Abstract
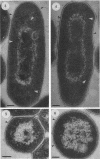
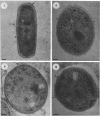
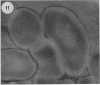
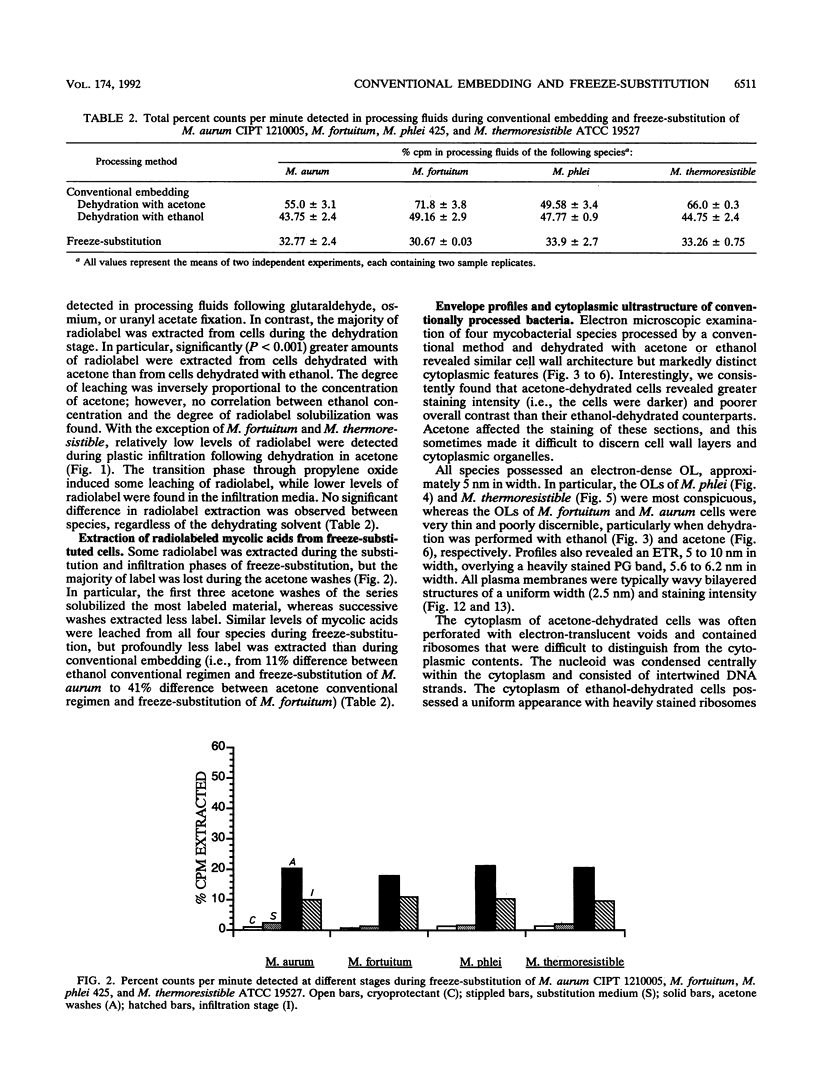
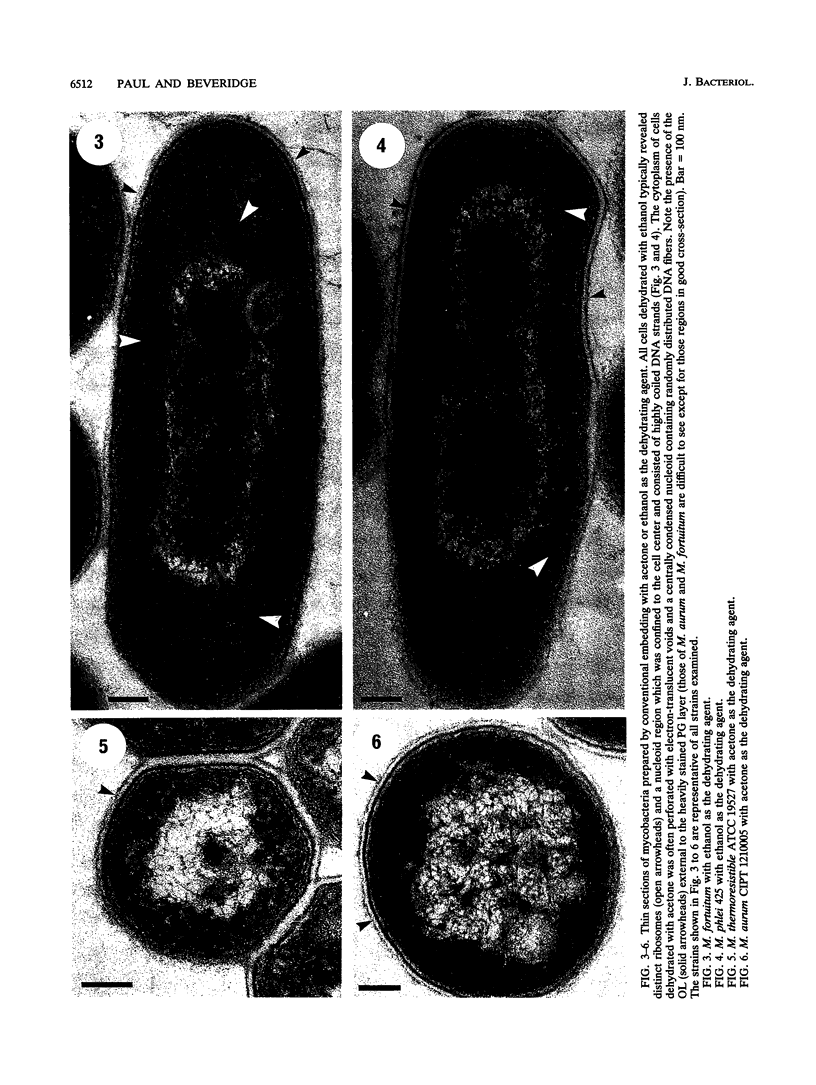
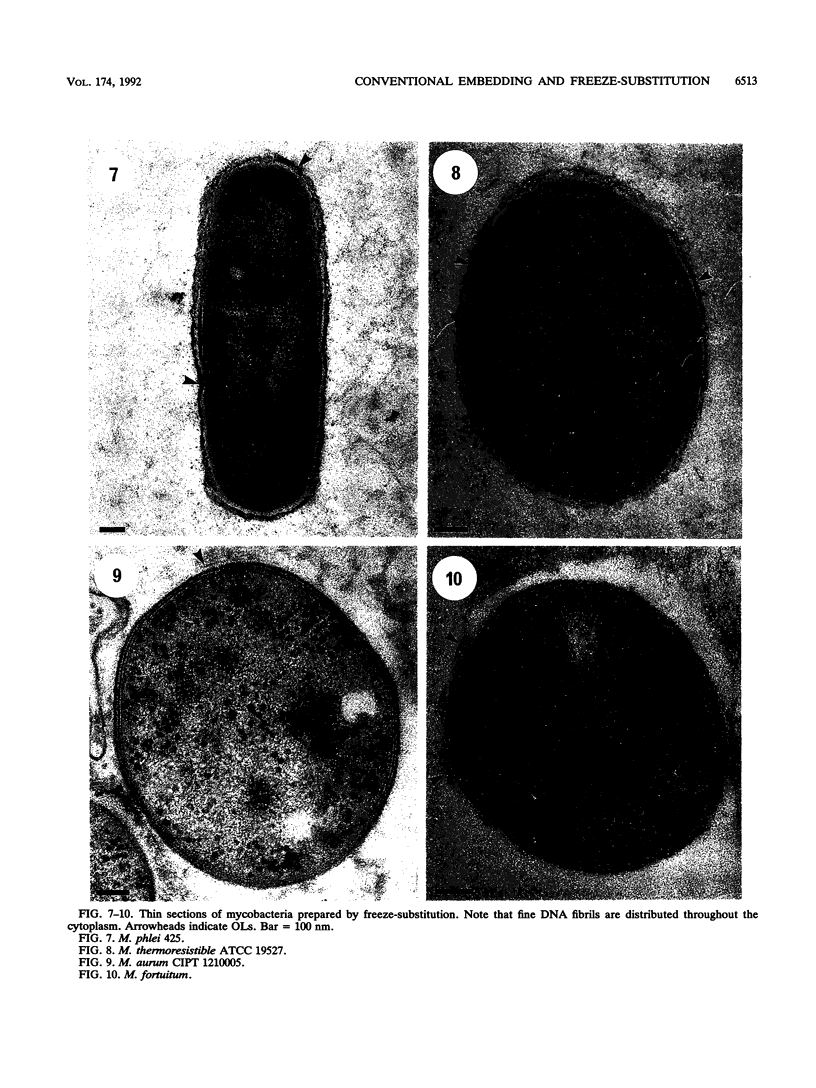
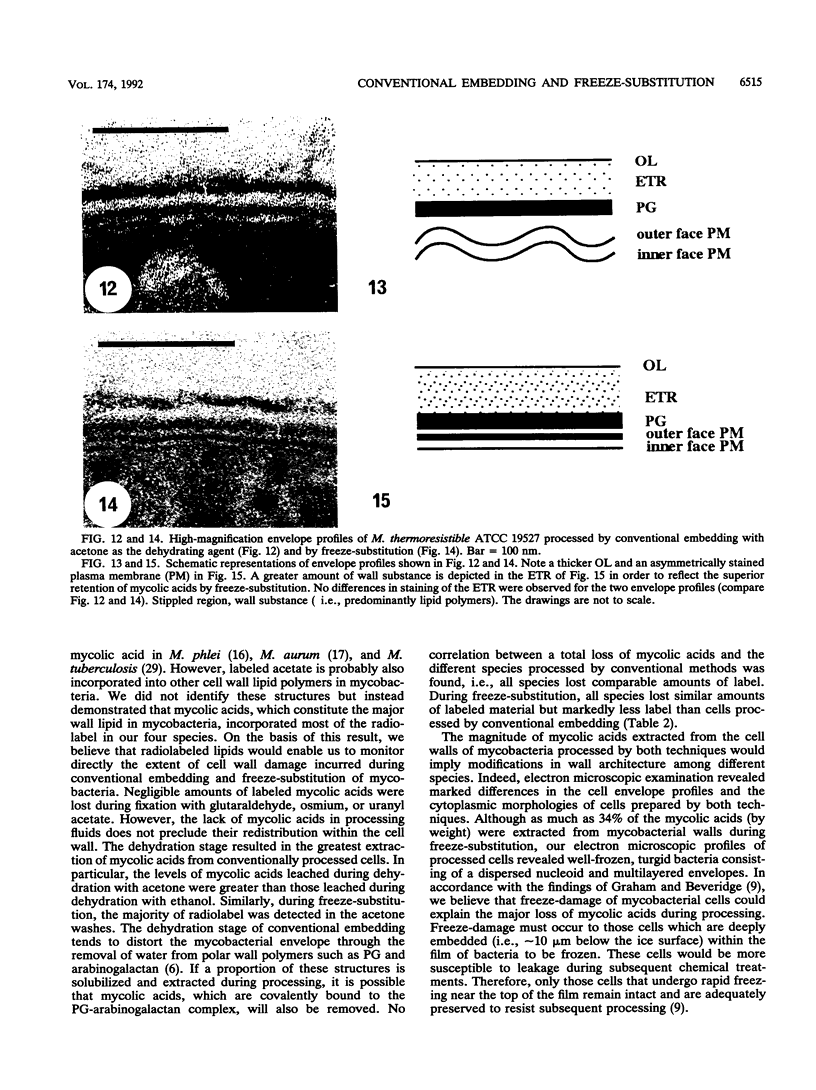
The cell envelope architectures and cytoplasmic structures of Mycobacterium aurum CIPT 1210005, M. fortuitum, M. phlei 425, and M. thermoresistible ATCC 19527 were compared by conventional embedding and freeze-substitution methods. To ascertain the integrity of cells during each stage of the processing regimens, [1-14C]acetate was incorporated into the mycolic acids of mycobacterial walls, and the extraction of labeled mycolic acids was monitored by liquid scintillation counting. Radiolabeled mycolic acids were extracted by both processing methods; however, freeze-substitution resulted in the extraction of markedly less radiolabel. During conventional processing of cells, most of the radiolabel was extracted during the dehydration stage, whereas postsubstitution washes in acetone yielded the greatest loss of radiolabel during freeze-substitution. Conventional embedding frequently produced cells with condensed fibrous nucleoids and occasional mesosomes. Their cell walls were relatively thick (approximately 25 nm) but lacked substance. Freeze-substituted cells appeared more robust, with well-dispersed nucleoids and ribosomes. The walls of all species were much thinner than those of their conventionally processed counterparts, but these stained well, which was an indication of more wall substance; the fabric of these walls, in particular the plasma membrane, appeared highly condensed and tightly apposed to the peptidoglycan. Some species possessed a thick, irregular outer layer that was readily visualized in the absence of exogenous stabilizing agents by freeze-substitution. Since freeze-substituted mycobacteria retained a greater percentage of mycolic acids in their walls, and probably other labile wall and cytoplasmic constituents, we believe that freeze-substitution provides a more accurate image of structural organization in mycobacteria than that achieved by conventional procedures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- David H. L. Basis for lack of drug susceptibility of atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):878–884. doi: 10.1093/clinids/3.5.878. [DOI] [PubMed] [Google Scholar]
- Draper P. The structure of the mycobacterial cell envelope is not yet understood. Res Microbiol. 1991 May;142(4):420–422. doi: 10.1016/0923-2508(91)90113-o. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Effect of chemical fixatives on accurate preservation of Escherichia coli and Bacillus subtilis structure in cells prepared by freeze-substitution. J Bacteriol. 1990 Apr;172(4):2150–2159. doi: 10.1128/jb.172.4.2150-2159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Harris R., Villiger W., Beveridge T. J. Freeze-substitution of gram-negative eubacteria: general cell morphology and envelope profiles. J Bacteriol. 1991 Mar;173(5):1623–1633. doi: 10.1128/jb.173.5.1623-1633.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D. M. Freeze-substitution. J Microsc. 1982 Aug;127(Pt 2):209–221. doi: 10.1111/j.1365-2818.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T., Kanetsuna F., Galindo B. Ultrastructure of cell walls of genus Mycobacterium. J Ultrastruct Res. 1968 Oct;25(1):46–63. doi: 10.1016/s0022-5320(68)80059-0. [DOI] [PubMed] [Google Scholar]
- Lacave C., Laneelle M. A., Daffe M., Montrozier H., Laneelle G. Mycolic acid metabolic filiation and location in Mycobacterium aurum and Mycobacterium phlei. Eur J Biochem. 1989 May 1;181(2):459–466. doi: 10.1111/j.1432-1033.1989.tb14747.x. [DOI] [PubMed] [Google Scholar]
- Lacave C., Quémard A., Lanéelle G. Cell-free synthesis of mycolic acids in Mycobacterium aurum: radioactivity distribution in newly synthesized acids and presence of cell wall in the system. Biochim Biophys Acta. 1990 Jun 28;1045(1):58–68. doi: 10.1016/0005-2760(90)90203-a. [DOI] [PubMed] [Google Scholar]
- McNeil M. R., Brennan P. J. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res Microbiol. 1991 May;142(4):451–463. doi: 10.1016/0923-2508(91)90120-y. [DOI] [PubMed] [Google Scholar]
- Puzo G. The carbohydrate- and lipid-containing cell wall of mycobacteria, phenolic glycolipids: structure and immunological properties. Crit Rev Microbiol. 1990;17(4):305–327. doi: 10.3109/10408419009105730. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Rastogi N. Recent observations concerning structure and function relationships in the mycobacterial cell envelope: elaboration of a model in terms of mycobacterial pathogenicity, virulence and drug-resistance. Res Microbiol. 1991 May;142(4):464–476. doi: 10.1016/0923-2508(91)90121-p. [DOI] [PubMed] [Google Scholar]
- Ridgway H. F., Rigby M. G., Argo D. G. Adhesion of a Mycobacterium sp. to cellulose diacetate membranes used in reverse osmosis. Appl Environ Microbiol. 1984 Jan;47(1):61–67. doi: 10.1128/aem.47.1.61-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. The interpretation of the ultrastructure of mycobacterial cells in transmission electron microscopy of ultrathin sections. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):225–234. [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. Ultrastructure of Mycobacterium leprae and other acid-fast bacteria as influenced by fixation conditions. Ann Microbiol (Paris) 1982 Jul-Aug;133(1):59–73. [PubMed] [Google Scholar]
- Supplement on future research in tuberculosis. Prospects and priorities for elimination. Endorsement of the American Thoracic Society Board of Directors in March 1986. Am Rev Respir Dis. 1986 Aug;134(2):401–423. [PubMed] [Google Scholar]
- Takayama K., Wang L., David H. L. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1972 Jul;2(1):29–35. doi: 10.1128/aac.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C., Christiansson A., Carlemalm E. Extraction of membrane lipids during fixation, dehydration and embedding of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1983 Feb;129(Pt 2):201–207. doi: 10.1111/j.1365-2818.1983.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Weibull C., Christiansson A. Extraction of proteins and membrane lipids during low temperature embedding of biological material for electron microscopy. J Microsc. 1986 Apr;142(Pt 1):79–86. doi: 10.1111/j.1365-2818.1986.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Weibull C., Villiger W., Carlemalm E. Extraction of lipids during freeze-substitution of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1984 May;134(Pt 2):213–216. doi: 10.1111/j.1365-2818.1984.tb02513.x. [DOI] [PubMed] [Google Scholar]