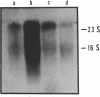
Abstract
In a screen for Escherichia coli genes whose products are required for high-temperature growth, we identified and characterized a mini-Tn10 insertion that allows the formation of wild-type-size colonies at 30 degrees C but results in microcolony formation at 36 degrees C and above (Ts- phenotype). Mapping, molecular cloning, and DNA sequencing analyses showed that the mini-Tn10 insertion resides in the cydB gene, the distal gene of the cydAB operon (cytochrome d). The Ts- growth phenotype was also shown to be associated with previously described cyd alleles. In addition, all cyd mutants were found to be extremely sensitive to hydrogen peroxide. Northern (RNA) blot analysis showed that cyd-specific mRNA levels accumulate following a shift to high temperature. Interestingly, this heat shock induction of the cyd operon was not affected in an rpoH delta background but was totally absent in an arcA or arcB mutant background. Extragenic suppressors of the Cyd Ts- phenotype are found at approximately 10(-3). Two extragenic suppressors were shown to be null alleles in either arcA or arcB. One interpretation of our results is that in the absence of ArcA or ArcB, which are required for the repression of the cyo operon (cytochrome o), elevated levels of Cyo are produced, thus compensating for the missing cytochrome d function. Consistent with this interpretation, the presence of the cyo gene on a multicopy plasmid suppressed the Ts- and hydrogen peroxide-sensitive phenotypes of cyd mutants.
Full text
PDF

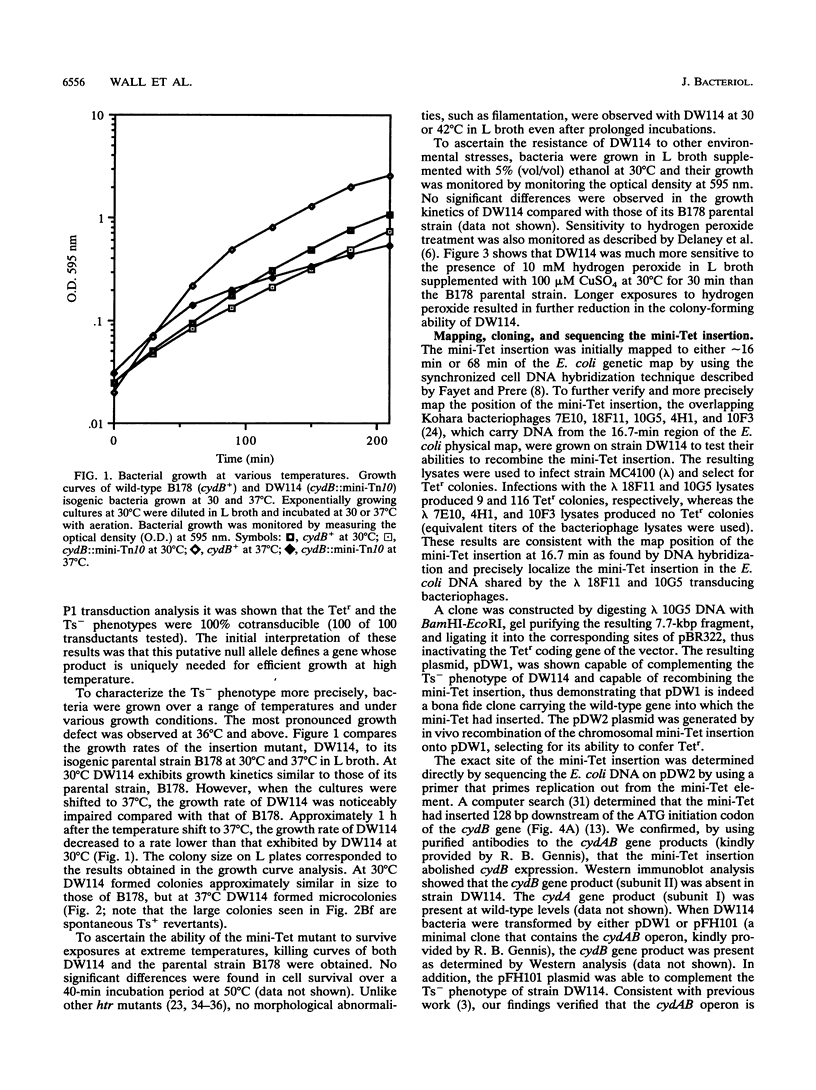
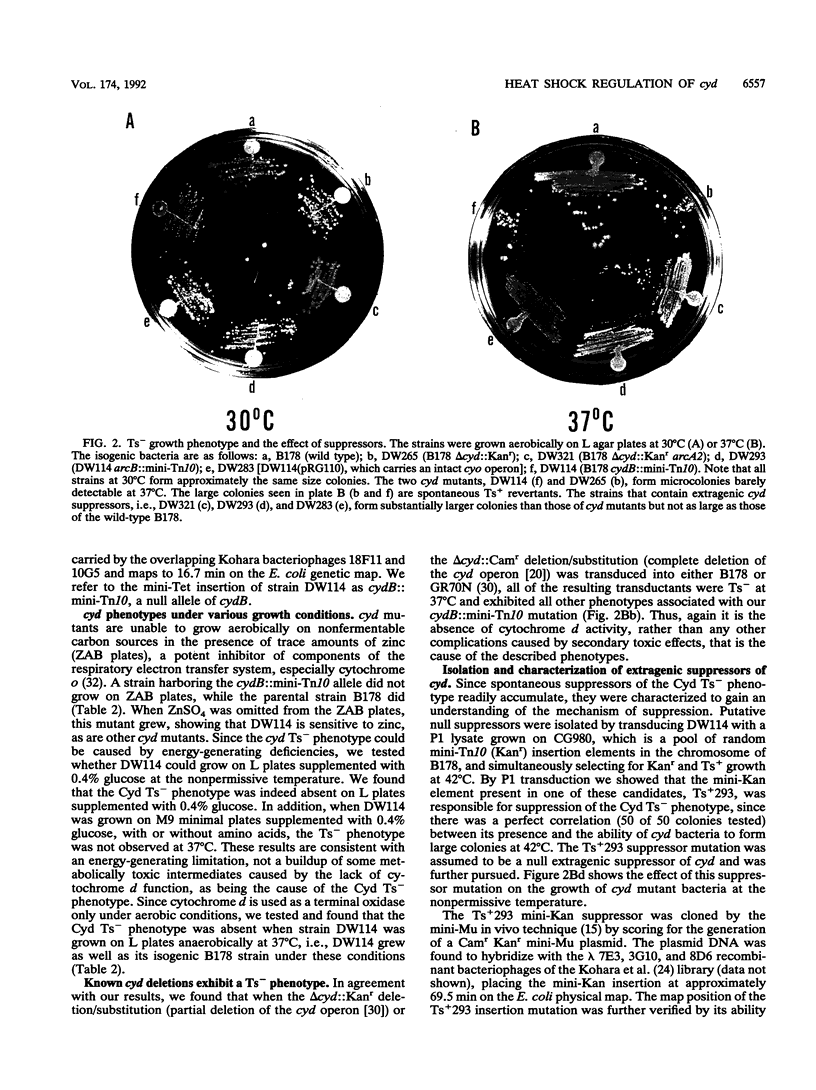
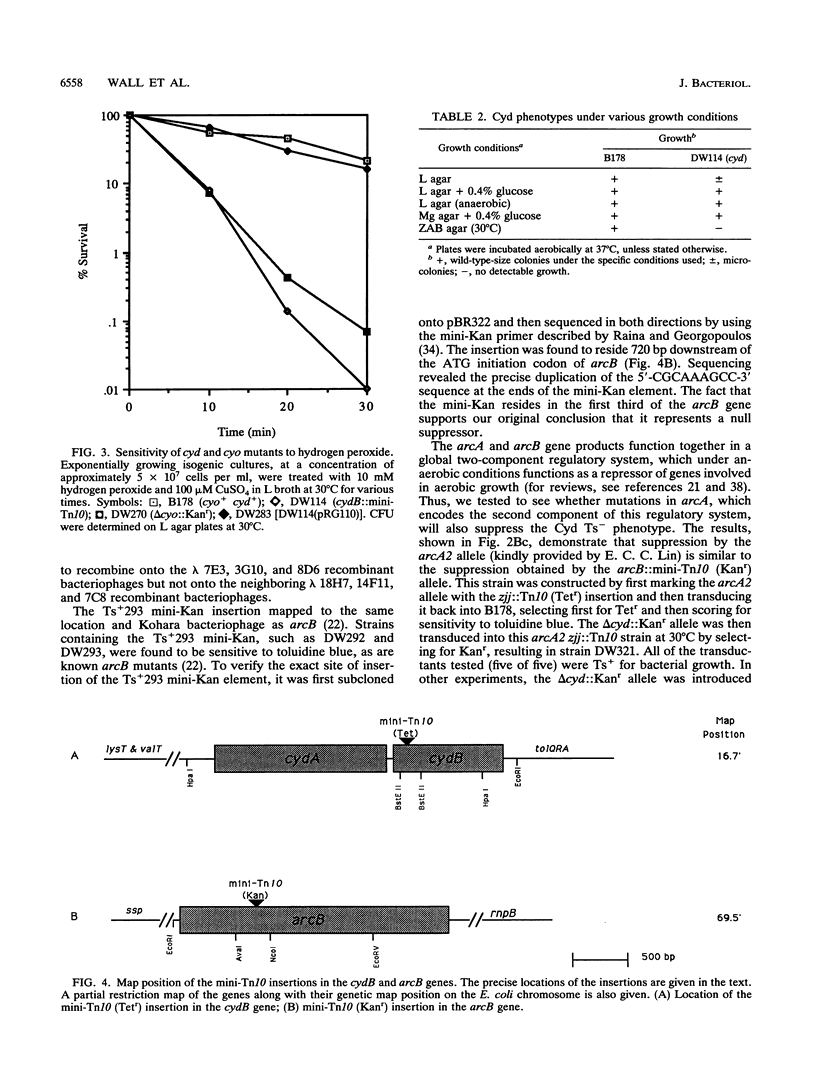

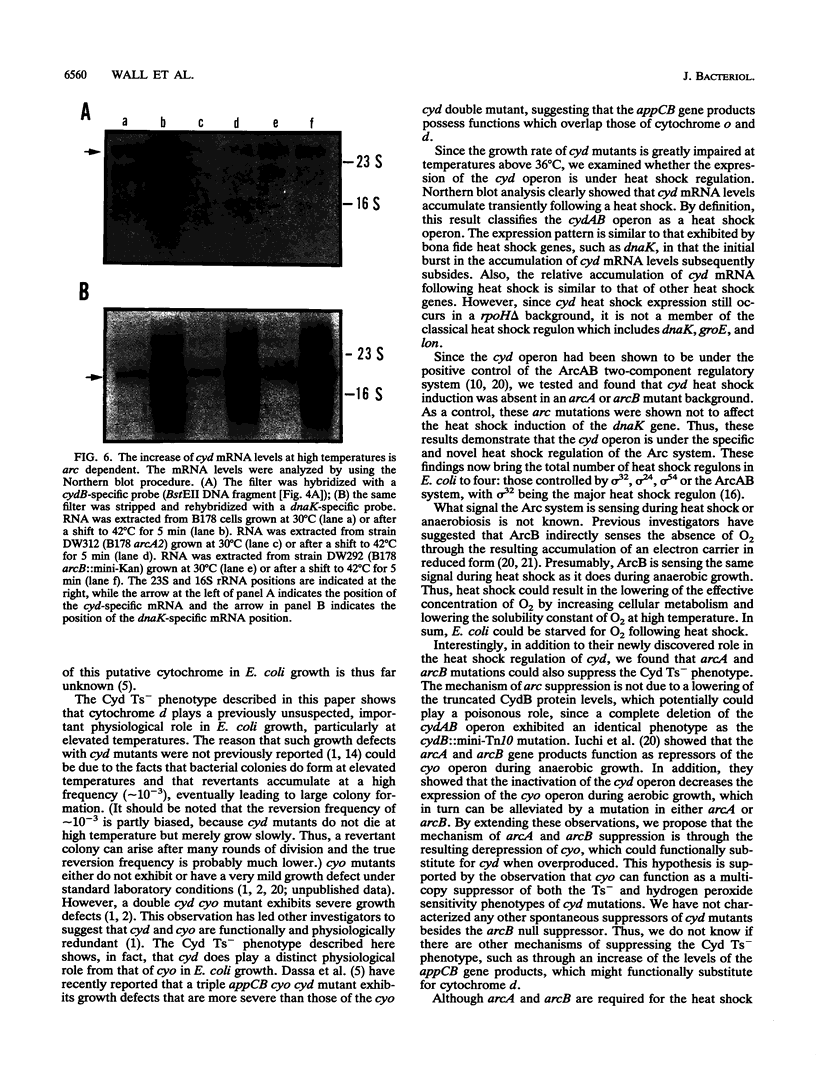


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au D. C., Lorence R. M., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking the cytochrome o terminal oxidase. J Bacteriol. 1985 Jan;161(1):123–127. doi: 10.1128/jb.161.1.123-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun M. W., Newton G., Gennis R. B. E. coli map. Physical map locations of genes encoding components of the aerobic respiratory chain of Escherichia coli. J Bacteriol. 1991 Mar;173(5):1569–1570. doi: 10.1128/jb.173.5.1569-1570.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. A., Chepuri V., Gennis R. B., Gunsalus R. P. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol. 1990 Nov;172(11):6333–6338. doi: 10.1128/jb.172.11.6333-6338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa J., Fsihi H., Marck C., Dion M., Kieffer-Bontemps M., Boquet P. L. A new oxygen-regulated operon in Escherichia coli comprises the genes for a putative third cytochrome oxidase and for pH 2.5 acid phosphatase (appA) Mol Gen Genet. 1991 Oct;229(3):341–352. doi: 10.1007/BF00267454. [DOI] [PubMed] [Google Scholar]
- Delaney J. M., Ang D., Georgopoulos C. Isolation and characterization of the Escherichia coli htrD gene, whose product is required for growth at high temperatures. J Bacteriol. 1992 Feb;174(4):1240–1247. doi: 10.1128/jb.174.4.1240-1247.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayet O., Prere M. F. Method for localization of cloned DNA fragments on the Escherichia coli chromosome. J Bacteriol. 1987 Dec;169(12):5641–5647. doi: 10.1128/jb.169.12.5641-5647.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B., Jänel G., Michelsen U., Kersten H. Mutations in the Escherichia coli fnr and tgt genes: control of molybdate reductase activity and the cytochrome d complex by fnr. J Bacteriol. 1989 Mar;171(3):1524–1530. doi: 10.1128/jb.171.3.1524-1530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H. A., Iuchi S., Lin E. C. The requirement of ArcA and Fnr for peak expression of the cyd operon in Escherichia coli under microaerobic conditions. Mol Gen Genet. 1991 Apr;226(1-2):209–213. doi: 10.1007/BF00273605. [DOI] [PubMed] [Google Scholar]
- Georgiou C. D., Dueweke T. J., Gennis R. B. Regulation of expression of the cytochrome d terminal oxidase in Escherichia coli is transcriptional. J Bacteriol. 1988 Feb;170(2):961–966. doi: 10.1128/jb.170.2.961-966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Fang H., Lin R. J., Newton G., Mather M., Georgiou C. D., Gennis R. B. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J Biol Chem. 1988 Sep 15;263(26):13138–13143. [PubMed] [Google Scholar]
- Groisman E. A., Casadaban M. J. Mini-mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986 Oct;168(1):357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Effect of RNase III on efficiency of translation of bacteriophage T7 lysozyme mRNA. J Virol. 1978 Jun;26(3):793–804. doi: 10.1128/jvi.26.3.793-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Hill S., Viollet S., Smith A. T., Anthony C. Roles for enteric d-type cytochrome oxidase in N2 fixation and microaerobiosis. J Bacteriol. 1990 Apr;172(4):2071–2078. doi: 10.1128/jb.172.4.2071-2078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Chepuri V., Fu H. A., Gennis R. B., Lin E. C. Requirement for terminal cytochromes in generation of the aerobic signal for the arc regulatory system in Escherichia coli: study utilizing deletions and lac fusions of cyo and cyd. J Bacteriol. 1990 Oct;172(10):6020–6025. doi: 10.1128/jb.172.10.6020-6025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Adaptation of Escherichia coli to respiratory conditions: regulation of gene expression. Cell. 1991 Jul 12;66(1):5–7. doi: 10.1016/0092-8674(91)90130-q. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Matsuda Z., Fujiwara T., Lin E. C. The arcB gene of Escherichia coli encodes a sensor-regulator protein for anaerobic repression of the arc modulon. Mol Microbiol. 1990 May;4(5):715–727. doi: 10.1111/j.1365-2958.1990.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Karow M., Georgopoulos C. Sequencing, mutational analysis, and transcriptional regulation of the Escherichia coli htrB gene. Mol Microbiol. 1991 Sep;5(9):2285–2292. doi: 10.1111/j.1365-2958.1991.tb02159.x. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Sharma S., Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988 Nov 11;16(21):10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville S. B., Gunsalus R. P. Mutations in fnr that alter anaerobic regulation of electron transport-associated genes in Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18733–18736. [PubMed] [Google Scholar]
- Nakamura H., Yamato I., Anraku Y., Lemieux L., Gennis R. B. Expression of cyoA and cyoB demonstrates that the CO-binding heme component of the Escherichia coli cytochrome o complex is in subunit I. J Biol Chem. 1990 Jul 5;265(19):11193–11197. [PubMed] [Google Scholar]
- Oden K. L., DeVeaux L. C., Vibat C. R., Cronan J. E., Jr, Gennis R. B. Genomic replacement in Escherichia coli K-12 using covalently closed circular plasmid DNA. Gene. 1990 Nov 30;96(1):29–36. doi: 10.1016/0378-1119(90)90337-q. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Williams H. D., Downie J. A., Gibson F. Mutations affecting the cytochrome d-containing oxidase complex of Escherichia coli K12: identification and mapping of a fourth locus, cydD. J Gen Microbiol. 1989 Jul;135(7):1865–1874. doi: 10.1099/00221287-135-7-1865. [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Induction of superoxide dismutase in Escherichia coli by heat shock. Proc Natl Acad Sci U S A. 1987 May;84(9):2723–2726. doi: 10.1073/pnas.84.9.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S., Georgopoulos C. A new Escherichia coli heat shock gene, htrC, whose product is essential for viability only at high temperatures. J Bacteriol. 1990 Jun;172(6):3417–3426. doi: 10.1128/jb.172.6.3417-3426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S., Georgopoulos C. The htrM gene, whose product is essential for Escherichia coli viability only at elevated temperatures, is identical to the rfaD gene. Nucleic Acids Res. 1991 Jul 25;19(14):3811–3819. doi: 10.1093/nar/19.14.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S., Mabey L., Georgopoulos C. The Escherichia coli htrP gene product is essential for bacterial growth at high temperatures: mapping, cloning, sequencing, and transcriptional regulation of htrP. J Bacteriol. 1991 Oct;173(19):5999–6008. doi: 10.1128/jb.173.19.5999-6008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Adaptive responses to oxygen limitation in Escherichia coli. Trends Biochem Sci. 1991 Aug;16(8):310–314. doi: 10.1016/0968-0004(91)90125-f. [DOI] [PubMed] [Google Scholar]
- Storz G., Jacobson F. S., Tartaglia L. A., Morgan R. W., Silveira L. A., Ames B. N. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J Bacteriol. 1989 Apr;171(4):2049–2055. doi: 10.1128/jb.171.4.2049-2055.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardat B., Touati D. Two global regulators repress the anaerobic expression of MnSOD in Escherichia coli::Fur (ferric uptake regulation) and Arc (aerobic respiration control). Mol Microbiol. 1991 Feb;5(2):455–465. doi: 10.1111/j.1365-2958.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Weiner L., Brissette J. L., Model P. Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on sigma 54 and modulated by positive and negative feedback mechanisms. Genes Dev. 1991 Oct;5(10):1912–1923. doi: 10.1101/gad.5.10.1912. [DOI] [PubMed] [Google Scholar]