). This effect is similar to that seen with the stresses described above. Increasing hydrostatic pressure increases the membrane order and reduces the lateral diffusion in both artificial and biological membranes, causing decreased fluidity of the membranes (31). Therefore, we hypothesize that hydrostatic pressure would affect the activity of tryptophan permease either directly, through changes in the protein conformation, or, most probably, through changes in the lipid bilayer structure.
In studies of the TOR-signaling pathway, Tat2 and the general amino acid permease Gap1 were shown to be inversely regulated in a manner dependent on Rsp5 ubiquitin ligase (also known as Npi1 and Mdp1 [64, 73]) (11, 55) Upon starvation or treatment with rapamycin, Tat2 is ubiquitinated and then degraded in the vacuole, whereas Gap1 is induced and delivered to the plasma membrane (11). The high-pressure sensing pathway is distinct from the TOR-signaling pathway, as evidenced by the fact that degradation of both Tat2 and Gap1 is stimulated by increasing pressure and is independent of the downstream protein kinase Npr1 (3).
It has been demonstrated that the activation volume (ΔV≠), representing the difference in volume between the initial state and the activated state, for the overall tryptophan uptake process of the cells has a large positive value of 46.2 ml/mol, indicating that there is a net volume increase during tryptophan import (3). Such a large volume change cannot be accounted for only by an interaction between tryptophan molecules and the permease protein; therefore, it is evident that a protein conformational change must also take place in the plasma membrane during tryptophan import. Moreover, upon incubation of the cells at 25 MPa for 5 h, Tat2 protein is degraded, tryptophan uptake is down-regulated, and ΔV≠ increases to 83.0 ml/mol (3). The increase in the ΔV≠ of tryptophan uptake (from 46.2 to 83.0 ml/mol) led us to speculate that the tryptophan permease Tat2 underwent posttranslational modification, likely via ubiquitination, under high-pressure conditions (3). Therefore, at least two aspects of the unique pressure-sensitive property of tryptophan permease should be investigated: the function-structure relationship within the lipid bilayer and posttranslational modification. A new model that accounts for lipid diversity was developed by Simons and Ikonen (60), who proposed the existence in biological membranes of lipid microdomains, so-called lipid rafts, that have high sphingolipid and cholesterol contents. Lipid rafts are tightly packed and ordered, unlike disordered bulk lipids. Such a difference in lipid order between lipid rafts and bulk lipids would affect the ΔV≠ for integral membrane protein functions.
Chung et al. (17) demonstrated that phytosphingosine (PHS) inhibited the uptake of tryptophan as well as that of leucine and histidine, suggesting that PHS is likely to be embedded in the lipid bilayer and to interfere with membrane protein functions. Unlike the application of high pressure, treatment of the cells with PHS did not cause degradation of Tat2 (17).
We have started to isolate mutants of S. cerevisiae capable of growth at high pressure, referred to as high-pressure growth (HPG) mutants, in hopes that phenotypic characterization of the HPG mutants and cloning of the genes would provide insights into the subsets of components required for regulation of the tryptophan permeases Tat1 and Tat2 as well as other factors affecting the function of these permeases. Here we report that one of the four HPG mutations, HPG1, is allelic to RSP5 and that Rsp5 together with its binding proteins Bul1 and Bul2 differentially regulates Tat1 and Tat2 in response to increasing hydrostatic pressure. Moreover, we demonstrate that ubiquitination is involved in the cellular distribution of the permeases and their partitioning in lipid rafts.
MATERIALS AND METHODS
Yeast strains and media.
All strains used in this study were isogenic derivatives of the wild-type haploid strain YPH499 (58) and are listed in Table 1. Cells were grown in yeast extract-peptone-dextrose (YPD) medium, synthetic minimal (SD) medium, or synthetic complete (SC) medium (29) under various pressure conditions. Unless otherwise specified, cells were grown at 24°C. SC medium was prepared with the following modifications. Tryptophan (40 mg/liter), leucine (90 mg/liter), lysine (30 mg/liter), and histidine (20 mg/liter) were added, and valine and uracil were removed (3). The optical density at 600 nm (OD600) was measured after appropriate dilution of the samples. Under these culture conditions, an OD600 of 0.1 corresponded to a cell density of (9.27 ± 0.57) × 105 cells/ml (mean ± standard deviation; n = 9), as counted under a microscope.
TABLE 1.
Strains used in this studya
| Strain | Genotype | Source or reference |
|---|---|---|
| YPH499 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 | 58 |
| YPH500 | MATα his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 | 58 |
| FAA3 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 YCplac33 [ARS1 CEN4 URA3] | Laboratory stock |
| FAA4 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 YEplac195 [2μm URA3] | Laboratory stock |
| FAY18A | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-1 | This study |
| FAY12C | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-2 | This study |
| FAY171E | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-3 | This study |
| FAY29E | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-4 | This study |
| FAY51R | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 rsp5-1 | This study |
| FAY113M | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 mdp1-13 | This study |
| FAB306 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 tat2Δ::URA3 | This study |
| FAB205 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 tat2Δ::HIS3 | This study |
| FAB210 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-1 tat2Δ::HIS3 | This study |
| FAB214 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-2 tat2Δ::HIS3 | This study |
| FAB219 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-3 tat2Δ::HIS3 | This study |
| FAB223 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-4 tat2Δ::HIS3 | This study |
| FAB229 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 rsp5-1 tat2Δ::HIS3 | This study |
| FAB233 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 mdp1-13 tat2Δ::HIS3 | This study |
| FAC65 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 tat1Δ::URA3 | This study |
| FAC70 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-1 tat1Δ::URA3 | This study |
| FAC75 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-2 tat1Δ::URA3 | This study |
| FAC80 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-3 tat1Δ::URA3 | This study |
| FAC85 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 HPG1-4 tat1Δ::URA3 | This study |
| FAC90 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 rsp5-1 tat1Δ::URA3 | This study |
| FAC95 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 mdp1-13 tat1Δ::URA3 | This study |
| FAJ1 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 bul1Δ::URA3 | This study |
| FAJ5 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 bul1Δ::HIS3 | This study |
| FAJ2 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 bul2Δ::URA3 | This study |
| FAJ3 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 bul2Δ::HIS3 | This study |
| FAJ72 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 bul1Δ::HIS3 bul2Δ::URA3 | This study |
| FAJ75 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 bul1Δ::HIS3 bul2Δ::LEU2 | This study |
| FAC37 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 tat1Δ::URA3 | This study |
| FAC40 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 tat1Δ::URA3 bul1Δ::HIS3 | This study |
| FAB158 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 tat2Δ::HIS3 | This study |
| FAB176 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 tat2Δ::HIS3 bul1Δ::URA3 | This study |
| FAF30 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 gap1Δ::URA3 | This study |
| FAF31 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 gap1Δ::URA3 bul1Δ::HIS3 | This study |
| FAM7 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 doa4Δ::URA3 | This study |
| FAM405b | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 doa4Δ::URA3 | This study |
| FAM4 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 vps27Δ::URA3 | This study |
| FAM105b | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 vps27Δ::URA3 | This study |
| FAM5 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 vps45Δ::URA3 | This study |
| FAM202b | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 vps45Δ::URA3 | This study |
| FAM6 | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 pep12Δ::URA3 | This study |
| FAM301b | MATahis3-Δ200 leu2-Δ1 lys2-801 trp1-Δ1 ade2-101 ura3-52 pep12Δ::URA3 | This study |
All strains are isogenic derivatives of strain YPH499.
These strains are segregants obtained from diploid strains.
Isolation of HPG mutants.
Strain YPH499 was used to isolate the Hpg− mutants. Since increasing pressure and decreasing temperature had analogous effects, causing a reduction in membrane fluidity, we obtained low-temperature growth mutants and high-pressure survival mutants. Cells were cultured in YPD medium at 24°C and were treated with or without 2% ethylmethanesulfonate for 10 to 30 min, so that the survival fraction of the cells decreased to 10 to 60%. After a wash with 0.1 M sodium phosphate buffer (pH 7.0), the cells were spread on YPD agar and either incubated at 4 to 10°C and 0.1 MPa for 1 to 3 months or subjected to pressures of 30 to 50 MPa in YPD medium for 1 to 3 months at 24°C by use of hydrostatic vessels (Teramecs Co. Ltd., Kyoto, Japan). Approximately 400 strains were obtained. For the high-pressure growth assay, a small number of colonies were placed on YPD agar, and the agar was covered with low-melting-point YPD agar. The plate was wrapped in transparent sheets and incubated at 18 or 25 MPa and 24°C.
Genetic analysis.
Forty-four of the 400 HPG mutants were backcrossed to the mating strain YPH500. Tetrad analysis showed that the HPG phenotype was segregated 2:2 in 34 mutants. All 34 mutants had dominant single nuclear mutations. Twenty of these 34 mutants were crossed with each other and were classified into four semidominant linkage groups, HPG1, HPG2, HPG3, and HPG4, that consisted of 10, 8, 1, and 1 mutant strain, respectively. The 10 HPG1 mutants were analyzed further.
Cloning of HPG1.
A genomic library containing 10- to 20-kb DNA fragments from HPG1 strain FAY12C was constructed by using plasmid YCplac111 (ARS1 CEN4 LEU2). The wild-type strain YPH499 was transformed with the library, and the transformants were mixed with low-melting-point SD agar, followed by incubation at 18 or 25 MPa and 24°C for 2 to 7 days to select transformants capable of high-pressure growth. Plasmids were purified from the transformants and were reintroduced into strain YPH499 to confirm the ability to grow at high pressure. To identify a base substitution in plasmid p3F1-8 (see Table 2), the entire coding region (2.4 kb) of HPG1 (RSP5) and its upstream (1.1-kb) and downstream (780-bp) noncoding regions were sequenced.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| YCplac33 | ARS1 CEN4 URA3 | 26 |
| YEplac195 | 2μm URA3 | 26 |
| YIplac211 | URA3 | 26 |
| YCplac111 | ARS1 CEN4 LEU2 | 26 |
| YEplac181 | 2μm LEU2 | 26 |
| pRS313 | ARSH4 CEN6 HIS3 | 26 |
| pTAT2c | 2.6-kb fragment containing TAT2 promoter-TAT2 in YCplac33 | 3 |
| pTAT1e | 2.6-kb fragment containing TAT1 promoter-TAT1 in YEplac195 | This study |
| pTAT2e | 2.6-kb fragment containing TAT2 promoter-TAT2 in YEplac195 | 3 |
| p3HA-TAT1c | 2.7-kb fragment containing TAT2 promoter-3HA-TAT1 in YCplac33 | This study |
| p3HA-TAT2c | 2.7-kb fragment containing TAT1 promoter-3HA-TAT2 in YCplac33 | This study |
| p3F1-8 | 10.5-kb fragment containing DSE1, RSP5, and NSA1 in YCplac111 | This study |
| pFA499Rc | 4.3-kb fragment containing RSP5 promoter-RSP5 in YCplac33 | This study |
| pFA499Re | 4.3-kb fragment containing RSP5 promoter-RSP5 in YEplac195 | This study |
| pFA18Ac | 4.3-kb fragment containing RSP5 promoter-HPG1-1 in YCplac33 | This study |
| pFA18Ae | 4.3-kb fragment containing RSP5 promoter-HPG1-1 in YEplac195 | This study |
| pFA12Cc | 4.3-kb fragment containing RSP5 promoter-HPG1-2 in YCplac33 | This study |
| pFA12Ce | 4.3-kb fragment containing RSP5 promoter-HPG1-2 in YEplac195 | This study |
| pFA171Ec | 4.3-kb fragment containing RSP5 promoter-HPG1-3 in YCplac33 | This study |
| pFA171Ee | 4.3-kb fragment containing RSP5 promoter-HPG1-3 in YEplac195 | This study |
| pFA29Ec | 4.3-kb fragment containing RSP5 promoter-HPG1-4 in YCplac33 | This study |
| pFA29Ee | 4.3-kb fragment containing RSP5 promoter-HPG1-4 in YEplac195 | This study |
| pFA51Rc | 4.3-kb fragment containing RSP5 promoter-rsp5-1 in YCplac33 | This study |
| pFA51Re | 4.3-kb fragment containing RSP5 promoter-rsp5-1 in YEplac195 | This study |
| pFA51Ri | 4.3-kb fragment containing RSP5 promoter-rsp5-1 in YIplac211 | This study |
| pFA113Mc | 4.3-kb fragment containing RSP5 promoter-mdp1-13 in YCplac33 | This study |
| pFA113Me | 4.3-kb fragment containing RSP5 promoter-mdp1-13 in YEplac195 | This study |
| YEpnpi1ΔC2 | 6.5-kb fragment containing RSP5 promoter-NPI1/RSP5 containing a deletion of codons for the C2 domain in plasmid 2μm | 65 |
| YEpnpi1ΔWWP | 6.5-kb fragment containing RSP5 promoter-NPI1/RSP5 containing a deletion of codons for the WW domain in plasmid 2μm | 65 |
| YEpnpi1ΔCys | 6.5-kb fragment containing RSP5 promoter-NPI1/RSP5 containing a deletion of codons for the active-site cysteine in plasmid 2μm | 65 |
| pAS55c | TAT2 promoter-2HA-TAT2 in YCplac33 | 11 |
| pTB306c | pAS55c containing a deletion of codons 2 through 10 of TAT2 | 11 |
| pTB307c | pAS55c containing a deletion of codons 2 through 17 of TAT2 | 11 |
| pTB313c | pAS55c containing a deletion of codons 2 through 20 of TAT2 | 11 |
| pTB288c | pAS55c containing a deletion of codons 2 through 29 of TAT2 | 11 |
| pTB373c | pTB288c with a nucleotide substitution changing lysine codon 31 of TAT2 to an arginine codon | 11 |
| pTB359c | pAS55c containing a deletion of codons 2 through 31 of TAT2 | 11 |
| pTB294c | pAS55c containing a deletion of codons 17 through 31 of TAT2 | 11 |
| pTB355c | pAS55c with nucleotide substitutions changing lysine codons 10, 17, 20, 29, and 31 of TAT2 to arginine codons | 11 |
| pBUL1e | 6.5-kb fragment containing BUL1 promoter-BUL1 in YEplac195 | 70, 71 |
| pBUL2e | 6.5-kb fragment containing BUL2 promoter-BUL2 in YEplac195 | 70, 71 |
| YEp105LEU | c-Myc-tagged ubiquitin regulated by the copper-inducible CUP1 promoter in YEp105 in which TRP1 was replaced with LEU2 | 22 |
Plasmid construction.
Plasmids used in this study are listed in Table 2. The HPG1 allele was recovered from the wild-type strain and the 10 HPG1 mutants by using the gap repair method. The XhoI/BseRI fragment containing HPG1 was removed from plasmid 3F1-8, and the gapped plasmid was introduced into the HPG1 mutants to recover the mutant alleles. Plasmids were isolated from the transformants, and the HindIII/BseRI fragments containing HPG1 were inserted into YCplac33 (ARS1 CEN4 URA3) and YEplac195 (2 μm URA3) to produce plasmids pFA499Rc (wild-type CEN4), pFA499Re (wild-type 2μm), pFA18Ac (HPG1-1 CEN4), pFA18Ae (HPG1-1 2 μm), pFA12Cc (HPG1-2 CEN4), pFA12Ce (HPG1-2 2μm), pFA171Ec (HPG1-3 CEN4), pFA171Ee (HPG1-3 2μm), pFA29Ec (HPG1-4 CEN4), and pFA29Ee (HPG1-4 2μm). The entire coding regions and the upstream and downstream noncoding regions for the four HPG1 alleles were sequenced to identify base substitutions. 3HA-TAT1 and 3HA-TAT2, each encoding an N-terminally hemagglutinin (HA)-tagged, fully functional Tat1 or Tat2 protein, respectively, under the control of its own promoter, were constructed as described previously (3) by using primers listed in Table 2. Each DNA fragment was inserted into YCplac33 to yield p3HA-TAT1c and p3HA-TAT2c, respectively. YEp105, encoding c-Myc-tagged ubiquitin regulated by the copper-inducible CUP1 promoter, was kindly provided by M. J. Ellison of the University of Alberta, Edmonton, Canada (22). TRP1 of YEp105 was replaced with LEU2 as a selective marker, yielding YEp105LEU. YEpnpi1ΔC2, YEpnpi1ΔWWP, and YEpnpi1ΔCys were kindly provided by B. André of the Université Libre de Bruxelles, Brussels, Belgium (65). pAS55, pTB306, pTB307, pTB313, pTB288, pTB373, pTB359, pTB294, and pTB355 were kindly provided by M. N. Hall of the University of Basel, Basel, Switzerland (11). The EcoRI/SphI fragments containing TAT2 variants were inserted into YCplac33 to yield pAS55c, pTB306c, pTB307c, pTB313c, pTB288c, pTB373c, pTB359c, pTB294c, and pTB355, respectively. pHY66 (YEUp3-BUL1) and pHY32 (pRS316-BUL2) were kindly provided by Y. Kikuchi of the University of Tokyo, Tokyo, Japan (70, 71). The EcoRI/SalI fragment of pHY66 containing BUL1 was inserted into YEplac195 to yield pBUL1e. The SacI/SalI fragment of pHY32 containing BUL2 was inserted into YEplac195 to yield pBUL2e.
Gene disruption.
PCR-based gene disruption was performed for the creation of tat1Δ, tat2Δ, gap1Δ, bul1Δ, bul2Δ, doa4Δ, vps27Δ, vps45Δ, or pep12Δ by using relevant primers (Table 3) and plasmid pRS313 (ARSH4 CEN6 HIS3) or YEplac195 (2μm URA3). In the case of doa4Δ, vps27Δ, vp45Δ, or pep12Δ, replacement with URA3 was carried out in both the haploid strain YPH499 and a diploid strain formed by YPH499 and YPH500, followed by tetrad analysis. Deletion of each gene was confirmed by PCR amplification of genomic DNA using the appropriate primers.
TABLE 3.
Oligonucleotide primers used in this study
| Primer | Deletion and/or HA tag | Sequencea |
|---|---|---|
| hisBUL1a | BUL1 | 5′-ATGGCCAAAGATTTGAACGATTCGGGGTTTCCACCGAAGAGGAAGCCTTTGCTGCGTCCT-GGTGAGCGCTAGGAGTCACT-3′ |
| hisBUL1b | BUL1 | 5′-TTATTTTGTCACTTGCCTAACAGAAATAGGGATATCAATCTTCGCTACGCCAAGATGGTT-ACACCGCATATGATCCGTCG-3′ |
| hisBUL2a | BUL2 | 5′-ATGACTTTTACATTCTCCACTTCATCAAGGAAAAATGGGAGACCTCCTTTAAAATCAGTT-GGTGAGCGCTAGGAGTCACT-3′ |
| hisBUL2b | BUL2 | 5′-CTAAATTTGTAACTTTTTCACATCAACCGGAATATCAACTTTCATAGAACCCAAGTGGTT-ACACCGCATATGATCCGTCG-3′ |
| uraBUL1a | BUL1 | 5′-ATGGCCAAAGATTTGAACGATTCGGGGTTTCCACCGAAGAGGAAGCCTTTGCTGCGTCCT-AATTCAATTCATCATTTTTT-3′ |
| uraBUL1b | BUL1 | 5′-TTATTTTGTCACTTGCCTAACAGAAATAGGGATATCAATCTTCGCTACGCCAAGATGGTT-TGGATTAGTCTCATCCTTCA-3′ |
| uraBUL2a | BUL2 | 5′-ATGACTTTTACATTCTCCACTTCATCAAGGAAAAATGGGAGACCTCCTTTAAAATCAGTT-AATTCAATTCATCATTTTTT-3′ |
| uraBUL2b | BUL2 | 5′-CTAAATTTGTAACTTTTTCACATCAACCGGAATATCAACTTTCATAGAACCCAAGTGGTT-TGGATTAGTCTCATCCTTCA-3′ |
| leuBUL2a | BUL2 | 5′-ATGACTTTTACATTCTCCACTTCATCAAGGAAAAATGGGAGACCTCCTTTAAAATCAGTT-ATCAAATTCGATGACTGGAA-3′ |
| leuBUL2b | BUL2 | 5′-CTAAATTTGTAACTTTTTCACATCAACCGGAATATCAACTTTCATAGAACCCAAGTGGTT-AACTGGCAAACCGAGGAACT-3′ |
| uraTAT1a | TAT1 | 5′-ATGGACGATAGTGTCAGTTTCATTGCCAAAGAGGCCAGTCCAGCACAATATTCGCACAGT-AATTCAATTCATCATTTTTT-3′ |
| uraTAT1b | TAT1 | 5′-TTAGCACCAGAAATTGGTCATCCTCTTAAAAAACTTTCTTGAACGCGAGCTGTTTGGATT-TGGATTAGTCTCATCCTTCA-3′ |
| uraTAT2a | TAT2 | 5′-ATGACCGAAGACTTTATTTCTTCTGTCAAGCGTTCAAATGAGGAGCTGAAGGAGCGAAAAAATTCAATTCATCATTTTTT-3′ |
| uraTAT2b | TAT2 | 5′-TTAACACCAGAAATGGAACTGTCTCACGTACCATGGACGGGAATCTAGGTACATTTTCTTTGGATTAGTCTCATCCTTCA-3′ |
| hisTAT2a | TAT2 | 5′-ATGACCGAAGACTTTATTTCTTCTGTCAAGCGTTCAAATGAGGAGCTGAAGGAGCGAAAA-GGTGAGCGCTAGGAGTCACT-3′ |
| hisTAT2b | TAT2 | 5′-TTAACACCAGAAATGGAACTGTCTCACGTACCATGGACGGGAATCTAGGTACATTTTCTT-ACACCGCATATGATCCGTCG-3′ |
| uraGAP1a | GAP1 | 5′-ATGAGTAATACTTCTTCGTACGAGAAGAATAATCCAGATAATCTGAAACACAATGGTATT-AATTCAATTCATCATTTTTT-3′ |
| uraGAP1b | GAP1 | 5′-TTAACACCAGAAATTCCAGATTCTATACCATCTTGGCTTTGTGGCCATAATTGCCTTTTC-TGGATTAGTCTCATCCTTCA-3′ |
| uraDOA4a | DOA4 | 5′-ATGGAGCAGAATATTATTAGTACCATAAGGGATGAGTGTATTCGTCACCGGTCGAAGTAC-TTTCAATTCAATTCATCATTTTTT-3′ |
| uraDOA4b | DOA4 | 5′-TCAAACACCGTAGACGCGGTGATAAAACAAAACGTATGCATTAGAGTTAATTGCATCGGC-CAATTGGATTAGTCTCATCCTTCA-3′ |
| uraVPS27a | VPS27 | 5′-ATGTCCGTTAGCACGCCAAGTGAGTTAGACGCATTAATAGAACAAGCCACTAGTGAGAGCAATTCAATTCATCATTTTTT-3′ |
| uraVPS27b | VPS27 | 5′-TTAAAGCTCTATTAGCAGTTCCTCTTGAGGACTAGGCGGCCTTTCCTCTTTGATCGGAATTGGATTAGTCTCATCCTTCA-3′ |
| uraVPS45a | VPS45 | 5′-ATGAACCTTTTTGATGTGGCTGACTTTTATATAAACAAAATTGTGACTTCCCAATCGAAAAATTCAATTCATCATTTTTT-3′ |
| uraVPS45b | VPS45 | 5′-TTATTTTGCAGATCTAATAGAATCCATATATTCTTTAGTTGAAAGTATAGAGGTGCCTCCTGGATTAGTCTCATCCTTCA-3′ |
| uraPEP12a | PEP12 | 5′-ATGTCGGAAGACGAATTTTTTGGTGGTGATAATGAAGCCGTTTGGAACGGTTCCAGATTCAATTCAATTCATCATTTTTT-3′ |
| uraPEP12b | PEP12 | 5′-TTACAATTTCATAATGAGAAAAATAAAAAGAAGCATTACGAGAAGCACAATCAACAAATATGGATTAGTCTCATCCTTCA-3′ |
| rsp5-1a | rsp5-1 | 5′-AGAGCCCGTTCATTGCAGTTC-3′ |
| rsp5-1b | rsp5-1 | 5′-GAACTGCAATGAACGGGCTCT-3′ |
| mdp1-13a | mdp1-13 | 5′-GATTATCGTGATTACCAAGAG-3′ |
| mdp1-13b | mdp1-13 | 5′-CTCTTGGTAATCACGATAATC-3′ |
| 5′-3HA-TAT1 | 3HA-TAT1 | 5′-GCTCTAGATGCATAGTCCGGGACGTCATAGGGATAGCCCGCATAGTCAGGAACATCGTATGGGTAGCCAGCGTAGTCTGGGACGTCGTATGGGTACATTTTTACGCCTTTTTATCGAGAAA-3′ |
| 3′-3HA-TAT1 | 3HA-TAT1 | 5′-CGTCTAGAGACGATAGTGTCAGTTTCATTGCC-3′ |
| 5′-3HA-TAT2 | 3HA-TAT2 | 5′-CGTCTAGATGCATAGTCCGGGACGTCATAGGGATAGCCCGCATAGTCAGGAACATCGTATGGGTAGCCCGCATAGTCAGGAACATCGTATGGGTACATATGAGAGTGTGTTGCGTAATTT-3′ |
| 3′-3HA-TAT2 | 3HA-TAT2 | 5′-CGTCTAGAACCGAAGACTTTATTTCTTCTGTC-3′ |
Base substitutions are boldfaced.
Site-directed mutagenesis.
We generated an rsp5-1 (67) or mdp1-13 (73) mutation within the genome of strain YPH499 by site-directed mutagenesis using complementary primers containing base substitutions. Base substitutions within the primers (rsp5-1a and rsp5-1b) are boldfaced in Table 3. Plasmid pFA499Re (RSP5 in YEplac195) was used as a template. The resulting fragment containing rsp5-1 was digested with HindIII and inserted into YCplac33 and YEplac195 to yield pFA51Rc and pFA51Re, respectively, and into YIplac211 to yield plasmid pFA51Ri, which was used to integrate the rsp5-1 gene within the YPH499 genome. Ura+ strains were subjected to a subsequent 5-fluoroorotic acid selection, yielding strain FAY51R (rsp5-1) (13). The same procedure was used to generate an mdp1-13 strain by using primers mdp1-13a and mdp1-13b, yielding strain FAY113 M (mdp1-13) and corresponding plasmids pFA113Mc and pFA113Me (Tables 2 and 3).
Prediction of the 3D structure of the Rsp5 HECT domain.
The three-dimensional (3D) structure of the Rsp5 HECT domain was predicted using 3D-JIGSAW (version 2.0; Cancer Research, London, United Kingdom), an automated system for construction of 3D models of proteins based on homologues of known structures. The crystal structure of the human E6-AP HECT domain was used as a template (37). The structure was visualized using a WebLab ViewerLite 3.2 viewer (Molecular Simulations Inc., San Diego, Calif.).
Amino acid uptake assay.
The following radiolabeled amino acids were purchased from Amersham Pharmacia Biotech UK Limited (Little Chalfont, Buckinghamshire, United Kingdom): l-[5-3H]tryptophan (TRK460; 1.18 TBq/mmol), l-[4,5-3H]leucine (TRK510; 6.33 TBq/mmol), l-[4,5-3H]lysine monohydrochloride (TRK520; 2.66 TBq/mmol), and l-[2,5-3H]histidine (TRK199; 1.18 TBq/mmol). Cells were grown in SD or SC medium at 24°C at densities up to 1.0 × 107/ml, and the amino acid uptake assay was performed as described previously (3). Data are expressed as picomoles of amino acid incorporated per 107 cells per minute (means ± standard deviations obtained from more than three independent experiments).
Western blot analysis.
To prepare whole-cell extracts for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blot analysis, 108 cells were collected by centrifugation, washed once with 10 mM NaN3-10 mM NaF, and washed once in lysis buffer A (50 mM Tris-HCl, 5 mM EDTA, 10 mM NaN3, 1 mM phenylmethylsulfonyl fluoride, and one tablet of EDTA-free Complete [Roche, Mannheim, Germany] for 50 ml [pH 7.5]). The cells were broken with glass beads. Unbroken cells and debris were removed by centrifugation at 300 × g for 2 min, and the cleared lysates were treated with 5% SDS and 5% 2-mercaptoethanol at 37°C for 10 min to denature proteins. Western blot analysis was performed as described previously (3) using monoclonal antibodies for HA (16B12; BabCO, Richmond, Calif.), c-Myc (9E10; BabCO), Pep12 (2C3; Molecular Probes, Inc., Eugene, Oreg.), Vps10 (18C8; Molecular Probes, Inc.), alkaline phosphatase (ALP) (to detect vacuolar membrane ALP) (1D3; Molecular Probes, Inc.), or Pma1 (kindly provided by R. Serrano of the Universidad Politecnica de Valencia CSIC, Valencia, Spain). An image analyzer (LAS-1000 plus; Fujifilm, Tokyo, Japan) was used for signal detection.
Subcellular fractionation.
Cells were fractionated using differential centrifugation as described by Horazdovsky and Emr (36) with a slight modification. Whole-cell lysates from 108 cells were prepared as described above. One hundred microliters of the cleared lysates containing 75 μg of total proteins was mixed with an equal volume of STE20 buffer (20% sucrose, 50 mM Tris-HCl, 5 mM EDTA [pH 7.5]) and was subjected to centrifugation at 13,000 × g for 10 min to yield a P13 (pellet) fraction. The resulting supernatant fraction was subjected to centrifugation at 100,000 × g for 30 min, yielding a P100 (pellet) fraction. The P13 and P100 fractions were treated with 5% SDS and 5% 2-mercaptoethanol at 37°C for 10 min to denature proteins. To isolate the plasma membrane, the P13 fraction obtained from 3 × 108 cells was subjected to centrifugation on a sucrose density gradient (10, 20, 40, and 50%) at 100,000 × g for 18 h. The pellet was collected. Cells were also fractionated using centrifugation on a sucrose density gradient (54). Whole-cell extracts were subjected to centrifugation on a sucrose density gradient (10 to 60%) at 100,000 × g for 18 h by using an SW55 Ti rotor (Beckman Instruments, Fullerton, Calif.). Eleven fractions (500 μl each) were collected from the top, and the proteins were precipitated with 6% trichloroacetic acid in the presence of 0.015% deoxycholate. When a TLS55 rotor (Beckman Instruments) was used, sample volumes were reduced and centrifugation was carried out at 100,000 × g for 12 h. Eleven fractions were collected from the top.
Immunoprecipitation of Tat1 and Tat2 from P13 and P100 fractions.
To detect ubiquitinated forms of Tat1 and Tat2, we performed immunoprecipitation of these proteins followed by detection of c-Myc-tagged ubiquitin as reported by Beck et al. (11). Cells containing either p3HA-TAT1c or p3HA-TAT2c were transformed with YEp105LEU (CUP1 promoter-dependent, c-Myc-tagged ubiquitin [Table 2] [22]), and the transformants were cultured in SC medium (<107 cells/ml). Expression of c-Myc-tagged ubiquitin was induced by addition of 0.1 mM CuSO4 for 3 h. For immunoprecipitation, P13 and P100 fractions were prepared from 75 μg of whole-cell lysates and were precleared with Sepharose CL-4B (Sigma, St. Louis, Mo.) in buffer B (buffer A containing 1% Triton X-100 and 150 mM NaCl) at 4°C for 30 min. After removal of Sepharose CL-4B by centrifugation, the supernatants were mixed with 10 μl of an anti-HA antibody-bound affinity matrix (Roche) and incubated for 2 h at 4°C with shaking. The affinity matrix was collected by centrifugation and washed four times with buffer B. Samples containing immunoprecipitated 3HA-Tat2 or 3HA-Tat1 were subjected to Western blot analysis using an anti-HA antibody (16B12) to detect 3HA-Tat2 or 3HA-Tat1. The antibody was stripped from the polyvinylidene difluoride membrane, and the membrane was reprobed with an anti-c-Myc antibody (9E10).
Purification of detergent-insoluble membranes and the membrane flotation assay.
Nonionic detergent-insoluble membranes, so-called lipid rafts, were prepared basically as described by Bagnat et al. (8). A P13 fraction was prepared from 108 cells harboring p3HA-TAT1c or p3HA-TAT2c as described above. The P13 fraction or purified plasma membranes were incubated in 75 μl of TXNE1 buffer (50 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100 [pH 7.5]) for 30 min in an ice bath. After extraction with Triton X-100, the lysates were adjusted to 40% Optiprep (Nycomed, Oslo, Norway) by addition of 150 μl of Optiprep solution and were overlaid with 900 μl of 30% Optiprep in TXNE0.1 buffer (50 mM Tris-HCl, 5 mM EDTA, 150 mM NaCl, 0.1% Triton X-100 [pH 7.5]) and 100 μl of TXNE0.1 buffer. The samples were centrifuged at 55,000 rpm for 2 h in a TLS55 rotor. Four fractions (300 μl each) were collected from the top, and the samples were mixed with 700 μl of deionized distilled water, 100 μl of 0.15% deoxycholate, and 100 μl of 72% trichloroacetic acid for precipitation of proteins.
RESULTS
Classification of HPG mutants.
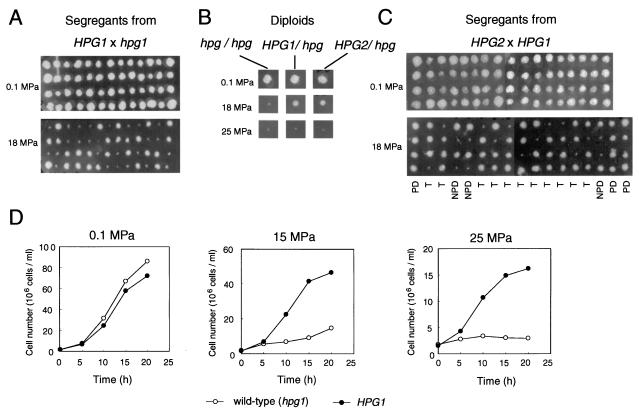
Of the 400 Hpg− mutants isolated, 44 strains were subjected to genetic analysis. Tetrad analysis showed that the Hpg phenotype segregated 2:2 in 34 strains (Fig. 1A); the affected loci were designated hpg. Note that Hpg− represents the high-pressure growth phenotype. In other words, Hpg− cells can grow under high-pressure conditions, whereas Hpg+ cells, i.e., wild-type cells, cannot. Diploid strains, which were formed by a cross between each of the 34 mutants and the mating strain YPH500, grew at 18 MPa but were unable to grow at 25 MPa, indicating that the Hpg− phenotypes were semidominant (Fig. 1B). Next, 20 of the 34 mutants that showed a clearer phenotype than the others were crossed with each other, and the segregants were subjected to a high-pressure growth assay. Figure 1C shows a typical example segregating as the parental ditype:nonparental ditype:tetra type at 3:3:11 (nearly 1:1:4). Finally, those 20 mutants were classified into four semidominant linkage groups, HPG1, HPG2, HPG3, and HPG4, consisting of 10, 8, 1, and 1 representative, respectively, suggesting that at least four genes are involved in cell growth at high pressure. The 10 HPG1 mutants were analyzed further. The growth properties of one of the HPG1 mutants, FAY12C, are shown in Fig. 1D. Because of the semidominant character of the mutant alleles, they are designated HPG1-X, in capital letters, where X stands for an allele number.
FIG. 1.
Isolation and classification of semidominant HPG mutants. Typical examples of results of high-pressure growth assays are shown. (A) The HPG phenotype of each tetrad derived from the HPG1/hpg1 heterozygous diploid segregated 2 Hpg+:2 Hpg− on YPD agar at 18 MPa. The YPD plates were incubated for 2 days at 0.1 or 18 MPa. Note that capital letters are used for the semidominant mutant allele and lowercase letters are used for the wild-type allele. (B) Heterozygous diploids (HPG1/hpg1 and HPG2/hpg2) were grown on YPD agar at 0.1, 18, and 25 MPa for 2 days. (C) Tetrad distribution of segregants derived from an HPG2/HPG2 diploid strain. Segregants were grown on YPD agar at 0.1 and 18 MPa for 2 days. PD, parental ditype; NPD, nonparental ditype; T, tetra type. (D) Cell growth of the wild-type strain and an HPG1 mutant in liquid YPD medium under different pressure conditions. Immediately after decompression, the cell number was counted under a microscope using a hemocytometer.
The HPG1 mutation is allelic to RSP5.
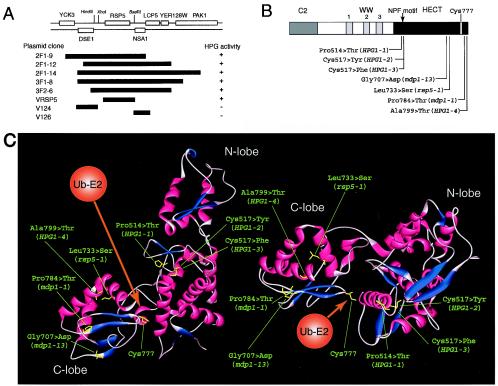
The wild-type cells of YPH499 were transformed with the genomic library from the mutant strain FAY12C (HPG1), followed by incubation at high pressure. Selection of clones conferring high-pressure growth yielded five plasmids carrying overlapping fragments, each including RSP5, which encodes Rsp5 ubiquitin ligase (Fig. 2A). Among candidate genes on the plasmids, only the RSP5 gene from the HPG1-2 mutant conferred high-pressure growth on the wild-type strain. All of the RSP5-containing DNA fragments recovered from the 10 HPG1 mutants conferred high-pressure growth ability, although wild-type RSP5 did not, suggesting that HPG1 was a mutant allele of RSP5.
FIG. 2.
HPG1 mutation sites and predicted tertiary structure of the Rsp5 HECT domain. (A) Map of DNA fragments containing RSP5 from an HPG1 genome library that can confer high-pressure growth. (B) Four HPG1 mutations together with some known rsp5 mutation sites are located in the HECT domain of Rsp5. (C) Predicted 3D structure of the Rsp5 HECT domain, obtained by use of 3D-JIGSAW, version 2.0. Predicted α-helices and β-sheets are shown in red and blue, respectively. The HPG1 mutation and some known rsp5 mutation sites are shown in yellow. The ubiquitin-bound E2 enzyme(s) is assumed to have access to the HECT domain from the direction shown in orange.
To confirm that HPG1 is allelic to RSP5, we integrated plasmid YIplac-RSP5 (URA3) into each HPG1 strain. Stable transformants (MATa HPG1-X RSP5 URA3) were crossed with the mating strain YPH500 (MATα HPG1 RSP5 ura3), and the segregation of the Hpg+ and Ura+ phenotypes was determined for 20 asci. Only the segregation pattern of 2:2 for Hpg− Ura+:Hpg+ Ura− was obtained, indicating that the plasmid was integrated into a genomic region tightly linked with HPG1. This is genetic evidence that HPG1 is allelic to RSP5. Based on DNA sequencing and computational polypeptide analysis, the 10 HPG1 mutants were classified into four alleles: HPG1-1, which has the Pro514→Thr (CCA→ACA) substitution; HPG1-2, which has the Cys517→Tyr (TGT→TAT) substitution; HPG1-3, which has the Cys517→Phe (TGT→TTT) substitution; and HPG1-4, which has the Ala799→Thr (GCC→ACC) substitution (Fig. 2B). All four mutation sites were located within the catalytic HECT domain of Rsp5 ubiquitin ligase (Fig. 2B). Taking the results together, we concluded that HPG1 is a semidominant allele of RSP5 which allows cells to proliferate at high pressure.
The 3D localization of the HPG1 mutation sites in terms of the HECT domain was predicted by the method of homology modeling. As observed for the human E6-AP (37), the predicted Rsp5 HECT domain consisted of two lobes, a large N-terminal lobe and a small C-terminal lobe (C-lobe), packed across a small interface (Fig. 2C). The active cysteine (Cys777) forming the thioester bond with ubiquitin was found in the C-lobe at the junction of the two lobes. Interestingly, the mutation sites of HPG1-1 (Pro514→Thr) (in strain FAY18A), HPG1-2 (Cys517→Tyr) (in strain FAY12C), HPG1-3 (Cys517→Phe) (in strain FAY171E), HPG1-4 (Ala799→Thr) (in strain FAY29E), and mdp1-1 (Pro784→Thr) and rsp5-1 (Leu733→Ser) (in strain FAY51R) were mapped at the interface of the HECT domain, although the mdp1-13 (Gly707→Asp) mutation site (in strain FAY113 M) is located behind the C-lobe (Fig. 2C). As described in detail below, the mdp1-13 mutation neither confers high-pressure growth nor enhances the Tat2 level. These results suggest that the interface of the HECT domain is important for the function of Rsp5. Rsp5 is known to localize at multiple sites within the endocytic pathway (68). Since the HPG1-1, HPG1-2, and HPG1-3 mutation sites are located within or beside the NPF (Asn-Pro-Phe) motif (53, 69), this motif is likely to have a role in interaction with the EH domains of endocytic components rather than in ubiquitin ligase activity.
Growth properties of HPG1 mutants.
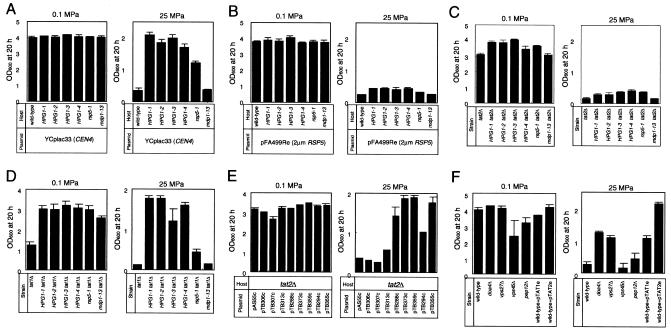
Next we show quantitative results on the growth profiles of the mutant strains at high hydrostatic pressure. The starting OD600 in SC medium was adjusted to 0.13 (approximately 1.2 × 106 cells/ml). In agreement with the previous result obtained with Tat2 (3), overexpression of TAT1 on a multicopy plasmid conferred high-pressure growth on the wild-type strain, although it was less effective than TAT2 overexpression (Fig. 3F). The four HPG1 mutants (HPG1-1, HPG1-2, HPG1-3, and HPG1-4) grew well at 25 MPa, and the well-characterized rsp5-1 mutant displayed moderate growth at this pressure, but the mdp1-13 mutant did not (Fig. 3A). Similar results were obtained with the rsp5Δ mutant bearing either the RSP5, HPG1, rsp5-1, or mdp1-13 gene in YCplac33 (data not shown). These results suggest that the HPG1 and rsp5-1 mutations stabilize Tat2 and/or Tat1 under high-pressure conditions, as observed with the stabilization of Tat2 in rsp5-1 cells treated with rapamycin (11). Overexpression of the wild-type RSP5 gene on a multicopy plasmid suppressed high-pressure growth of the HPG1-X and rsp5-1 mutants (Fig. 3B). In contrast, overexpression of either the HPG1-X or the rsp5-1 allele conferred high-pressure growth ability on the wild-type strain (data not shown). Overexpression of Rsp5 lacking the active-site cysteine (YEpnpi1ΔCys) also conferred high-pressure growth, but overexpression of Rsp5 lacking the C2 domain (YEpnpi1ΔC2) or the WW domain (YEpnpi1ΔWWP) did not (data not shown). These results suggest that Rsp5 and the mutant form Hpg1 compete for Tat2, or that some interacting proteins and abundant Hpg1 or Rsp5/Npi1ΔCys protein sequester Tat2 or the interacting proteins. Yeast two-hybrid experiments have shown that Rsp5 interacts with itself and exists as an Rsp5-Rsp5 multimer (19). Thus, another possible explanation is that Rsp5 and Hpg1 form less-functional heteromers, causing less-efficient Tat2 ubiquitination. The four HPG1 tat2Δ mutants (HPG1-1 tat2Δ, HPG1-2 tat2Δ, HPG1-3 tat2Δ, and HPG1-4 tat2Δ mutants) containing YCplac33 failed to grow at 25 MPa (Fig. 3C), although the HPG1 tat1Δ mutants grew at that pressure (Fig. 3D). These results clearly indicate that the high-pressure growth phenotype of the HPG1 mutants is achieved through the function of Tat2 but not through that of Tat1. The details regarding protein expression are described below.
FIG. 3.
Growth properties of HPG1 mutants and some gene disruptants under high-pressure conditions. (A) Cells of the various mutants harboring the empty vector YCplac33 were grown in SC medium at 0.1 and 25 MPa for 20 h. After decompression, the OD600 was determined. (B) Cells of the various mutants containing the wild-type RSP5 gene in a multicopy plasmid were grown at 0.1 and 25 MPa for 20 h. (C through F) Cells of the following mutants were grown at 0.1 and 25 MPa for 20 h: HPG1 tat2Δ mutants (C), HPG1 tat1Δ mutants (D), the tat2Δ mutant harboring TAT2 plasmids containing N-terminal mutations (E), and the doa4Δ, vps27Δ, vps45Δ, and pep12Δ mutants (F). All data are means and standard deviations from three independent experiments.
With rapamycin treatment, ubiquitin is transferred to at least one lysine residue within the N-terminal 31 amino acid residues of Tat2, leading to degradation in the vacuole. We verified that strains with one of the Tat2 variant proteins Tat25K→R (pTB355c; replacement of five lysines with arginines), Tat2Δ31 (pTB359c; deletion of the 2nd through the 31st amino acid residue), Tat2Δ29K31R (pTB373c; deletion of the 2nd through the 29th amino acid residue and replacement of the 31st lysine with arginine), and Tat2Δ29 (pTB288c; deletion of the 2nd through the 29th amino acid residue) were able to grow at 25 MPa, although other strains did not (Fig. 3E), suggesting that the lysine residue(s), most likely Lys29 and/or Lys31, is ubiquitinated at high pressure, as reported for the rapamycin effect (11).
Beck et al. have demonstrated that with rapamycin treatment, intracellular Tat2 is delivered from the Golgi apparatus to the vacuole via the vacuolar protein sorting (VPS) pathway without passing through the cell surface, based on the finding that Tat2 is stabilized in pep12, vps45, and vps27 mutant cells when they are treated with rapamycin (11). If Tat2 is stabilized at high pressure in these mutants, the cells could exhibit high-pressure growth. We found that the vps27Δ mutants (FAM4 and FAM105) grew well at 25 MPa, although the vps45Δ mutants (FAM5 and FAM202) and the pep12Δ mutants (FAM6 and FAM301) did not (Fig. 3F). Therefore, it is likely that a blockage by the vps27Δ mutation preventing exit from the prevacuolar compartment (PVC) in the forward direction to the vacuole or back to the Golgi apparatus causes accumulation of Tat2 and/or Tat1 in the plasma membrane at high pressure, but a blockage by the vps45Δ or pep12Δ mutation in the fusion of Golgi apparatus-derived vesicles with the PVC is not likely. Doa4 is one of 17 deubiquitinating enzymes in yeast and plays a role in ubiquitin-dependent proteolysis and ubiquitin homeostasis (5, 66). doa4Δ mutants (FAM7 and FAM405) exhibited high-pressure growth at 25 MPa (Fig. 3F), suggesting that the doa4Δ mutation results in the stabilization of Tat2, probably due to a defect in replenishing the pool of free ubiquitin or a defect causing accumulation of ubiquitinated Tat2 that is not followed by degradation.
The rsp5-1 mutant displays high-temperature sensitivity (67, 68). Among strains tested, the HPG1-4 mutant, like the rsp5-1 mutant, showed marked high-temperature sensitivity at 37°C, indicating that temperature sensitivity is allele specific in RSP5 (data not shown).
The HPG1 mutation enhances amino acid uptake.
Cells were incubated at 0.1 or 25 MPa for 5 h in SC medium prior to an amino acid uptake assay at 0.1 MPa using 3H-labeled materials (3). The HPG1 mutations were revealed to enhance steady-state levels of tryptophan uptake at 0.1 MPa 1.3- to 1.7-fold over the level for the wild-type strain (Table 4). Upon high-pressure incubation, the uptake activity was reduced to 20.7% in wild-type cells. Strikingly, the uptake activity was maintained at almost 100% in the HPG1 mutants upon high-pressure incubation (Table 4). These results suggest that the HPG1 mutations increase steady-state expression of tryptophan permeases and stabilize them under high-pressure conditions.
TABLE 4.
Effects of high-pressure incubation on amino acid uptake
| Genotype | Uptakea (fold change relative to wild type) of the following amino acid at the indicated pressure, and comparison (% uptake)b
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptophan
|
Leucine
|
Lysine
|
Histidine
|
|||||||||
| 0.1 MPa | 25 MPa, 5 h | % Uptake | 0.1 MPa | 25 MPa, 5 h | % Uptake | 0.1 MPa | 25 MPa, 5 h | % Uptake | 0.1 MPa | 25 MPa, 5 h | % Uptake | |
| Wild type | 8.3 ± 1.2 (1.0) | 1.7 ± 0.4 (1.0) | 20.7 | 55.4 ± 6.3 (1.0) | 10.6 ± 0.8 (1.0) | 19.1 | 90.3 ± 14.5 (1.0) | 4.9 ± 0.2 (1.0) | 5.4 | 21.0 ± 1.6 (1.0) | 4.3 ± 5.0 (1.0) | 20.3 |
| HPG1-1 | 11.3 ± 1.2 (1.4) | 11.8 ± 2.1 (6.8) | 103.9 | 70.3 ± 12.5 (1.3) | 65.1 ± 16.6 (6.1) | 92.6 | 102.0 ± 7.2 (1.1) | 88.4 ± 7.9 (18.0) | 86.7 | 27.8 ± 4.0 (1.3) | 29.7 ± 2.9 (7.0) | 106.8 |
| HPG1-2 | 11.5 ± 0.9 (1.4) | 11.6 ± 1.9 (6.7) | 100.7 | 66.2 ± 6.2 (1.2) | 68.5 ± 13.9 (6.5) | 106.5 | 93.2 ± 11.4 (1.0) | 75.6 ± 5.0 (15.4) | 81.1 | 28.2 ± 3.6 (1.3) | 30.6 ± 4.3 (7.2) | 108.6 |
| HPG1-3 | 13.9 ± 2.0 (1.7) | 13.3 ± 1.9 (7.7) | 96.2 | 66.9 ± 8.4 (1.2) | 67.0 ± 11.3 (6.3) | 100.1 | 106.0 ± 9.4 (1.2) | 84.6 ± 10.5 (17.2) | 79.7 | 28.6 ± 3.1 (1.4) | 33.8 ± 3.8 (7.9) | 118.3 |
| HPG1-4 | 10.8 ± 1.6 (1.3) | 10.2 ± 2.7 (5.9) | 94.3 | 61.1 ± 7.4 (1.1) | 58.2 ± 9.8 (5.5) | 95.3 | 122.0 ± 10.7 (1.4) | 110.0 ± 14.3 (22.3) | 89.8 | 26.2 ± 5.0 (1.3) | 28.3 ± 6.2 (6.6) | 107.9 |
| rsp5-1 | 9.0 ± 0.6 (1.1) | 6.4 ± 0.8 (3.7) | 71.1 | 69.4 ± 3.4 (1.3) | 43.5 ± 6.8 (4.1) | 62.6 | 109.0 ± 13.0 (1.2) | 61.4 ± 8.0 (12.5) | 56.4 | 27.7 ± 1.5 (1.3) | 11.8 ± 2.5 (2.8) | 42.5 |
| mdp1-13 | 8.9 ± 1.2 (1.1) | 2.0 ± 0.1 (1.1) | 22.1 | 63.8 ± 3.7 (1.2) | 14.1 ± 1.2 (1.3) | 22.1 | 86.7 ± 16.5 (1.0) | 5.6 ± 0.4 (1.2) | 6.5 | 23.7 ± 4.1 (1.1) | 2.9 ± 1.7 (0.7) | 12.2 |
| pBUL1ec | 2.7 ± 0.3 (0.3) | 4.0 ± 0.5 (2.3) | 148.3 | 16.0 ± 0.8 (0.3) | 14.5 ± 3.5 (1.4) | 90.7 | 22.3 ± 2.9 (0.3) | 10.2 ± 1.0 (2.1) | 45.6 | 4.57 ± 0.3 (0.2) | 2.7 ± 0.2 (0.6) | 57.9 |
| pBUL2ed | 10.8 ± 1.6 (1.3) | 4.3 ± 1.6 (2.5) | 39.6 | 74.4 ± 10.7 (1.3) | 21.6 ± 2.8 (2.0) | 29.0 | 90.0 ± 8.1 (1.0) | 11.3 ± 1.7 (2.3) | 12.6 | 21.1 ± 3.0 (1.0) | 3.8 ± 0.6 (0.9) | 17.8 |
| bul1Δ | 11.8 ± 1.6 (1.4) | 12.0 ± 3.0 (6.9) | 101.7 | 99.9 ± 7.6 (1.8) | 89.3 ± 18.1 (8.4) | 89.4 | 110.0 ± 8.9 (1.2) | 90.5 ± 2.7 (18.4) | 82.4 | 31.8 ± 4.6 (1.5) | 27.9 ± 6.4 (6.5) | 87.7 |
| bul2Δ | 9.1 ± 1.8 (1.1) | 4.2 ± 3.1 (2.4) | 45.4 | 61.8 ± 11.8 (1.1) | 16.1 ± 4.8 (1.5) | 26.0 | 101.0 ± 9.1 (1.1) | 7.8 ± 4.0 (1.6) | 7.7 | 24.2 ± 2.5 (1.2) | 4.0 ± 1.2 (0.9) | 16.5 |
| bul1Δ bul2Δ | 12.4 ± 0.9 (1.5) | 12.7 ± 2.2 (7.3) | 102.4 | 71.3 ± 2.4 (1.3) | 61.5 ± 2.8 (5.8) | 86.3 | 55.4 ± 2.2 (0.6) | 48.1 ± 3.5 (9.8) | 86.8 | 17.3 ± 1.2 (0.8) | 20.9 ± 2.8 (4.9) | 120.8 |
Expressed in picomoles per 107 cells per minute.
Uptake by cells incubated at 25 MPa for 5 h as a percentage of uptake at 0.1 MPa.
Wild-type strain containing pBUL1e.
Wild-type strain containing pBUL2e.
The HPG1 mutations also enhanced the steady-state uptake of other amino acids; the effect was most remarkable for tryptophan at 0.1 MPa. The order of uptake enhancement (from highest to lowest), derived from means of results with the four HPG1 mutations, was as follows: tryptophan (1.42- ± 0.16-fold; n = 4), histidine (1.32- ± 0.05-fold; n = 4), leucine (1.19- ± 0.05-fold; n = 4), and lysine (1.17- ± 0.13-fold; n = 4) (Table 4). During incubation at 25 MPa, the uptake of these amino acids was reduced in wild-type cells, as observed in the case of tryptophan (Table 4), whereas it was maintained in HPG1 mutants. These results suggest that the HPG1 mutation also stabilizes amino acid permeases to various degrees.
Bul1 is a negative regulator of Tat2, but Bul2 is not.
Bul1 was first identified as a binding protein of Rsp5 (70), and Bul2 is its functional homologue (71). Both Bul1 and Bul2 possess the PPxY motif, an interaction module that has an affinity for the WW domain of Rsp5. We asked whether Bul1 and Bul2 have a role in tryptophan uptake under high-pressure conditions. Table 4 shows that overexpression of BUL1 reduced the uptake of the four amino acids at 0.1 MPa, whereas overexpression of BUL2 did not. Inversely, the bul1Δ mutation enhanced the uptake of the four amino acids at 0.1 MPa, and high uptake activity was maintained upon high-pressure incubation at 25 MPa (Table 4). The bul2Δ mutation did not influence uptake. These results indicate that Bul1, but not Bul2, is a negative regulator of the uptake of tryptophan and other amino acids at high pressure.
At 25 MPa, the growth of all four HPG1 mutants and the rsp5-1 mutant was restricted by overexpression of BUL1 but was not restricted to the same extent by overexpression of BUL2 (Fig. 4A). The bul1Δ mutation or the bu1Δ bul2Δ double mutation conferred growth at 25 MPa on the wild-type cells, but the bul2Δ mutation did not, suggesting that the bul1Δ mutation stabilized Tat2 and/or Tat1 at high pressure (Fig. 4B). To test the possibility that the bul1Δ mutation might stabilize other permeases for tryptophan, we introduced the tat2Δ, tat1Δ, or gap1Δ mutation into the bul1Δ genetic background. We verified that both the bul1Δ gap1Δ mutant and the bul1Δ tat1Δ mutant grew at 25 MPa as efficiently as the bul1Δ mutant (Fig. 4C). The bul1Δ tat2Δ mutant grew normally at 0.1 MPa, although growth was significantly impaired at 25 MPa (Fig. 4C). These results clearly indicate that Tat2, but neither Tat1 nor Gap1, is required for high-pressure growth in the bul1Δ mutant, probably via its stabilization.
FIG. 4.
Overexpression and deletion of BUL1 have inverse effects. (A) Overexpression of BUL1 but not BUL2 suppresses high-pressure growth of HPG1 mutants. Cells of the indicated genotype containing YEplac195, pBUL1e, or pBUL2e were grown in SC medium at 0.1 and 25 MPa for 20 h. (B) Deletion of BUL1 confers high-pressure growth, but deletion of BUL2 does not. Cells of the wild-type strain and the bul1Δ, bul2Δ, and bul1Δ bul2Δ mutants were grown in SC medium at 0.1 and 25 MPa for 20 h. (C) Cells of the bul1Δ, bul1Δ tat1Δ, bul1Δ tat2Δ, and bul1Δ gap1Δ mutants were grown in SC medium at 0.1 and 25 MPa for 20 h. All data are means and standard deviations from three independent experiments.
Rsp5-Bul1-Bul2 differentially regulates Tat1 and Tat2 in response to increasing pressure.
Although there are apparent similarities between Tat1 and Tat2 in terms of primary structure (7, 47), Tat2 is distinct from Tat1 in terms of the essential role in high-pressure growth (Fig. 3C and D). To determine the reason for this puzzling difference, we first analyzed the expression of Tat1 and Tat2 by Western blotting (Fig. 5). Interestingly, the chemiluminescent signal from Tat1 was ninefold stronger than that from Tat2 in the wild-type strain (data not shown). Assuming that protein blotting and detection of the HA antigen had almost the same efficiency for these two 3HA-tagged proteins, we suggest that the amount of Tat1 is ninefold greater than that of Tat2.
FIG. 5.
Tat1 and Tat2 are differentially regulated by the HPG1 or bul1Δ bul2Δ mutation. Cells of the indicated mutants containing a functional 3HA-TAT2 or 3HA-TAT1 on YCplac33 (p3HA-TAT2c or p3HA-TAT1c, respectively) were grown in SC medium, and whole-cell extracts were subjected to Western blot analysis. (A) Steady-state levels of Tat1 and Tat2 at 0.1 MPa. (B) Changes in Tat1 and Tat2 levels after the shift to high-pressure conditions at 25 MPa for 2 or 5 h. Typical data from at least four independent experiments are shown. D, degradation; S, stabilization.
At atmospheric pressure, the steady-state level of Tat2 in the four HPG1 mutants was enhanced approximately twofold, suggesting that Tat2 is regulated by Rsp5 in growing wild-type cells (Fig. 5A). While the bul1Δ or bul2Δ mutation hardly affected the Tat2 level, the bul1Δ bul2Δ double mutation caused a fourfold increase in the Tat2 level at 0.1 MPa (Fig. 5A), indicating that Bul1 and Bul2 have overlapping effects on the stabilization of Tat2 protein at 0.1 MPa. Upon high-pressure incubation at 25 MPa, the Tat2 level decreased in the wild-type cells (Fig. 5B). However, the Tat2 level was maintained in the HPG1, rsp5-1, bul1Δ, and bul1Δ bul2Δ mutants but not in the mdp1-13 and bul2Δ mutants (Fig. 5B), consistent with the results for growth properties at 25 MPa (Fig. 3 and 4). Pulse-chase experiments with Tat2 are necessary in order to understand the effect of pressure on de novo Tat2 synthesis.
In contrast to the remarkable enhancement of Tat2 abundance in HPG1 mutants and the bul1Δ bul2Δ mutant, the steady-state level of Tat1 was not increased by the HPG1 mutation or the bul1Δ or bul2Δ mutation, and the bul1Δ bul2Δ double mutation caused a reduction in the Tat1 level (Fig. 5A). This result suggests that Tat1 is not substantially regulated by Rsp5 ubiquitin ligase under normal growth conditions. Upon high-pressure incubation at 25 MPa, the Tat1 level decreased in the wild-type cells (Fig. 5B). As observed with the Tat2 level, the Tat1 level was also maintained in the HPG1 and rsp5-1 mutants but not in the mdp1-13 mutant at 25 MPa (Fig. 5B). In addition, the Tat1 level was no longer maintained in the bul1Δ and bul1Δ bul2Δ mutants at 25 MPa (Fig. 5B), although the two mutants exhibited remarkable high-pressure growth (Fig. 4B and C). There are two possibilities to explain the lack of dependence of Tat1 ubiquitination on Bul1 and Bul2. The first is that Rsp5 and Hpg1-X, a mutant form of Rsp5, have sufficient activity to ubiquitinate Tat1 in the absence of Bul1 and Bul2 but are insufficient for Tat2. The second is that another ubiquitin ligase is involved in Tat1 ubiquitination. These results showing the pressure-induced differential regulation of Tat1 and Tat2 prompted us to characterize further the localization and properties of these permeases.
Subcellular localization of Tat1 and Tat2.
We next investigated the subcellular localization of Tat1 and Tat2. Immunofluorescence microscopy has revealed that Tat2 is localized in the endoplasmic reticulum (ER), the Golgi apparatus, and, to a smaller extent, the plasma membrane in wild-type cells (11). To the best of our knowledge, however, there has been no report on the subcellular localization of Tat1. Whole-cell extracts from wild-type cells containing either p3HA-TAT1c or p3HA-TAT2c were subjected to subcellular fractionation using a sucrose density gradient (10 to 60%). The localization of Tat1 and Tat2 was determined by Western blot analysis using the following membrane marker proteins: the plasma membrane H+-ATPase Pma1, the vacuolar membrane ALP, the Golgi membrane protein Vps10, and the endosomal multifunctional yeast syntaxin Pep12. Tat2 appeared to localize mainly with Pep12, partly with Vps10 and ALP, and to a smaller extent with Pma1 (Fig. 6A), indicating that Tat2 is more abundant in internal membranes than in the plasma membrane, which is consistent with the previous observation (11). Strikingly, in contrast to Tat2, Tat1 was found to be localized mainly in the plasma membrane, like Pma1 (Fig. 6A). Even though Tat1 is supposed to be a low-affinity tryptophan permease, we speculate that it contributes to tryptophan uptake, because the majority of Tat1 is in the plasma membrane and the tat1Δ mutation resulted in a growth defect in the wild-type strain (Fig. 3D, left). The differential localization of the two permeases is surprising, raising possibilities that the individual localization is due to their primary structure or is ensured by specific and regulated mechanisms, likely via ubiquitination.
FIG. 6.
Rsp5 ubiquitin ligase is involved in the subcellular localization of Tat2 but not of Tat1. (A) Subcellular localization of Tat1 and Tat2 by sucrose density gradient centrifugation in wild-type cells. Whole-cell extracts were prepared from the wild-type strain containing either p3HA-TAT1c or p3HA-TAT2c and were subjected to centrifugation on a sucrose density gradient (10 to 60%). Eleven fractions from the top were collected and subjected to Western blot analysis to detect Tat1 and Tat2 together with the four membrane marker proteins Pma1, Pep12, Vps10, and ALP. (B) Subcellular localization of Tat1 and Tat2 in wild-type or mutant cells analyzed by differential centrifugation. Whole-cell extracts prepared from wild-type, HPG1-1, and bu1Δ bul2Δ cells containing either p3HA-TAT1c or p3HA-TAT2c and from Tat25K→R cells (tat2Δ cells containing pTB355c [2HA-Tat25K→R]) were subjected to differential centrifugation to yield the P13 and P100 fractions. Western blotting was performed to detect Tat1 and Tat2 in these fractions together with marker proteins. Typical data from three independent experiments are shown.
To elucidate the role of ubiquitination in the distribution of Tat1 and Tat2, we next carried out subcellular fractionation essentially as described by Horazdovsky and Emr (36). Whole-cell extracts prepared from wild-type, HPG1-1, and bu1Δ bul2Δ cells containing either p3HA-TAT1c or p3HA-TAT2c and from Tat25K→R cells (tat2Δ cells containing pTB355c [2HA-Tat25K→R]) were subjected to differential centrifugation to obtain the P13 and P100 fractions. Large membrane structures such as the plasma membrane, the ER, and the vacuolar membranes are expected to be collected mainly in the P13 fraction, and the Golgi membrane and endosomes are in the P100 fraction. Figure 6B shows that there was no significant difference in the distribution of the four marker proteins between the wild-type, HPG1-1, and bul1Δ bul2Δ cells. In the wild-type cells, Tat2 was revealed to localize almost equally in the P13 and P100 fractions (Fig. 6B). Taking these findings together with the results of fractionation on a sucrose density gradient (Fig. 6A), we conclude that Tat2 is abundant in internal membranes, probably in the ER, the Golgi apparatus, or endosomes. Notably, Tat2 was enriched in the P13 fraction in HPG1-1 or bul1Δ bul2Δ mutants. In addition, Tat25K→R was also enriched in the P13 fraction (Fig. 6B). These results indicate that Tat2 is localized to membranes collected in the P13 fraction because of a ubiquitination defect, caused either by low Rsp5 activity or by five K→R substitutions on Tat2. In contrast, however, neither the HPG1-1 nor the bul1Δ bul2Δ mutation affected the localization of Tat1, which was localized in the P13 fraction in both wild-type and mutant cells. As shown in Fig. 5, the Tat1 level was lower in the bul1Δ bul2Δ strain; the reduction was equal in the P13 and P100 fractions (Fig. 6B). The localization of Tat1 and Tat2 in other HPG1 mutants was identical to that observed in the HPG1-1 mutant (data not shown). These results indicate that Tat2 localization is regulated by Rsp5 and Bul1-Bul2 but that Tat1 localization is not.
The HPG1 mutation differentially affects the ubiquitination levels of Tat1 and Tat2.
To characterize further the role of ubiquitination in the localization of Tat1 and Tat2, the ubiquitination level was examined for the two proteins as described by Beck et al. (11). Wild-type, HPG1-1, and bu1Δ bul2Δ mutant cells containing either p3HA-TAT1c or p3HA-TAT2c, and cells of the tat2Δ mutant containing pTB355c (2HA-Tat25K→R), were transformed with plasmids containing the CUP1 promoter-dependent c-Myc-tagged ubiquitin (YEp105LEU for the wild type and the HPG1-1 and Tat25K→R mutants; YEp105 for the bul1Δ bul2Δ mutant) (22). After preparation of the P13 and P100 fractions, the membranes were solubilized with 1% Triton X-100, followed by immunoprecipitation of Tat1 or Tat2 protein. For the wild-type cells, ubiquitinated forms of Tat2 were detected in immunoprecipitates from the P13 fraction but not from the P100 fraction (Fig. 7A), suggesting that the ubiquitinated forms of Tat2 could localize predominantly to the plasma membrane or the ER rather than to the Golgi apparatus or endosomes. The HPG1-1 mutation reduced the levels of the ubiquitinated forms of Tat2 in the P13 fraction (Fig. 7A). In Tat25K→R cells, the ubiquitinated forms of Tat2 were detected in trace amounts in the P13 fraction. The result indicates that Hpg1, a mutant form of Rsp5, has a reduced ability to ubiquitinate Tat2. The ubiquitinated forms of Tat1 were detected in immunoprecipitates from the P13 fraction but not from the P100 fraction, suggesting that the ubiquitinated forms of Tat1 could also localize predominantly to the plasma membrane or the ER rather than to the Golgi apparatus or endosomes (Fig. 7B). Unlike that of Tat2, the ubiquitination level of Tat1 seemed to be almost unchanged by the HPG1-1 mutation. This result suggests that the mutant Hpg1-1 protein still has the ability to ubiquitinate Tat1 or that, otherwise, there is another ubiquitin ligase for Tat1.
FIG. 7.
Visualization of ubiquitinated forms of Tat2 (A) or Tat1 (B) in immunoprecipitated proteins present in the P13 and P100 fractions. Cells of the wild-type and HPG1-1 strains containing either YCplac33 (empty vector), p3HA-TAT1c, or p3HA-TAT2c, or tat2Δ cells containing pTB355c (2HA-Tat25K→R), were transformed with YEp105LEU (containing CUP1 promoter-dependent, c-Myc-tagged ubiquitin). After induction of c-Myc-tagged ubiquitin by addition of 0.1 mM CuSO4, whole-cell extracts were prepared and subjected to differential centrifugation to yield the P13 and P100 fractions. Each fraction was solubilized and subjected to immunoprecipitation using an anti-HA antibody-bound affinity matrix as described in Materials and Methods. The immunoprecipitated Tat2 or Tat1 proteins were analyzed by Western blotting using an anti-HA antibody (left) or an anti-c-Myc antibody (right). (Ub)n-Tat2 or -Tat1, ubiquitinated form of Tat2 or Tat1; −, cells containing YEp105LEU and YEplac195 (empty vector); +, cells containing YEp105LEU and either p3HA-TAT2 or p3HA-TAT1; asterisk, nonspecific band.
The bul1Δ bul2Δ mutant has not been analyzed for the following reasons. During sample preparation, we found that cell pellets of the bul1Δ bul2Δ mutant became greenish brown after the addition of 0.1 mM CuSO4, probably due to the accumulation of copper transporters in the plasma membrane, and that c-Myc-tagged ubiquitin molecules were highly expressed in bul1Δ bul2Δ cells, causing enhanced c-Myc ubiquitination on numerous cellular proteins (unpublished data). Such an abnormality would preclude the evaluation of ubiquitination levels of Tat1 and Tat2 in the bul1Δ bul2Δ mutant.
Tat2 becomes associated with lipid rafts upon introduction of a ubiquitination defect.
In yeasts, glycosphingolipids and ergosterol are progressively enriched in membranes as they pass through the secretory pathway, reaching the highest level at the plasma membrane, where they form lipid rafts (60, 72). Association with lipid rafts has been shown to play a role in the biosynthetic delivery of Pma1 to the plasma membrane (8, 9, 27). Since the mutant Pma1 protein (Pma1-7) fails to arrive at the plasma membrane and instead is targeted for vacuolar degradation (9), recognition of distinct conformational defects is likely the mechanism for delivery from the Golgi apparatus to the vacuolar membrane. To understand the role of ubiquitination in the differential localization of Tat1 and Tat2 in the cell, we investigated whether these permeases are associated with lipid rafts. The P13 fraction was collected from the cells and extracted with ice-cold 1% Triton X-100, and the samples were then centrifuged to generate detergent-insoluble fractions. We confirmed that Pma1 was associated with detergent-insoluble lipid rafts (Fig. 8A, Pma1, fraction 1) and that Pep12 remained in detergent-soluble bulk lipids (Fig. 8A, Pep12, fraction 4), indicating that the extraction of lipid rafts was properly carried out. In wild-type cells, Tat2 appeared to remain in the detergent-soluble fraction (Fig. 8A, Tat2, fraction 4). However, Tat2 became associated with lipid rafts in the HPG1-1 or bul1Δ bul2Δ mutant. Tat25K→R was also associated with the detergent-insoluble fraction (Fig. 8A, Tat2, fraction 1). It is possible that excess amounts of Tat2 leaked from the detergent-soluble to the detergent-insoluble fraction in the mutant cells, because the amount of Tat2 was greater in mutant cells than in wild-type cells, but this protein remained predominantly in the detergent-soluble fraction when expressed to approximately 10-fold-greater amounts from a multicopy plasmid (Fig. 8A, Tat2, fraction 4). These results suggest that ubiquitination is involved in the association of Tat2 with lipid rafts. Unlike Tat2, however, Tat1 was associated mainly with lipid rafts in wild-type cells and in HPG1-1 and bul1Δ bul2Δ cells (Fig. 8A, Tat1, fraction 1). Therefore, Tat2 association with lipid rafts occurs in the absence of ubiquitination, whereas Tat1 association with lipid rafts is independent of ubiquitination. Unlike Pma1, Tat2 in the mutant cells or Tat1 in the wild-type and mutant cells remained in intermediate fractions 2 and 3, suggesting that these permeases exist in more heterogeneous lipid microenvironments or that the permeases are more loosely associated with lipid rafts than Pma1. We discuss below why the ubiquitination defect resulted in the association of Tat2 with lipid rafts.
FIG. 8.
Tat2 associates with lipid rafts in ubiquitination-defective cells, whereas Tat1 associates with lipid rafts in an Rsp5-independent manner. (A) Raft association of Tat1 and Tat2 in P13 membrane fractions. Whole-cell extracts from wild-type, HPG1-1, or bul1Δ bul2Δ cells containing either p3HA-TAT1 or p3HA-TAT2, or from tat2Δ cells containing pTB355c (2HA-Tat25K→R), were prepared and subjected to differential centrifugation to yield the P13 fractions. The P13 fractions were solubilized with ice-cold 1% Triton X-100 and subjected to the membrane flotation assay. Four fractions were collected from the top, and the protein samples were analyzed by Western blotting. (B) Raft association of Tat1 and Tat2 in purified plasma membranes. The P13 fractions were prepared as described above and subjected to centrifugation on a sucrose density gradient to yield purified plasma membranes. The plasma membrane samples were solubilized with ice-cold 1% Triton X-100 and subjected to the membrane flotation assay. Four fractions were collected from the top, and the protein samples were analyzed by Western blotting.
We next examined the raft association of Tat1 and Tat2 within the plasma membrane, where these permeases function. The P13 fraction was subjected to centrifugation on a sucrose density gradient to purify the plasma membrane. A small proportion of Pma1 still remained in bulk lipids or in intermediate fractions, probably due to partial disorganization of lipid rafts during the preparation of the plasma membrane samples (Fig. 8B, Pma1, fractions 2 to 4). We found that Tat2 remained in the bulk lipids within the plasma membranes of wild-type cells (Fig. 8B, Tat2, fraction 4), whereas Tat2 in cells of the HPG1-1, bul1Δ bul2Δ, and Tat25K→R mutants became associated with lipid rafts (Fig. 8B, Tat2, fraction 1), although large amounts of this protein existed in intermediate fractions (Fig. 8B, Tat2, fractions 2 to 4). However, Tat1 was associated with lipid rafts within the plasma membrane in wild-type cells as well as in HPG1-1 and bul1Δ bul2Δ cells (Fig. 8B, Tat1, fraction 1), even though certain amounts of this protein existed in intermediate fractions (Fig. 8B, Tat1, fractions 2 to 4). As lipid rafts are thought to consist of sphingolipids and ergosterol, the molecular motion of lipid rafts is highly restricted compared with that of bulk lipids. Therefore, we conclude that Tat1 exists in the highly ordered lipid microdomain, whereas Tat2 exists in disordered bulk lipids, in wild-type cells. The effect of hydrostatic pressure on the raft association of these permeases is under investigation.
Tat1 undergoes a dramatic conformational change during tryptophan import.
ΔV≠ represents the difference in volume between the initial state and the activated state of the reaction and can be obtained only by measuring the rate constants of the reaction as a function of hydrostatic pressure. To determine ΔV≠ for tryptophan import through either Tat1 or Tat2, we first constructed a tat1Δ mutant (tat1Δ TAT2; strain FAC37) and a tat2Δ mutant (TAT1 tat2Δ; strain FAB306). The ΔV≠ associated with the overall process of tryptophan uptake is calculated by the equation (∂lnk/∂p)T = −ΔV≠/RT, where k is the rate constant, p is pressure (in megapascals), T is absolute temperature (Kelvin), and R is the gas constant (in milliliters megapascal per Kelvin per mole) (4, 59). ΔV≠ is the apparent volume change of activation (in milliliters per mole). Tat1 is known to import tyrosine as well as tryptophan (55). To eliminate potential complexity in calculating ΔV≠ for Tat1 activity, we used SD medium that lacked tyrosine and phenylalanine in this assay rather than SC medium. The growth of the tat2Δ mutant is inhibited on YPD agar or by high concentrations of tyrosine and phenylalanine (Fig. 9), suggesting that tryptophan uptake through Tat1 is competitively inhibited by the aromatic amino acids.
FIG. 9.
The growth of the tat2Δ mutant is impaired by high concentrations of aromatic amino acids. Cells of the indicated mutants were grown on either SD medium, SC medium, SD medium plus 100 μg of tyrosine/ml (SD+Tyr100), SD medium plus 100 μg of phenylalanine/ml (SD+Phe100), SD medium plus 100 μg of tyrosine/ml and 100 μg of phenylalanine/ml (SD+Try100+Phe100), or YPD agar for 3 days. Typical data from three independent experiments are shown.
As shown in Table 5, the rate constants of tryptophan uptake activity decreased with increasing pressure in the three strains. Notably, there was a marked difference in ΔV≠ between Tat1 and Tat2. The ΔV≠ for Tat2 activity was 50.8 ± 13.1 ml/mol (n = 5), and that for Tat1 was 89.3 ± 17.1 ml/mol (n = 11), indicating that Tat1 underwent a larger volume change and thus is more significantly impaired by increasing pressure than Tat2. The large ΔV≠s cannot be accounted for by a single chemical process. According to a proposed model (41, 45, 46), ΔV≠ can be accounted for by summing three volume values: those associated with (i) the interaction between the substrate and amino acid residues of proteins, (ii) the protein conformational change, and (iii) hydration. The conformation of membrane proteins would be affected by the structure of lipid bilayers where the membrane proteins localize. Therefore, we hypothesize that a membrane protein localized in lipid rafts, such as Tat1, could undergo a remarkable conformational change during its activation, whereas a membrane protein existing in bulk lipids, such as Tat2, could undergo a relatively smaller conformational change during its activation. In this sense, hydrostatic pressure would be a useful parameter for probing lipid microenvironments where proteins of interest are localized by measuring ΔV≠ for membrane protein activities.
TABLE 5.
ΔV ≠ of tryptophan uptake through Tat1 and/or Tat2
| Genotype (permease[s]) | Rate constant (pmol of tryptophan 107 cells−1 min−1)a at the following pressure:
|
ΔV≠ (ml/mol)a of overall process of tryptophan uptake | ||
|---|---|---|---|---|
| 0.1 MPa | 25 MPa | 50 MPa | ||
| Wild type (Tat1 and Tat2) | 3.75 ± 0.69 | 2.14 ± 0.69 | 0.65 ± 0.35 | 91.7 ± 17.3 |
| tat1Δ (Tat2) | 2.40 ± 0.56 | 1.57 ± 0.45 | 0.89 ± 0.34 | 50.8 ± 13.1 |
| tat2Δ (Tat1) | 3.76 ± 0.62 | 2.17 ± 0.43 | 0.65 ± 0.28 | 89.3 ± 17.1 |
Data are means ± standard deviations from 6 (wild type), 5 (tat1Δ), or 11 (tat2Δ) experiments. SD medium rather than SC medium was used to determine rate constants.
The ΔV≠ of tryptophan uptake of the wild-type strain was 91.7 ± 17.3 ml/mol (n = 6), which was similar to that of the tat2Δ mutant, suggesting that Tat1 is the major tryptophan permease in the wild-type cells in the absence of tyrosine and phenylalanine in SD medium. The ΔV≠ previously obtained for the wild-type strain by using SC medium (46.2 ± 3.9 ml/mol) (3) was smaller than the value for the wild-type strain obtained in this analysis by using SD medium (91.7 ± 17.3 ml/mol) but similar to the value for the tat1Δ mutant (50.8 ± 13.1 ml/mol). These results suggest that Tat2 is the major tryptophan permease in the wild-type cells in the presence of tyrosine and phenylalanine in SC medium and probably in YPD medium.
DISCUSSION
In this study, we used a unique parameter, hydrostatic pressure, to determine the distinctive features of yeast tryptophan uptake. Pressure-induced regulation of tryptophan uptake was illustrated by the dramatic difference between the two permeases Tat1 and Tat2 in terms of Rsp5-Bul1-Bul2-mediated regulation, subcellular localization, and lipid microdomains where individual permeases are localized. Our findings highlighted a novel aspect of yeast in investigations of microbial adaptation to high-pressure environments as well as an understanding of membrane protein dynamics through the determination of volume changes.
Ubiquitination is responsible for pressure-induced degradation of tryptophan permeases.
Analysis of high-pressure growth mutants defined four distinct loci, HPG1, HPG2, HPG3, and HPG4. HPG1 was identical to RSP5. Rsp5 ubiquitin ligase consists of a C2 calcium-binding domain, three WW domains, and a catalytic HECT domain and has been implicated in numerous processes including transcription (35), mitochondrial inheritance (23, 73), the stability of cytosolic proteins (6), and endocytosis of membrane proteins (11, 19, 20, 21, 34, 63, 65, 67, 73). Among the four HPG1 mutants, only the HPG1-4 (Ala799→Thr) mutant showed significant high-temperature lethality, as observed in the well-characterized rsp5-1 (Leu733→Ser) (67, 68) and mdp1-1 (Pro784→Thr) (73) mutants. The Rsp5-1 protein shows impaired ubiquitin-thioester formation and impaired catalysis of substrates in vitro (67). Based on the tertiary structure of the HECT domain predicted by homology modeling (Fig. 2C), we found that the HPG1-4, rsp5-1, and mdp1-1 mutation sites are located in the C-lobe and are close to the active cysteine (Cys777). Since the three mutations generate an OH group at the amino acid residues, we suggest that hydrogen bond formation could alter the tertiary structure of the catalytic site or, most probably, interfere with the access of the ubiquitin-bound E2 enzymes. Since the HPG1-1, HPG1-2, and HPG1-3 mutants, for which the mutation sites are located within or adjacent to the NPF motif in the N-lobe, display normal growth at 37°C, the Hpg1-1, Hpg1-2, and Hpg1-3 proteins are likely to possess a substantial level of catalytic activity. Dunn and Hicke noted that deletion of the NPF motif of Rsp5 does not affect ubiquitination and subsequent endocytosis of the α-factor receptor Ste2, suggesting that the NPF motif has no role in the down-regulation of Ste2 (20). Therefore, the three mutations at the NPF motif are likely to alter the ability of Rsp5 to recognize Tat1 and Tat2 directly or indirectly, and the NPF motif specifies the recruitment of Rsp5 protein to the permeases at the plasma membrane by interacting with the endocytic EH-domain proteins such as Pan1 or End3. In addition, Wang et al. (68) reported that Rsp5 was distributed in a punctate pattern at the plasma membrane as well as at perivacuolar sites, and the catalytic activity as well as the C2 domain of Rsp5 is an important determinant of the localization of this protein.
The WW domain is characterized by two conserved tryptophan residues and a proline residue and is assumed to be a module interacting with proline-rich sequences or the PPxY motif. Bul1 and Bul2 are homologous proteins that contain the PPxY motif (70, 71). The WW domain of Rsp5 has been shown to be essential for endocytosis or the interaction with the actin cytoskeleton (38). Taken together with our results, Bul1 would have a role in the endocytosis of Tat2 as well as supporting Rsp5 activity. It is still unclear why the bul1Δ mutation caused the stabilization of Tat2 but the destabilization of Tat1 upon high-pressure incubation of the cells and why the bul1Δ bul2Δ double mutation enhanced the Tat2 level but decreased the Tat1 level. Bul1 and Bul2 are required for the polyubiquitination of Gap1, directing the sorting of this permease to the vacuole instead of the plasma membrane (34, 63). Whether Bul1 and Bul2 control the polyubiquitination of Tat2 remains to be determined.
Most recently, we have confirmed that HPG2 is allelic to TAT2 and that multiple HPG2 mutation sites are located in the N-terminal and C-terminal cytoplasmic domains of the Tat2 protein, not all of which are at lysine residues (unpublished data). Therefore, the cytoplasmic tails of Tat2 may be recognized by the Rsp5-Bul1-Bul2 complex and/or endocytic components in response to increasing hydrostatic pressure, followed by ubiquitination and subsequent internalization.
Vps27 is known to control membrane traffic through the PVC and the endosomal compartment (49, 50). vps27 cells accumulate vacuolar, Golgi, and endocytosed proteins in the PVC adjacent to the vacuoles (49, 50). Recent results indicate that the ubiquitin-interacting motifs of Vps27 are necessary to sort biosynthetic and endocytic cargo into vesicles that bud into the lumen of a late endosomal compartment, the multivesicular bodies (12, 40, 51, 52, 57). Deletion of either VPS27 or DOA4 produced a marked high-pressure growth phenotype (Fig. 3F), suggesting that accumulation of the ubiquitinated form of Tat1 and/or Tat2 leads to stabilization of these permeases in the plasma membrane at high pressure.
Subcellular localization and raft association.
Although Tat1 and Tat2 are homologous proteins, there was a striking difference in their localization: Tat1 localized predominantly in the plasma membrane, whereas Tat2 was abundant in the internal membranes. Moreover, Tat1 was associated with lipid rafts, whereas Tat2 existed in bulk lipids in both the P13 fraction and the plasma membrane. We propose a model depicting the subcellular localization and raft association of the two permeases focusing on the nature of these proteins and the role of ubiquitination. Protein hydrophobicity according to the model of Kyte and Doolittle (42) indicates that Tat2 is a remarkably hydrophobic protein (hydrophobicity index score, 0.4356) compared with other integral membrane proteins such as the amino acid permeases Tat1 (0.2452), Bap2 (0.2394), Bap3 (0.2275), Lyp1 (0.2959), Hip1 (0.2985), Put4 (0.2962), and Gap1 (0.3776), the uracil permease Fur4 (0.2352), and the H+-ATPase Pma1 (0.1229). Due to the high hydrophobicity of Tat2, its transmembrane domains are likely to undergo direct hydrophobic interactions with aliphatic chains of phospholipids. However, many membrane proteins, including Tat1, may not stably localize within the lipid bilayer due to low hydrophobicity. In this situation, ergosterol is likely to mediate the contact between such membrane proteins and lipids through a chemical attraction through π electrons of the sterol. This hypothesis is consistent with the facts that Pma1 (8, 9) Fur4 (21, 32), and Tat1 (Fig. 8), each with a relatively low hydrophobicity index value, have been shown to localize in lipid rafts and that proton extrusion mediated by Pma1 is highly sensitive to increasing pressure compared with proton incorporation, i.e., vacuolar acidification, mediated by vacuolar H+-ATPase (1, 2).
One of the critical issues in this study is that Tat2 became associated with lipid rafts when ubiquitination was abolished, whereas Tat1 localization was independent of ubiquitination. In rat basoleukemia cells, ubiquitinated forms of the high-affinity receptor for immunoglobulin E, FcɛRI, are clustered in lipid rafts upon receptor activation (43). Moreover, Nedd4, the mammalian homologue of Rsp5, has been found to associate with clustered FcɛRI rafts (43). In yeast, the association of Fur4 with lipid rafts has been shown to be independent of ubiquitination (21). We speculate that Rsp5 functions at lipid rafts to ubiquitinate Tat2 and probably other proteins as a protein quality control system. One possible mechanism is the Rsp5-dependent, ER-associated degradation termed Hrd1-independent proteolysis (30). Upon the loss of the Rsp5 ubiquitination activity, misfolded Tat2 proteins in lipid rafts are delivered to the plasma membrane along with Pma1 and Tat1. In this sense, the Tat2 protein associated with lipid rafts in the plasma membrane is less functional in HPG1-X, bul1Δ bul2 Δ, and Tat25K→R mutants. In contrast to our findings, a mutant form of Pma1 produced by the pma1-7 mutant is missorted to the vacuole and thus is unable to associate with lipid rafts (9).
The lipid microenvironment is a determining factor for ΔV≠.
One of our most notable findings is that there is a significant difference in the ΔV≠ for tryptophan import between Tat1 and Tat2. The inhibition of tryptophan uptake is attributable to positive ΔV≠s associated with catalysis and the reduced probability of attaining these volumes in a more ordered lipid environment. According to a proposed model (41, 45, 46), ΔV≠ is accounted for by summing three volumes, ΔV(interaction)≠ + ΔV(conformation)≠ + ΔV(hydration)≠, where ΔV(interaction)≠ is the volume change associated with the formation and deformation of various interactions between the substrate and amino acid residues, ΔV(conformation)≠ is the volume change of the protein conformation, and ΔV(hydration)≠ is the volume associated with modifying the hydration density resulting from the movement of certain amino acid residues into or away from contact with water. Assuming that ΔV(interaction)≠ is almost identical for Tat1 and Tat2, the sum of the ΔV(conformation)≠ and ΔV(hydration)≠ of Tat1 would be larger than that of Tat2. This indicates that Tat1 undergoes a dramatic conformational change during the activation associated with tryptophan import. According to the results obtained with mammalian Na+,K+-ATPase (15, 18, 39), the ATPase activity is accompanied by a smaller ΔV≠ (53 ml/mol) when the membrane maintains fluidity (pressure, 0.1 to 24 MPa) (18), which is a situation similar to that of Tat2 (ΔV≠ = 50.8 ml/mol [Table 5]), and is accompanied by a higher ΔV≠ (83 ml/mol) (18) when the membrane is ordered (pressure, >24 MPa), which is a situation similar to that of Tat1 (ΔV≠ = 89.3 ml/mol [Table 5]), indicating that the Na+,K+-ATPase underwent larger conformational changes at higher pressures because of an increase in the order parameter effected by shifting the melting transitions in phospholipids and aliphatic chains.
Because both Tat1 and Tat2 import the same substrate, tryptophan, their activated states are likely to be similar. Therefore, the remarkable difference in the ΔV≠ (89.3 versus 50.8 ml/mol) could be accounted for by the difference in the volume of the initial states (Fig. 10). This explanation is consistent with the findings that Tat1 is localized in the highly ordered lipid phase, in which the volume is small, whereas Tat2 is localized in the disordered phase, in which the volume is large. Some reports have supported the idea that factors affecting membrane order are crucial for tryptophan uptake. For example, membrane fluidity as measured by electron spin resonance is significantly lowered in ergosterol-deficient mutants (44), and the erg6 mutant, which is defective in ergosterol biosynthesis, has a defect in tryptophan uptake, suggesting that appropriate membrane order and/or ergosterol are required for normal tryptophan uptake activity (25). The addition of the volatile anesthetic isoflurane impairs cell growth in trp1 strains, probably by disrupting membrane order (48). It is still unclear whether inhibition of tryptophan uptake by PHS is due to disruption of membrane order or rather to specific effects on the membrane proteins (16, 17). It is worthwhile to systematically compare the effect of hydrostatic pressure with the effects of these drugs or the defect in ergosterol synthesis.
FIG. 10.
A model for the differential localization and dynamics of the two tryptophan permeases. Tat1 is associated with lipid rafts, whereas Tat2 is localized in bulk lipids. The large ΔV≠s for Tat1- and Tat2-mediated tryptophan import are accounted for mainly by volume changes associated with protein conformational changes. The initial volume of Tat1 is smaller than that of Tat2 because Tat1 is localized in the highly ordered lipid microdomain of lipid rafts. V, volume of the permease in the initial state; V≠, volume of the permease in the activated state; ΔV≠, activation volume associated with tryptophan import through the permease.
Finally, we suggest that hydrostatic pressure is a useful tool for probing lipid rafts and the resident proteins by measurement of ΔV≠s in combination with the detergent insolubility method to elucidate the dynamics of membrane protein function in living cells. In our studies of piezophysiology, we use hydrostatic pressure to bring minor species out of the background noise to the foreground for direct observation and discovery of novel biological processes employed by living cells (1-4).
Acknowledgments
We thank K. Horikoshi for discussion and encouragement; M. N. Hall, Y. Kikuchi, B. André, and M. J. Ellison for providing plasmids; and R. Serrano for the anti-Pma1 monoclonal antibody. We also thank T. Miura and A. Nagayama for technical assistance and S. Kaneshina, N. Matsuda, M. Kato, and T. Ushimaru for valuable discussions.
REFERENCES
- 1.Abe, F., and K. Horikoshi. 1998. Analysis of intracellular pH in the yeast Saccharomyces cerevisiae under elevated hydrostatic pressure: a study in baro- (piezo-) physiology. Extremophiles 2:223-228. [DOI] [PubMed] [Google Scholar]
- 2.Abe, F., C. Kato, and K. Horikoshi. 1999. Pressure-regulated metabolism in microorganisms. Trends Microbiol. 7:447-452. [DOI] [PubMed] [Google Scholar]
- 3.Abe, F., and K. Horikoshi. 2000. Tryptophan permease gene TAT2 confers high-pressure growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 20:8093-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe, F., and K. Horikoshi. 2001. The biotechnological potential of piezophiles. Trends Biotechnol. 19:102-108. [DOI] [PubMed] [Google Scholar]
- 5.Amerik, A. Y., J. Nowak, S. Swaminathan, and M. Hochstrasser. 2000. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol. Biol. Cell 11:3365-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andoh, T., Y. Hirata, and A. Kikuchi. 2000. Yeast glycogen synthase kinase 3 is involved in protein degradation in cooperation with Bul1, Bul2, and Rsp5. Mol. Cell. Biol. 20:6712-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.André, B. 1995. An overview of membrane transport proteins in Saccharomyces cerevisiae. Yeast 11:1575-1611. [DOI] [PubMed] [Google Scholar]
- 8.Bagnat, M., S. Keranen, A. Shevchenko, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagnat, M., A. Chang, and K. Simons. 2001. Plasma membrane protein ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell 12:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett, D. H. 1999. Microbial adaptations to the psychrosphere/piezosphere. J. Mol. Microbiol. Biotechnol. 1:93-100. [PubMed] [Google Scholar]
- 11.Beck, T., A. Schmidt, and M. N. Hall. 1999. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J. Cell Biol. 146:1227-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilodeau, P. S., J. L. Urbanowski, S. C. Winistorfer, and R. C. Piper. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4:534-539. [DOI] [PubMed] [Google Scholar]
- 13.Boeke, J. D., F. LaCrout, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine 5′-phosphase decarboxylase activity in yeast: 5-fluoroorotic acid resistance. Mol. Gen. Genet. 197:345-346. [DOI] [PubMed] [Google Scholar]
- 14.Chen, X. H., Z. Xiao, and M. Fitzgerald-Hayes. 1994. SCM2, a tryptophan permease in Saccharomyces cerevisiae, is important for cell growth. Mol. Gen. Genet. 244:260-268. [DOI] [PubMed] [Google Scholar]
- 15.Chong, P. L.-G, P. A. G. Fortes, and D. M. Jameson. 1985. Mechanisms of inhibition of (Na, K)-ATPase by hydrostatic pressure studied with fluorescent probes. J. Biol. Chem. 260:14484-14490. [PubMed] [Google Scholar]
- 16.Chung, N., G. Jenkins, Y. A. Hannun, J. Heitman, and L. M. Obeid. 2000. Sphingolipids signal heat stress-induced ubiquitin-dependent proteolysis. J. Biol. Chem. 275:17229-17232. [DOI] [PubMed] [Google Scholar]
- 17.Chung, N., C. Mao, J. Heitman, Y. A. Hannun, and L. M. Obeid. 2001. Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae. J. Biol. Chem. 276:35614-35621. [DOI] [PubMed] [Google Scholar]
- 18.de Smedt, H., R. Borghgraef, F. Ceuterick, and K. Heremans. 1979. Pressure effects on lipid-protein interactions in (Na+ + K+)-ATPase. Biochim. Biophys. Acta 556:479-489. [DOI] [PubMed] [Google Scholar]
- 19.Dunn, R., and L. Hicke. 2001. Domains of the Rsp5 ubiquitin-protein ligase required for receptor-mediated and fluid-phase endocytosis. Mol. Biol. Cell 12:421-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn, R., and L. Hicke. 2001. Multiple roles for Rsp5p-dependent ubiquitination at the internalization step of endocytosis. J. Biol. Chem. 276:25974-25981. [DOI] [PubMed] [Google Scholar]
- 21.Dupré, S., and R. Haguenauer-Tsapis. 2003. Raft partitioning of the yeast uracil permease during trafficking along the endocytic pathway. Traffic 4:83-96. [DOI] [PubMed] [Google Scholar]
- 22.Ellison, M. J., and M. Hochstrasser. 1991. Epitope-tagged ubiquitin. A new probe for analyzing ubiquitin function. J. Biol. Chem. 266:21150-21157. [PubMed] [Google Scholar]
- 23.Fisk, H. A., and M. P. Yaffe. 1999. A role for ubiquitination in mitochondrial inheritance in Saccharomyces cerevisiae. J. Cell Biol. 145:1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friant, S., B. Zanolari, and H. Riezman. 2000. Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. EMBO J. 19:2834-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaber, R. F., D. M. Copple, B. K. Kennedy, M. Vidal, and M. Bard. 1989. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol. Cell. Biol. 9:3447-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacing six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 27.Gong, X., and A. Chang. 2001. A mutant plasma membrane ATPase, Pma1-10, is defective in stability at the yeast cell surface. Proc. Natl. Acad. Sci. USA 98:9104-9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grzanowski, A., R. Needleman, and W. S. Brusilow. 2002. Immunosuppressant-like effects of phenylbutyrate on growth inhibition of Saccharomyces cerevisiae. Curr. Genet. 41:142-149. [DOI] [PubMed] [Google Scholar]
- 29.Guthrie, C., and G. R. Fink. 1991. Guide to yeast genetics and molecular biology. Academic Press, Inc., San Diego, Calif.
- 30.Haynes, C. M., S. Caldwell, and A. A. Cooper. 2002. An HRD/DER-independent ER quality control mechanism involves Rsp5p-dependent ubiquitination and ER-Golgi transport. J. Cell Biol. 158:91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hazel, J. R., and E. E. Williams. 1990. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid Res. 29:167-227. [DOI] [PubMed] [Google Scholar]
- 32.Hearn, J. D., R. L. Lester, and R. C. Dickson. 2003. The uracil transporter Fur4p associates with lipid rafts. J. Biol. Chem. 278:3679-3686. [DOI] [PubMed] [Google Scholar]
- 33.Heitman, J., A. Koller, J. Kunz, R. Henriquez, A. Schmidt, N. R. Movva, and M. N. Hall. 1993. The immunosuppressant FK506 inhibits amino acid import in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:5010-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helliwell, S. B., S. Losko, and C. A. Kaiser. 2001. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 153:649-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoppe, T., K. Matuschewski, M. Rape, S. Schlenker, H. D. Ulrich, and S. Jentsch. 2000. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 102:577-586. [DOI] [PubMed] [Google Scholar]
- 36.Horazdovsky, B. F., and S. D. Emr. 1993. The VPS16 gene product associates with a sedimentable protein complex and is essential for vacuolar protein sorting in yeast. J. Biol. Chem. 268:4953-4962. [PubMed] [Google Scholar]
- 37.Huang, L., E. Kinnucan, G. Wang, S. Beaudenon, P. M. Howley, J. M. Huibregtse, and N. P. Pavletich. 1999. Structure of an E6AP-UbcH7 complex: insight into ubiquitination by the E2-E3 enzyme cascade. Science 286:1321-1326. [DOI] [PubMed] [Google Scholar]
- 38.Kaminska, J., B. Gajewska, A. K. Hopper, and T. Zoladek. 2002. Rsp5p, a new link between the actin cytoskeleton and endocytosis in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 22:6946-6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato, M., R. Hayashi, T. Tsuda, and K. Taniguchi. 2002. High pressure-induced changes of biological membrane. Study on the membrane-bound Na+/K+-ATPase as a model system. Eur. J. Biochem. 269:110-118. [DOI] [PubMed] [Google Scholar]
- 40.Katzmann, D. J., M. Babst, and S. D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145-155. [DOI] [PubMed] [Google Scholar]
- 41.Kunugi, S., M. Fukuda, and N. Ise. 1982. Pressure dependence of trypsin-catalyzed hydrolyses of specific substrates. Biochim. Biophys. Acta 704:107-113. [DOI] [PubMed] [Google Scholar]
- 42.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 43.Lafont, F., and K. Simons. 2001. Raft-partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE-triggered cell signaling. Proc. Natl. Acad. Sci. USA 98:3180-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lees, N. D., M. Bard, M. D. Kemple, R. A. Haak, and F. W. Kleinhans. 1979. ESR determination of membrane order parameter in yeast sterol mutants. Biochim. Biophys. Acta 553:469-475. [DOI] [PubMed] [Google Scholar]
- 45.Low, P. S., and G. N. Somero. 1975. Activation volumes in enzymic catalysis: their sources and modification by low-molecular-weight solutes. Proc. Natl. Acad. Sci. USA 72:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makimoto, S., and Y. Taniguchi. 1987. Effect of pressure on the pre-steady-state kinetics of the hydrolysis of anilide substrates catalyzed by alpha-chymotrypsin. Biochim. Biophys. Acta 914:304-307. [DOI] [PubMed] [Google Scholar]
- 47.Nelissen, B., R. de Wachter, and A. Goffeau. 1997. Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21:113-134. [DOI] [PubMed] [Google Scholar]
- 48.Palmer, L. K., D. Wolfe, J. L. Keeley, and R. L. Keil. 2002. Volatile anesthetics affect nutrient availability in yeast. Genetics 161:563-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piper, R. C., A. A. Cooper, H. Yang, and T. H. Stevens. 1995. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131:603-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raymond, C. K., I. Howald-Stevenson, C. A. Vater, and T. H. Stevens. 1992. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3:1389-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reggiori, F., M. W. Black, and H. R. Pelham. 2000. Polar transmembrane domains target proteins to the interior of the yeast vacuole. Mol. Biol. Cell 11:3737-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reggiori, F., and H. R. Pelham. 2002. A transmembrane ubiquitin ligase required to sort membrane proteins into multivesicular bodies. Nat. Cell Biol. 4:117-123. [DOI] [PubMed] [Google Scholar]
- 53.Riezman, H., P. G. Woodman, G. van Meer, and M. Marsh. 1997. Molecular mechanisms of endocytosis. Cell 91:731-738. [DOI] [PubMed] [Google Scholar]
- 54.Roberg, K. J., N. Rowley, and C. A. Kaiser. 1997. Physiological regulation of membrane protein sorting late in the secretory pathway of Saccharomyces cerevisiae. J. Cell Biol. 137:1469-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt, A., M. N. Hall, and A. Koller. 1994. Two FK506 resistance-conferring genes in Saccharomyces cerevisiae, TAT1 and TAT2, encode amino acid permeases mediating tyrosine and tryptophan uptake. Mol. Cell. Biol. 14:6597-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmidt, A., T. Beck, A. Koller, J. Kunz, and M. N. Hall. 1998. The TOR nutrient signaling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17:6924-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shih, S. C., D. J. Katzmann, J. D. Schnell, M. Sutanto, S. D. Emr, and L. Hicke. 2002. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4:389-393. [DOI] [PubMed] [Google Scholar]
- 58.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva, J. L., D. Foguel, and C. A. Royer. 2001. Pressure provides new insights into protein folding, dynamics and structure. Trends Biochem. Sci. 26:612-618. [DOI] [PubMed] [Google Scholar]
- 60.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 61.Singh, A., and T. R. Manney. 1974. Genetic analysis of mutations affecting growth of Saccharomyces cerevisiae at low temperature. Genetics 77:651-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skrzypek, M. S., M. M. Nagiec, R. L. Lester, and R. C. Dickson. 1998. Inhibition of amino acid transport by sphingoid long chain bases in Saccharomyces cerevisiae. J. Biol. Chem. 273:2829-2834. [DOI] [PubMed] [Google Scholar]
- 63.Soetens, O., J.-O. De Craene, and B. André. 2001. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 276:43949-43957. [DOI] [PubMed] [Google Scholar]
- 64.Springael, J. Y., and B. André. 1998. Nitrogen-regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol. Biol. Cell 9:1253-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Springael, J.-Y., J.-O. De Craene, and B. André. 1999. The yeast Npi1/Rsp5 ubiquitin ligase lacking its N-terminal C2 domain is competent for ubiquitination but not for subsequent endocytosis of the Gap1 permease. Biochem. Biophys. Res. Commun. 257:561-566. [DOI] [PubMed] [Google Scholar]
- 66.Swaminathan, S., A. Y. Amerik, and M. Hochstrasser. 1999. The Doa4 deubiquitinating enzyme is required for ubiquitin homeostasis in yeast. Mol. Biol. Cell 10:2583-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, G., J. Yang, and J. M. Huibregtse. 1999. Functional domains of the Rsp5 ubiquitin-protein ligase. Mol. Cell. Biol. 19:342-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, G., J. M. McCaffery, B. Wendland, S. Dupré, R. Haguenauer-Tsapis, and J. M. Huibregtse. 2001. Localization of the Rsp5 ubiquitin-protein ligase at multiple sites within the endocytic pathway. Mol. Cell. Biol. 21:3564-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wendland, B., S. D. Emr, and H. Riezman. 1998. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr. Opin. Cell Biol. 10:513-522. [DOI] [PubMed] [Google Scholar]
- 70.Yashiroda, H., T. Oguchi, Y. Yasuda, A. Toh-e, and Y. Kikuchi. 1996. Bul1, a new protein that binds to the Rsp5 ubiquitin ligase in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:3255-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yashiroda, H., D. Kaida, A. Toh-e, and Y. Kikuchi. 1998. The PY-motif of Bul1 protein is essential for growth of Saccharomyces cerevisiae under various stress conditions. Gene 225:39-46. [DOI] [PubMed] [Google Scholar]
- 72.Zinser, E., F. Paltauf, and G. Daum. 1993. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 175:2853-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zoladek, T., A. Tobiasz, G. Vaduva, M. Boguta, N. C. Martin, and A. Hopper. 1997. MDP1, a Saccharomyces cerevisiae gene involved in mitochondrial/cytoplasmic protein distribution, is identical to the ubiquitin-protein ligase gene RSP5. Genetics 145:595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]