Abstract
The mitochondrial release of cytochrome c and Smac/DIABLO has been implicated in the activation of apoptosis in response to cell stress. Smac promotes cytochrome c-induced activation of caspases by sequestering the inhibitor of apoptosis protein (IAP) family of potent caspase suppressors. Differential release from mitochondria of cytochrome c and Smac can occur, but the underlying mechanism and physiological significance of this are unclear. Here we show that the mechanism by which fibroblast growth factor 2 (FGF-2) protects small cell lung cancer (SCLC) cells from etoposide-induced cell death involves inhibition of Smac release but not of cytochrome c release. This process is MEK dependent and correlates with an increased expression of XIAP and cellular IAP-1, mediated principally through translational regulation. Exogenous expression of XIAP is sufficient to inhibit caspase 9 activation, Smac release, and cell death induced by etoposide. Prevention of the FGF-2-promoted increase in levels of functional IAPs by RNA interference or the cell-permeant Smac amino-terminal peptide blocked FGF-2-induced protection. FGF-2 can thus protect SCLC cells from chemotherapeutic drugs by modulating IAP levels via posttranscriptional regulation, providing a mechanism for postmitochondrial survival signaling by the MEK/mitogen-activated protein kinase pathway.
Apoptosis is a form of physiological cell death involved in development and homeostasis (4, 8, 13). The caspase family of cysteinyl aspartate-specific proteases plays a central role in this process (44). A main trigger of caspase activation is the release of cytochrome c from mitochondria following an apoptotic stimulus (44). This leads to the cytoplasmic assembly of procaspase 9, cytochrome c, and apoptosis protease-activating factor 1 (Apaf-1) into an initiation complex known as the apoptosome (1, 32, 34). However, the activation of caspase 9 and the subsequent execution of the apoptotic program, including caspase 3 activation, is blocked by the inhibitor-of-apoptosis proteins (IAPs) (15). Recently, a second mitochondrial factor involved in the activation of caspases has been identified and termed Smac (16) or DIABLO (42). Once released from mitochondria upon an apoptotic stimulus, Smac/DIABLO docks to the IAPs via an amino-terminal Reaper motif. This displaces the IAPs from their caspase binding sites, thereby relieving the block on caspase activation (37, 43). Another protein, the serine protease HtrA2/Omi, is also released from mitochondria, binds to IAPs via an amino-terminal Reaper motif, and may serve a function similar to that of Smac (19, 26, 39, 41, 43).
The mechanism for Smac and HtrA2 release is not well understood. In particular, it is unclear whether it is the same as the mechanism for cytochrome c release. There has been one report of release of Smac without release of cytochrome c (10) and a number of reports of cytochrome c being released without Smac (2, 14, 38), suggesting differential regulation of these two events. However, the molecular mechanisms underlying these differences are not known. Moreover, it is unclear whether physiological survival signals might differentially regulate the release of Smac or HtrA2 and cytochrome c.
Earlier work has identified fibroblast growth factor 2 (FGF-2) as a survival factor capable of protecting small cell lung carcinoma (SCLC) cells from etoposide-induced apoptosis via a MEK-dependent pathway (30). This common disease has a very poor prognosis due to the rapid development of resistance to chemotherapeutic agents such as etoposide. Here we have explored the mechanism whereby activation of the MEK/mitogen-activated protein (MAP) kinase (ERK) pathway in response to FGF-2 treatment is able to protect SCLC cells from drug-induced apoptosis. We show that FGF-2 does not inhibit cytochrome c release from mitochondria following etoposide treatment but does block Smac release and caspase activation. This suggests that the ERK pathway protects these cells downstream of cytochrome c release from mitochondria. We demonstrate that the mechanism by which this occurs is through ERK pathway-induced increases in the protein levels of a number of IAPs, principally through a translational mechanism. This is both necessary and sufficient for FGF-2 protection of SCLC cells from etoposide-induced apoptosis. Our results suggest that agents which inhibit MEK or neutralize IAPs, such as Reaper motif mimetics, may be able to reverse the resistance of SCLC to chemotherapeutic agents.
MATERIALS AND METHODS
Cell culture.
H510 and H69 SCLC cell lines were maintained as previously described (31). For experimental purposes, the cells were grown in serum-free medium (SFM) (RPMI 1640 supplemented with 5 μg of insulin/ml, 10 μg of transferrin/ml, 30 nM sodium selenite, and 0.25% bovine serum albumin) and used after 3 to 7 days.
Establishment of H69 SCLC cell lines stably expressing XIAP or activated MEK.
H69 cells were transfected with a pcDNA3.1 vector (Invitrogen) containing the coding sequence for XIAP (U45880) by using Lipofectin reagent (Invitrogen) according to the manufacturer's instructions. Selection was carried out in the presence of 1 mg of G418 (Invitrogen)/ml, and transgene expression was assessed using Western blotting. H69 cells expressing activated MEK were generated using a murine retroviral system as previously described (30).
Establishment of H510 cells stably expressing RNAi against XIAP and cIAP-1 using a panretroviral system.
RNA interference (RNAi) sequences were obtained using previously published guidelines. For XIAP, the forward sequence GATCCCCGTATCCCCAAATTGCAGATTTCAAGAGAATCTGCAATTTGGGGATACTTTTTGGAAA (X1) was annealed with the reverse sequence AGCTTTTCCAAAAAGTATCCCCAAATTGCAGATTCTCTTGAAATCTGCAATTTGGGGATACGGG. For cIAP-1, the forward sequence GATCCCCCCAGGAACTCTGGAGTTCATTCAAGAGATGAACTCCAGAGTTCCTGGTTTTTGGAAA (I3)) was annealed with the reverse sequence AGCTTTTCCAAAAAGGAAATGCTGCGGCCAACATCTCTTGAATGTTGGCCGCAGCATTTCCGGG. Annealed oligonucleotides were ligated into the pRetro-Super plasmid by using the BglII and HindIII sites. Panretroviruses were obtained by following the manufacturer's instructions (Clontech) and were incubated for 8 h with H510 cells. Cells were then washed and grown in RPMI containing 10% fetal calf serum (FCS) for 48 h prior to selection using puromycin. Down-regulation of the target proteins was confirmed by Western blotting.
Isolation of type II pneumocytes.
Human lung tissue was obtained following lobectomy for carcinoma of the lung, and type II pneumocytes were isolated as described previously (31, 45).
Subcellular fractionation.
H510 cells (2 × 106 per condition) were centrifuged in 1.5-ml Eppendorf tubes, resuspended in 100 μl of ice-cold fractionation buffer (0.02% digitonin, 220 mM manitol, 68 mM sucrose, 2 mM MgCl2, 2 mM NaCl, 2.5 mM H2KPO4, 0.5 mM EGTA, 0.5 mM sodium pyruvate, 0.5 mM l-glutamine, 10 mM HEPES [pH 7.4]), and incubated on ice for 1 min. Cells were then centrifuged at 15,000 × g and 4°C for 30 s. The supernatant containing the cytoplasmic fraction was then isolated from the pellet containing the mitochondrial fraction. The purity of the cytoplasmic fraction was assessed by confirming the absence of cytochrome oxidase by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Immunofluorescence and confocal microscopy.
SCLC cells in SFM were treated with the relevant factors and then centrifuged in 1.5-ml Eppendorf tubes. The cell pellet was resuspended in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 4% formaldehyde and 0.05% saponin for 15 min at room temperature (RT). Cells were then washed twice in Ca2+- and Mg2+-free PBS containing 0.03% saponin and were resuspended in blocking solution (PBS-3% bovine serum albumin [BSA]) for 30 min. After one wash in PBS-1% BSA, the relevant primary antibody was added (in PBS-1% BSA for 1 h at RT). Following three washes in PBS-1% BSA, the fluorescein isothiocyanate (FITC)-labeled secondary antibody was added (in PBS-1% BSA for 1 h at RT in the dark). Samples were then washed four times in PBS, centrifuged onto glass slides, and mounted with Vectashield containing 4′,6′-diamidino-2-phenylindole (DAPI).
Cell proliferation assay.
H510 cells were treated with or without FGF-2 for 4 h, followed by incubation with or without 0.1 μM etoposide for 4 days. Cells from untreated and FGF-2- and/or etoposide-treated cultures were then replated at 2.5 × 104/ml in RPMI-10% FCS and incubated at 37°C under 5% CO2. Cell numbers were regularly determined over a period of 10 days by cell counting under the microscope. Each condition was set in quadruplicate, and three counts were performed per replicate.
Caspase inhibition.
A total of 2 × 106 cells were resuspended in 1 ml of fresh SFM and preincubated in the presence or absence of the caspase inhibitor zVAD (100 μM), zDEVD (30 μM), or zLEHD (20 μM). Cells were then treated with 0.1 μM etoposide for 5.5 h prior to lysis. Lysates were run on 4 to 20% gradient SDS-PAGE gels and Western blotted for the indicated caspase cleavage products.
Cell death assay.
H510 cells (5 × 104/ml of SFM) were pretreated with or without 0.1 ng of FGF-2/ml for the time indicated in the figure legend prior to treatment with 0.1 μM etoposide and were incubated at 37°C for 96 h. When required, a cell-permeant Smac peptide at 75 μM (see below) was added to the cells 1 h prior to the addition of FGF-2 and/or etoposide. Single-cell suspensions were generated by passing the samples through a 19-gauge needle four times, and live-cell numbers were determined using trypan blue exclusion.
Cell-permeant Smac peptide.
A Smac peptide consisting of the eight N-terminal amino acids of Smac (AVPIAQKC), the seventh residue being biotinylated, was linked by a disulfide bond to a sequence derived from the Antennapedia homeodomain, which mediates membrane translocation (DRQIKIWFQNRRMKWKKC). The activity of the peptide was assayed by streptavidin bead pulldown assays followed by immunoblotting for IAPs (data not shown).
Caspase 3 activity assay.
H510 cells (2 × 106/ml of SFM) were incubated with or without 0.1 ng of FGF-2/ml for 4 h before addition of 0.1 μM etoposide for 10 h. Caspase 3 activity was assayed in cell lysates with acetyl-DEVD-p-nitroaniline (Calbiochem) according to the manufacturer's instructions.
Clonogenic assay.
H69 cells in SFM were disaggregated into a single-cell suspension, and 104 viable cells were mixed with either RPMI, with or without 10% FCS, or SFM, with or without 0.1 μM etoposide. Melted agarose was then added to a final concentration of 0.3%, and the suspension was layered over a solid base of 0.5% agarose containing the same factors and/or agents in a 6-well plate. The cultures were incubated at 37°C under 5% CO2 for 21 days and then stained with nitroblue tetrazolium, and colonies were counted as described previously (31).
35S labeling and pulse-chase.
H510 cells (4 × 106 per condition) were washed three times in methionine-free RPMI and incubated for 1 h at 37°C in Met-free SFM containing [35S]Met/Cys Promix (150 μCi of [35S]Met/ml). Cells were then washed three times with 15 ml of SFM (containing Met) and incubated with or without 0.1 ng of FGF-2/ml. For the following 4 h, samples were lysed at 1-h intervals in lysis buffer (at 4°C for 15 min) and stored at −80°C until the last time of collection. Immunoprecipitation of XIAP was performed overnight at 4°C. Immunoprecipitates were resuspended in gel sample buffer and analyzed by SDS-PAGE followed by autoradiography.
mRNA quantification by real-time quantitative PCR (Taqman).
H510 cells (4 × 106 cells per condition) were incubated for 4 h in SFM with or without 0.1 ng of FGF-2/ml. mRNA was extracted by using RNAzol (Biogenesis), and reverse transcription was performed by using the avian myeloblastosis virus 1st-strand reverse transcription-PCR (RT-PCR) kit (Roche). Equal amounts of cDNA were then introduced in a Taqman real-time quantitative PCR. For XIAP, primers with the sequences 5′-GACAGTATGCAAGATGAGTCAAGTCA-3′ and 5′-GCAAAGCTTCTCCTCTTGCAG-3′ were used. For cIAP-1, primers with the sequences 5′-TGTTGTCAACTTCAGATACCACTGG-3′ and 5′-CATCATGACAGCATCTTCTGAAGA-3′ were used. Real-time PCR amplification was performed according to the Taqman Universal PCR Master Mix protocol, and PCR products were labeled with Cybergreen dye. Relative quantification of gene expression was performed as described by the manufacturer by using glyceraldehyde-3-phosphate dehydrogenase mRNA as an internal standard.
Reagents.
Etoposide, actinomycin D-mannitol, and an anti-rat FITC antibody were purchased from Sigma-Aldrich. The FITC-conjugated anti-mouse antibody, the anti-COX antibody, and Mitotracker Red were from Molecular Probes. The inhibitor PD98059 was from New England Biolabs. Antibodies against lamin B and actin were from Santa Cruz Biotechnology. The cIAP-1 antibody was from Transduction Laboratories, and the XIAP and cytochrome c antibodies were from Pharmingen. The livin antibody was from Abcam. The anti-Smac antibody was obtained from Alexis Corporation. The RT-PCR kit was purchased from Roche. Taqman Universal PCR Master Mix was from Perkin-Elmer Applied Biosystems. Promix and ECL reagents were from Amersham-Pharmacia. Human recombinant FGF-2 was from Calbiochem.
RESULTS
FGF-2 blocks Smac but not cytochrome c release from mitochondria of etoposide treated SCLC cells.
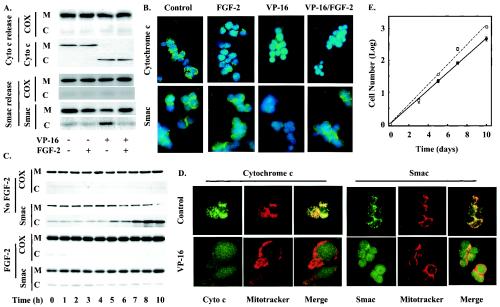
FGF-2 has previously been identified as a survival factor for SCLC cells (30). Preincubation with FGF-2 for 4 h substantially inhibited cell death induced by subsequent exposure of H510 SCLC cells to the chemotherapeutic drug etoposide (VP-16), a type II topoisomerase inhibitor. MEK inhibition abolished this effect, while expression of a constitutively active version of MEK rendered SCLC cells resistant to etoposide. These effects correlated with the inhibition of caspase activation and subsequent proteolytic cleavage of poly(ADP-ribose) polymerase (PARP) (30). Nevertheless, FGF-2 did not prevent cytochrome c release upon etoposide treatment. Indeed, subcellular fractionation experiments demonstrated that FGF-2 failed to prevent the release of cytochrome c from the mitochondria of H510 cells following a 4-h treatment with etoposide (Fig. 1A, upper panel). These results were confirmed by immunofluorescence staining experiments. Figure 1B (upper panel) shows that, while control untreated and FGF-2-treated cells displayed punctate cytochrome c staining, etoposide treatment led to diffuse cytoplasmic cytochrome c staining regardless of the presence or absence of FGF-2. In the absence of etoposide, cytochrome c colocalized with Mitotracker Red staining, consistent with the mitochondrial localization of this protein (Fig. 1D, left).
FIG. 1.
FGF-2 prevents Smac but not cytochrome c release by etoposide. H510 cells, pretreated with or without FGF-2 for 4 h, were incubated in the presence or absence of etoposide (VP-16) for 4 h (A, B, and D) or the times indicated (C). (A and C) Mitochondrial (M) and cytoplasmic (C) fractions were obtained by digitonin fractionation and analyzed by SDS-PAGE and Western blotting for the presence of Smac or cytochrome c (Cyto c). The absence of mitochondrial contamination of the cytoplasmic fraction was confirmed by detection of cytochrome oxidase (COX). (B) Smac and cytochrome c immunodetection, in fixed H510 cells, was visualized using FITC-labeled secondary antibodies (green). Cellular DNA was highlighted using DAPI staining (blue). (D) Cytochrome c and Smac colocalize with the mitochondria in untreated, but not in etoposide-treated, H510 cells. H510 cells were treated with 10 μM Mitotracker Red for 1 h to visualize the mitochondria, while FGF-2 and etoposide treatments were as described for panel A. Samples were then visualized by confocal microscopy. (E) FGF-2-rescued cells are still able to proliferate. Control untreated cells (open circles) and FGF-2-rescued cells (solid circles) were compared for their abilities to proliferate in RPMI containing 10% FCS. Results are representative of three independent experiments performed in quadruplicate. Error bars, standard errors of the means. For panels A through D, results are representative of at least three independent experiments.
Smac release from mitochondria also occurred following treatment of H510 cells with etoposide, although more slowly than cytochrome c release. Indeed, release of Smac was not observed until 5 h after etoposide addition (Fig. 1C, upper panel). As shown in Fig. 1B (lower panel), immunofluorescent detection of Smac in untreated control and FGF-2-only-treated cells showed a punctate staining consistent with mitochondrial localization. This staining became diffuse upon etoposide treatment. However, in contrast to cytochrome c, Smac was prevented from leaving the mitochondria in response to etoposide when cells were pretreated with FGF-2 (Fig. 1B). These immunofluorescence results were confirmed by cell fractionation data, where FGF-2 incubation caused Smac to remain in the mitochondrial subcellular fraction despite etoposide treatment (Fig. 1A, lower panel). This inhibition of Smac release by FGF-2 was sustained and was not due to a shift in time course, persisting for as long as 10 h following exposure of H510 cells to etoposide (Fig. 1C). These data demonstrate that the mechanisms of cytochrome c and Smac release are divergent and differentially regulated.
Etoposide-induced loss of cytochrome c from mitochondria might result in diminished long-term cellular viability. We therefore compared the abilities of FGF-2-rescued, etoposide-treated cells and control untreated cells to proliferate when returned to a serum-containing medium free of the drug. Figure 1E demonstrates that FGF-2-rescued cells had a proliferative potential comparable to that of control untreated cells. Therefore, we conclude that FGF-2 allowed H510 cells to retain viability following cytochrome c release from mitochondria.
The release of Smac from mitochondria is a caspase-dependent phenomenon.
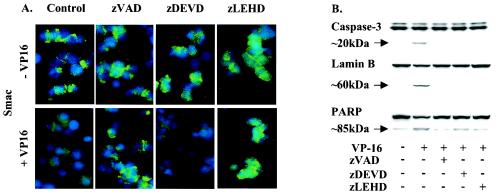
Because the release of Smac was delayed compared to the release of cytochrome c, we hypothesized that cytochrome c-mediated caspase activation may be required to initiate the transfer of Smac to the cytoplasm. We investigated this possibility using pharmacological inhibitors. Figure 2A demonstrates the requirement for caspase activation, as the broad-specificity caspase inhibitor zVAD prevented the release of Smac following the treatment of H510 cells with etoposide. Other peptide inhibitors that have been claimed to be more specific for individual caspases were also assessed. zLEHD, which has preferential activity toward caspase 9 in vitro, was able to block Smac release in response to etoposide, whereas zDEVD, a preferential caspase 3 inhibitor in vitro, was unable to block Smac release despite inhibiting PARP and lamin B cleavage (Fig. 2B). However, cytochrome c release was not affected by any of these caspase inhibitors (data not shown). Collectively, these data indicate that Smac release from mitochondria is dependent on caspase activation following cytochrome c release. It is possible that caspase 9, rather than caspase 3, is the critical activity required for Smac release, although the use of “caspase-specific” inhibitors must be viewed with caution. An alternative possibility is that zDEVD fails to inhibit Smac release, as it is slightly less potent than zVAD and zLEHD against a range of caspases under the conditions of these experiments.
FIG. 2.
Smac release is caspase dependent. H510 cells were treated with or without the caspase inhibitor zVAD (100 μM) (general caspase inhibitor), zDEVD (30 μM) (caspase 3 inhibitor), or zLEHD (20 μM) (caspase 9 inhibitor) and etoposide (VP-16). (A) Immunodetection of Smac was revealed using an FITC-conjugated secondary antibody (green). Nuclear DNA was highlighted by DAPI staining (blue). (B) The corresponding cell lysates were analyzed by SDS-PAGE and Western blotting for inhibition of caspase activity Results are representative of at least three independent experiments.
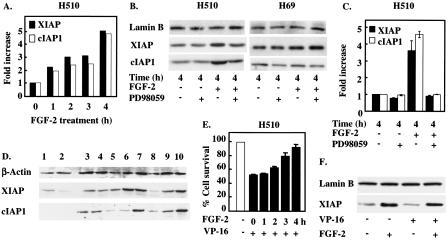
FGF-2 increases the levels of XIAP and cIAP-1 via a MEK-dependent pathway.
These results suggest that FGF-2-mediated inhibition of Smac release could be caused by the ability of this growth factor to inhibit caspase activation. Since the mechanism for FGF-2 inhibition of caspase activation is unclear, we investigated whether exposure of H510 cells to FGF-2 modified the levels of proteins known to impinge on caspase activity. One family of known caspase inhibitors that might be able to account for the observed effects is the IAPs (15). Western blot analysis revealed that two members of the IAP family, XIAP and cIAP-1, were overexpressed in most SCLC cell lines compared to their normal precursors, human type II pneumocytes (Fig. 3D), suggesting the possibility that these proteins may play a role in the aberrant protection of SCLC cells from apoptosis. In H510 SCLC cells, FGF-2 induced a further increase in both XIAP and cIAP-1 expression in a time-dependent fashion, up to fourfold at 4 h following addition of growth factor (Fig. 3A). Intriguingly, these results correlated with the minimum 4-h FGF-2 incubation required prior to challenge with etoposide for a maximal protective effect to be obtained (Fig. 3E). Together, these results indicate a possible involvement of XIAP and cIAP-1 in the prosurvival activity of FGF-2. In this respect, it is noteworthy that etoposide treatment does not down-regulate XIAP levels and that the prosurvival activity of FGF-2 is not therefore limited to counteracting the effects of etoposide treatment (Fig. 3F).
FIG. 3.
XIAP and cIAP-1 are overexpressed in SCLC cells and are up-regulated in response to FGF-2 treatment. (A, B, and C) H510 and H69 cells, incubated with or without 25 μM PD098059, were treated with FGF-2 for the times indicated. Cell lysates were analyzed by SDS-PAGE and Western blotting for XIAP and cIAP-1 expression levels. Lamin B immunodetection was used as a protein loading control. (A and C) Optical densitometry was performed on ECL-revealed bands. (D) Equal protein amounts from type II pneumocytes from two independent donors (lanes 1 and 2) and eight SCLC cell lines (lanes 3 to 10) were analyzed by SDS-PAGE and Western blotting for XIAP, cIAP-1, and actin expression levels. Lane 3, HC33; lane 4, H69; lane 5, H209; lane 6, H510; lane 7, H524; lane 8, H1045; lane 9, H1622; lane 10, H2171. (E) Maximal rescue of H510 cells by FGF-2 requires 4 h of preincubation prior to etoposide treatment. H510 cells were incubated with FGF-2 for the times indicated prior to etoposide (VP-16) addition. Cells were then left to incubate for 4 days, and cell survival was determined by cell counting. (F) H510 cells were treated for 4 h with FGF-2 prior to incubation with or without VP-16 for an additional 6 h. Samples were analyzed by SDS-PAGE and Western blotting for XIAP levels. Results shown are representative of three independent experiments (A through D and F). For panel E, each experiment was performed in quadruplicate.
The increase in XIAP and cIAP-1 protein levels was blocked by PD98059, an inhibitor of MEK (Fig. 3B and C), suggesting that activation of the MEK/ERK pathway is necessary for this FGF-2-induced effect. This notion was supported by the absence of an increase in XIAP and cIAP-1 levels in H69 SCLC cells in response to FGF-2 (Fig. 3B). H69 cells, unlike H510 cells, are unable to activate MEK/ERK signaling and do not resist etoposide killing, although other pathways are intact in response to FGF-2 (30, 31). It is thus possible that XIAP and cIAP-1 may be downstream targets of MEK in FGF-2-mediated survival signaling in SCLC cells.
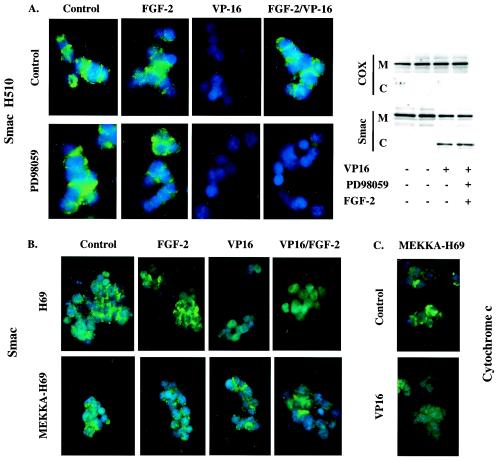
Inhibition of MEK signaling prevents FGF-2 from blocking Smac release.
In view of the MEK-dependent effects of FGF-2 on IAP levels, the possibility that the inhibition of Smac release from mitochondria was dependent on MEK activity was investigated. Both immunofluorescence (Fig. 4A, left) and cell fractionation (Fig. 4A, right) techniques revealed that in cells incubated with PD98059, Smac was released from mitochondria in response to etoposide regardless of the presence or absence of FGF-2. This suggested that MEK activation was central to the control of Smac release. This notion was confirmed by using H69 cells, where FGF-2 fails to activate ERK. In this system FGF-2 was unable to prevent Smac release from mitochondria upon etoposide treatment, but introduction of an activated version of MEK was sufficient to block Smac release in response to etoposide (Fig. 4B). However, release of cytochrome c was not prevented in activated MEK-expressing H69 cells (Fig. 4C), demonstrating that only the release of Smac from mitochondria, and not that of cytochrome c, is under the control of MEK signaling.
FIG. 4.
FGF-2 inhibition of Smac release is dependent on MEK activation. (A) Inhibition of MEK impairs FGF-2 control over Smac release. H510 cells were treated with or without 25 μM PD098059 and/or FGF-2 prior to etoposide (VP-16) addition. (B and C) Expression of a kinase-active MEK (MEKKA) in H69 cells prevents the release of Smac, but not that of cytochrome c, in response to VP-16. Smac or cytochrome c immunodetection was revealed using an FITC-conjugated secondary antibody (green), and nuclear DNA was visualized using DAPI staining (blue) (A [left panel], B, and C). Immunofluorescence results were confirmed by cellular fractionation (A, right panel). Mitochondrial (M) and cytoplasmic (C) fractions were analyzed by SDS-PAGE and Western blotting for the presence of Smac. The absence of mitochondrial contamination of the cytoplasmic fraction was confirmed by detection of cytochrome oxidase (COX). Results shown are representative of at least three independent experiments.
FGF-2-mediated increase in XIAP levels impairs caspase activation following etoposide treatment.
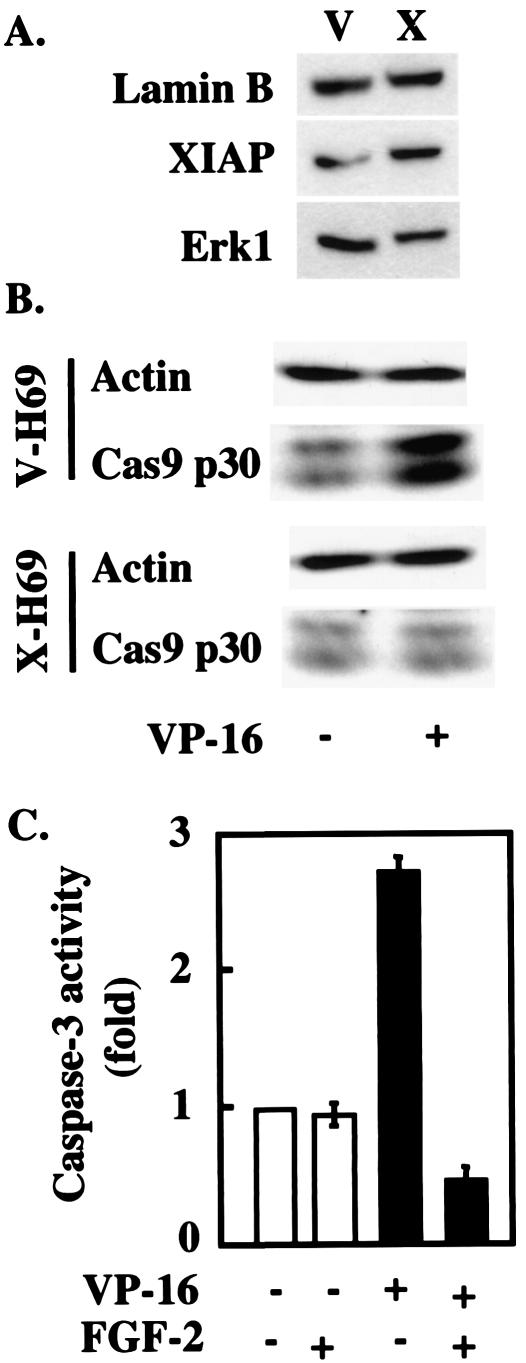
XIAP has previously been shown to inhibit apoptosis by binding to activated caspases and preventing subsequent activation of the apoptotic machinery (6, 37). Incubation of H510 cells with FGF-2 inhibited the ability of etoposide to activate caspase 3 (Fig. 5C), a substrate of caspase 9, suggesting that the FGF-2-driven increase in IAP levels might interfere with the activation of this enzyme. This was confirmed by using H69 cells stably transfected with XIAP (X-H69): as shown in Fig. 5B, these cells overexpress XIAP about three- to fourfold, an amount similar to the elevation achieved by FGF-2 treatment in H510 cells. Compared to control cells, X-H69 failed to activate caspase 9 in response to etoposide, as demonstrated by the absence of processing of procaspase 9 into its active p30 subunit (Fig. 5B). Taken together, these data suggest that the FGF-2-mediated increase in XIAP impairs the activation of the caspase cascade in response to etoposide.
FIG. 5.
XIAP transgene expression inhibits caspase activation in H69 cells. (A) H69 cells were stably transfected with XIAP (X), and the expression levels of XIAP were compared with those in cell transfected with the vector alone (V) by SDS-PAGE and Western blotting. Lamin B and ERK1 immunodetection was used as a protein loading control. (B) XIAP overexpression inhibits the processing of caspase 9 in response to etoposide. X-H69 and V-H69 were exposed to etoposide for 5.5 h and compared for their abilities to process caspase 9 into its active fragments. Actin detection was used as a protein loading control. (C) FGF-2 treatment inhibits etoposide-mediated caspase 3 activation. H510 cells were treated with FGF-2 (for 4 h) and/or etoposide (VP-16) for 8 h. Lysates were analyzed for caspase 3 activity by using a colorimetric substrate-based assay. Each assay condition was performed in triplicate.
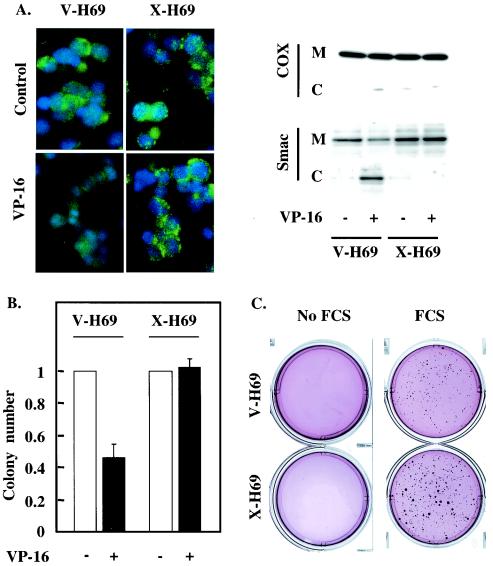
XIAP overexpression inhibits Smac release and increases cell survival.
Smac release from mitochondria requires caspase activity (Fig. 2), while an increase in XIAP levels, in the absence of additional signaling events, is sufficient to block caspase activation in response to etoposide (Fig. 5). We therefore investigated the effect of XIAP overexpression on the ability of etoposide to induce Smac release in H69 cells. As demonstrated by immunofluorescence (Fig. 6A, left) and cell fractionation (Fig. 6A, right) techniques, X-H69 retained mitochondrial localization of Smac in response to drug treatment, in contrast to vector control cells (V-H69) and parental H69 cells (Fig. 4B). Thus, increases in XIAP levels similar to those induced by FGF-2 treatment in H510 cells are able to prevent Smac release from mitochondria following etoposide treatment. Furthermore, inhibition of Smac release correlated with an improvement in the long-term survival of X-H69 relative to V-H69: the abilities of these cells to form colonies in soft agar were compared in the presence or absence of etoposide (Fig. 6B). While colony numbers dropped sharply in V-H69 as a result of etoposide treatment, the ability of X-H69 to form colonies was unaffected by drug treatment. In the presence of 10% FCS, X-H69 displayed both a higher number of colonies and a larger size than V-H69 (Fig. 6C).
FIG. 6.
XIAP expression prevents Smac release and cell death in response to etoposide. (A and B) X-H69 and V-H69 were incubated with or without etoposide. (A) Smac localization was probed by using immunofluorescent staining (left) and cell fractionation (right). (Left) Smac immunoreactivity was revealed using an FITC-conjugated secondary antibody (green). Nuclear DNA was stained using DAPI (blue). (Right) Mitochondrial (M) and cytoplasmic (C) fractions were analyzed by SDS-PAGE and Western blotting for the presence of Smac. The absence of mitochondrial contamination of the cytoplasmic fraction was confirmed by detection of cytochrome oxidase (COX). (B) Cells were subjected to a clonogenic assay, and colony numbers were determined under microscopic observation. Error bars, standard errors of the means. (C) X-H69 and V-H69 were subjected to a clonogenic assay in the presence or absence of 10% FCS. The colonies were stained using nitroblue tetrazolium, and the plates were photographed. Each result is representative of three independent experiments (A through C) performed in triplicate (B and C).
SCLC cells have a moderate background level of apoptotic cell death; thus, the results shown in Fig. 6 may reflect the ability of XIAP to inhibit this background cell death. Indeed, untreated X-H69 showed reduced annexin V staining relative to that of V-H69 (data not shown). XIAP expression and the subsequent control of Smac release may thus be a major element in determining the survival and tumorigenicity of SCLC cells, particularly in the presence of FGF-2.
XIAP induction by FGF-2 occurs through a translational mechanism.
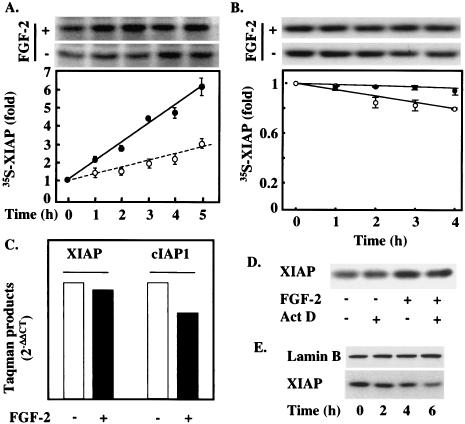
In order to establish how FGF-2 regulates XIAP protein levels, H510 cells were metabolically labeled with 35S-labeled amino acids in the presence or absence of FGF-2. The levels of 35S label incorporated into XIAP increased threefold more rapidly in the presence of FGF-2 than in its absence (Fig. 7A), in line with the results obtained by Western blotting (Fig. 4B). To confirm that this effect was due to an increase in new XIAP protein synthesis rather than to stabilization of existing XIAP by FGF-2, we performed pulse-chase experiments to determine the half-life of XIAP in the presence or absence of FGF-2. The half-life of XIAP far exceeded the time frame in which FGF-2-mediated changes in Smac release could be observed in H510 cells (Fig. 7B). FGF-2 slightly increased the stability of XIAP, with a difference of about 20% in [35S]methionine-labeled XIAP levels between FGF-2-treated and untreated cells at 4 h. However, this small difference in the stability of a long-lived protein would not account for the difference in the rate of 35S-labeled XIAP increase seen in Fig. 7A.
FIG. 7.
XIAP up-regulation occurs via a translational mechanism. (A) H510 cells were 35S labeled in the presence (solid circles) or absence (open circles) of FGF-2 for the times indicated. XIAP was immunoprecipitated from the cell lysates, and the amount of 35S-labeled XIAP was revealed by SDS-PAGE and autoradiography followed by optical densitometry. A representative autoradiogram is shown at the top. (B) H510 cells were pulsed with 35S-labeled amino acids and then chased in the presence or absence of FGF-2 for the times indicated. Samples were then analyzed as described for panel A. A representative autoradiogram is shown at the top. Results in panels A and B (graphs) are averages from at least three independent experiments. Error bars, standard errors of the means. (C) mRNAs extracted from FGF-2-treated and untreated H510 cells were analyzed by quantitative RT-PCR for XIAP and cIAP-1 mRNA levels. (D) H510 cells were treated with or without actinomycin D and FGF-2. Samples were then analyzed by SDS-PAGE and Western blotting for the presence of XIAP. (E) XIAP baseline levels depend on MEK activity. H510 cells were treated with 25 μM PD098059 for the times indicated, and lysates were analyzed by SDS-PAGE and Western blotting for XIAP expression. Results in panels C through E are representative of at least three independent experiments. For panel C, experiments were performed in triplicate.
To test whether transcriptional changes might contribute to alterations in XIAP protein levels, we investigated whether XIAP mRNA levels increased in response to FGF-2 treatment. Total mRNA was extracted from H510 cells treated in the presence or absence of FGF-2. cDNA obtained by RT-PCR was then probed by quantitative real-time PCR (Taqman). These experiments showed that mRNA levels for XIAP were not significantly modified by FGF-2 treatment (Fig. 7C). Similar results were obtained for cIAP-1 (Fig. 7C). Thus, an increase in XIAP transcription could not be responsible for the FGF-2-mediated induction of XIAP. This was further confirmed by the inability of actinomycin D, an inhibitor of RNA synthesis, to block the induction of XIAP in response to FGF-2 (Fig. 7D). The role of MEK in the induction of XIAP is further supported by the observation that PD98059 treatment decreased the basal levels of XIAP in a time-dependent manner in H510 cells (Fig. 7E). We propose that XIAP levels can be regulated through a translational mechanism involving the activation of MEK and that they are closely involved in determining cell fate at a post-cytochrome c level.
IAP function is required for FGF-2-mediated survival.
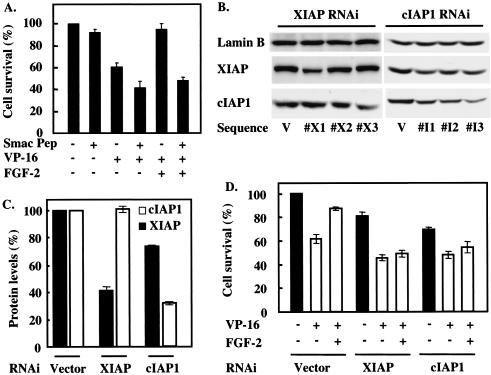
The results described above suggest that FGF-2 inhibits apoptosis in SCLC cells by causing up-regulation of IAP expression. This would block efficient caspase activation following cytochrome c release from mitochondria, resulting in failure to induce the release into the cytosol of Smac, and possibly that of other proteins with similar functions. Consequently, IAP activity would not be antagonized by Smac and would remain sufficient to suppress the activation of effector caspases. To test whether neutralizing the IAPs would block the protection afforded by FGF-2 from etoposide-induced apoptosis, we introduced a cell-permeant TAT peptide coupled by a disulfide bond to the amino-terminal region of Smac into H510 cells. This peptide efficiently enters cells and binds to several IAPs, including XIAP (data not shown). While incubation with the Smac peptide alone did not show significant toxicity on H510 cells, it slightly potentiated etoposide-induced cell death (Fig. 8A). However, pretreatment of H510 cells with this peptide very effectively prevented FGF-2-mediated rescue from etoposide-induced killing. A control TAT peptide had no effect on etoposide-induced cell death or protection by FGF-2 (data not shown). These results suggest that increased IAP levels and inhibition of Smac release from mitochondria are central to the antiapoptotic activity of FGF-2, as the growth factor is unable to rescue H510 cells from death once IAP-neutralizing Smac activity is present in the cytoplasm of these cells.
FIG. 8.
Down-regulation of IAPs blocks FGF-2 protective effects. (A) H510 cells were pretreated with 75 μM Smac peptide for 4 h prior to incubation with FGF-2 and/or etoposide. (B) H510 cells stably expressing one of three different RNAi sequences against either XIAP (X1 to X3) or cIAP-1 (I1 to I3) were compared with cells expressing vector alone (V) for their levels of XIAP and cIAP-1 by using SDS-PAGE and Western blotting. Lamin B was used as a control for protein loading. (C) H510 cells stably expressing either the RNAi sequence X1 for XIAP or the RNAi sequence I3 for cIAP-1 (see the legend to panel B) were analyzed by SDS-PAGE and Western blotting for their XIAP and cIAP-1 levels. The results obtained in three independent experiments were analyzed by optical densitometry and averaged, and protein levels are expressed as percentages of the levels found in vector-alone-infected cells. (D) H510 cells stably expressing RNAi for XIAP or cIAP-1 were treated with or without FGF-2 and etoposide, and their biological response was compared with that of vector-alone infected cells. (A and D) Cell death was determined 4 days later by cell counting. Each experimental condition was performed in quadruplicate. Results are representative of three independent experiments. (A, C, and D) Error bars, standard errors of the means.
In addition to neutralizing all IAPs with the Smac peptide, we also sought to impair expression of individual IAPs by using an RNAi approach. H510 cells were infected with retroviral constructs encoding short RNA hairpins that should be processed to target the mRNAs for XIAP and cIAP1. Three different sequences were tested against each protein (Fig. 8B), and constructs effective in reducing the expression levels of their target proteins by about 60% were selected (Fig. 8C). In the absence of etoposide, cells infected with RNAi constructs for XIAP and cIAP-1 showed a slight decrease in viability relative to that of cells infected with vector alone (Fig. 8D). This difference was more marked in the presence of etoposide, with a further increase of approximately 20% in cell death in RNAi-expressing cells. Crucially, the decrease in either XIAP or cIAP-1 expression levels by RNAi effectively prevented FGF-2 from rescuing etoposide-treated cells (Fig. 8D). Taken together, these data establish modulation of the level of expression of XIAP and cIAP-1 as indispensable to FGF-2 prosurvival activity.
DISCUSSION
The development of resistance to chemotherapy is one of the main factors preventing the cure of cancer patients (5, 22). Indeed, in the case of SCLC, while initial treatment often proves effective, the tumor generally reappears in a form resistant to chemotherapy, leading to a 5-year survival rate of less than 5%. Several mechanisms have been proposed to explain the onset of chemoresistance, including growth factor signaling (11, 24, 36). FGF-2 has previously been shown to induce broad-spectrum drug resistance in various tumor types (27, 36). It has been demonstrated that this growth factor rescues SCLC cells from etoposide-induced cell death via a MEK-dependent mechanism, resulting in failure to activate the apoptosis effector protease caspase-3 (30). This is of particular significance, because etoposide is one of the main agents used in the treatment of SCLC (28).
Cytochrome c release from mitochondria is one of the principal triggers of caspase activation in response to chemotherapeutic agents (35). The ability of Bcl-2 family members to regulate this phenomenon constitutes a prime mechanism of control over caspases (21, 46). It has previously been shown that FGF-2 stimulation of H510 SCLC cells led to an increase in the levels of two antiapoptotic members of the Bcl-2 family, Bcl-2 and Bcl-XL, and also prevented induction of the proapoptotic member Bad, following etoposide treatment (30). This suggests that the ability of FGF-2 to prevent caspase activation might be linked to inhibition of cytochrome c release from mitochondria. However, the data presented here demonstrate that etoposide-induced cytochrome c release still occurs regardless of FGF-2 treatment and inhibition of cell death. This suggests that etoposide-induced apoptosis can be inhibited at a postmitochondrial level downstream of cytochrome c release and that an apoptosis regulator other than Bcl-2 family proteins may be critical in modulating cell death in response to FGF-2.
Recently, a number of proteins in addition to cytochrome c have been shown to translocate from the mitochondria to the cytosol following an apoptotic insult to the cell (41). Two of these, AIF and endonuclease G, are involved in degradation of DNA, while two others, Smac/DIABLO and HtrA2/Omi, activate caspases by displacing the IAPs, which normally keep these proteases in an inactive state (9, 16). Translocation of both cytochrome c and Smac from the mitochondria to the cytosol has been found to be blocked by overexpression of antiapoptotic Bcl-2 family members or by loss of proapoptotic Bcl-2 family members such as Bax and Bak (2, 14, 38, 41). It has been reported previously that release of Smac from the mitochondria of stressed cells can be dependent on caspase activity under conditions where release of cytochrome c is not (2). A model has been proposed in which apoptotic stimuli lead to Bax-regulated release of cytochrome c, resulting in an initial increase in caspase activity. This in turn promotes Smac and HtrA2 release by an unknown mechanism that presumably differs from that of cytochrome c release. In the cytosol, Smac amplifies caspase activation by binding IAPs via its amino-terminal Reaper motif, leading to displacement of IAPs from caspases.
In this model, the levels of IAPs expressed in cells may be critical in the control of the amplification of caspase activation. Elevated expression of IAPs could suppress activation of caspases following cytochrome c release. This could lead to failure to promote the mitochondrial release of Smac and therefore to an absence of its restraint on the activity of the IAPs. Consequently, an increase in IAP levels could protect cells from apoptosis at multiple levels, including the release of Smac and similar molecules from mitochondria, and would be expected to increase the resistance of SCLC cells to apoptosis induced by chemotherapy. Since mice in which the Smac gene is deleted do not show obvious defects in the regulation of apoptosis (29), it is possible that other molecules with IAP-neutralizing activity, such as HtrA2, may also play an important role in the amplification of caspase activation following cytochrome c release.
Treatment of the SCLC cell line H510 with FGF-2 leads to increased levels of XIAP and cIAP-1 proteins (Fig. 3 and 7), two members of the IAP family previously shown to be potent caspase inhibitors. Intriguingly, this increase in protein levels is achieved principally through enhanced translation, with no changes seen in transcription and only minor changes in protein stability (Fig. 7). These effects were dependent on the activation of the MEK pathway and could not be observed in another SCLC cell line, H69, where, unusually, MEK is not activated following FGF-2 stimulation (30, 31). The increase in IAP levels induced following FGF-2 treatment of H510 cells was relatively modest, around fourfold for both XIAP and cIAP-1 after 4 h with growth factor. In addition, similar levels of increase in the expression of the IAP livin were also seen, but rather more rapidly (data not shown). Are these changes sufficient to generate the level of protection from apoptosis seen in these cells following FGF-2 treatment? Overexpression of XIAP to a similar level in H69 cells, which cannot be rescued by FGF-2, or expression of a constitutively activated form of MEK mimicked the protective effect obtained with FGF-2 in H510 cells (Fig. 4, 5, and 6). Furthermore, these XIAP- or activated-MEK-overexpressing H69 cells showed loss of etoposide-induced Smac release, but not of cytochrome c release, from mitochondria similar to that seen in FGF-2-treated H510 cells. It appears, therefore, that the levels of increase in IAPs seen with FGF-2 in H510 cells are sufficient to impair the feed-forward activation of caspases mediated by Smac release from mitochondria and thus to protect cells from apoptosis.
These changes in IAP levels are thus sufficient for the protective effect of FGF-2, but are they also necessary? Two approaches would suggest that this is the case (Fig. 8). In one, IAP family function was negated across the board by the introduction into the cells of an amino-terminal Reaper motif peptide based on the sequence of Smac. Similar peptides have been reported by others recently (3, 18). This leads to removal of the protective effect of FGF-2 in etoposide-treated cells without major induction of cell death in the absence of drug. The other approach, targeting expression of XIAP and cIAP-1 specifically by using RNAi retroviral vectors, gave comparable results.
Many growth factors mediate cell survival signaling through activation of the phosphatidylinositol 3-kinase and Akt/PKB pathways. This impacts on the apoptotic machinery primarily at the level of Bax regulation of the mitochondria, preventing cytochrome c release into the cytosol (7, 23). The Raf/ERK pathway has also been found to signal cell survival under various circumstances. In some cases this is due to activation of Akt via an autocrine pathway (33), while in others it has been suggested that Raf/ERK signaling impacts downstream of the mitochondria (17, 40) by an unknown mechanism. From the work reported here, it is likely that the postmitochondrial protective effect of the Raf/ERK pathway in SCLC cells is due to increased translation and protein levels of IAPs and the subsequent blockade of amplification of caspase activation by Smac and HtrA2 release from mitochondria. Since IAPs have been reported to promote the proteasomal degradation of Smac, small amounts of Smac released into the cytosol of cells with high levels of IAPs may be degraded rapidly, providing another means by which IAPs can neutralize Smac in addition to inhibiting its release from mitochondria (20, 25).
Altogether, these results suggest that the translational control of IAP levels downstream of growth factor-induced MEK/ERK signaling might be of central importance in the acquisition of chemoresistance in SCLC. Indeed, we found that XIAP, cIAP-1, and livin are naturally overexpressed in SCLC cells relative to their expression in normal human type II pneumocytes (Fig. 3 and data not shown), one of the cell types considered to be a common origin of lung cancers ((31). Lung tissues have been shown to be a major site of FGF-2 expression (12), possibly making FGF-2 signaling of particular relevance to the tumor type studied here. Mechanisms for targeting IAPs, such as molecules mimicking the amino-terminal Reaper motif of Smac, may hold promise as novel therapeutic approaches to the treatment of traditional-chemotherapeutic-drug-resistant SCLC and to that of other malignancies characterized by high IAP expression.
Acknowledgments
This work has been supported by Cancer Research UK.
REFERENCES
- 1.Acehan, D., X. Jiang, D. G. Morgan, J. E. Heuser, X. Wang, and C. W. Akey. 2002. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9:423-432. [DOI] [PubMed] [Google Scholar]
- 2.Adrain, C., E. M. Creagh, and S. J. Martin. 2001. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 20:6627-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnt, C. R., M. V. Chiorean, M. P. Heldebrant, G. J. Gores, and S. H. Kaufmann. 2002. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAPI in situ. J. Biol. Chem. 277:44236-44243. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, M. R., and J. J. Boyle. 1998. Apoptosis of vascular smooth muscle cells in atherosclerosis. Atherosclerosis 138:3-9. [DOI] [PubMed] [Google Scholar]
- 5.Borg, A. G., R. Burgess, L. M. Green, R. J. Scheper, and J. A. Yin. 1998. Overexpression of lung-resistance protein and increased P-glycoprotein function in acute myeloid leukaemia cells predict a poor response to chemotherapy and reduced patient survival. Br. J. Haematol. 103:1083-1091. [DOI] [PubMed] [Google Scholar]
- 6.Bratton, S. B., G. Walker, S. M. Srinivasula, X. M. Sun, M. Butterworth, E. S. Alnemri, and G. M. Cohen. 2001. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 20:998-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 8.Brill, A., A. Torchinsky, H. Carp, and V. Toder. 1999. The role of apoptosis in normal and abnormal embryonic development. J. Assist. Reprod. Genet. 16:512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai, J., C. Du, J. W. Wu, S. Kyin, X. Wang, and Y. Shi. 2000. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406:855-862. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan, D., T. Hideshima, S. Rosen, J. C. Reed, S. Kharbanda, and K. C. Anderson. 2001. Apaf-1/cytochrome c-independent and Smac-dependent induction of apoptosis in multiple myeloma (MM) cells. J. Biol. Chem. 276:24453-24456. [DOI] [PubMed] [Google Scholar]
- 11.Cole, S. P., and R. G. Deeley. 1996. Multidrug resistance associated with overexpression of MRP. Cancer Treat. Res. 87:39-62. [DOI] [PubMed] [Google Scholar]
- 12.Cordon-Cardo, C., I. Vlodavsky, A. Haimovitz-Friedman, D. Hicklin, and Z. Fuks. 1990. Expression of basic fibroblast growth factor in normal human tissues. Lab. Investig. 63:832-840. [PubMed] [Google Scholar]
- 13.DeLong, M. J. 1998. Apoptosis: a modulator of cellular homeostasis and disease states. Ann. N. Y. Acad. Sci. 842:82-90. [DOI] [PubMed] [Google Scholar]
- 14.Deng, Y., Y. Lin, and X. Wu. 2002. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deveraux, Q. L., and J. C. Reed. 1999. IAP family proteins—suppressors of apoptosis. Genes Dev. 13:239-252. [DOI] [PubMed] [Google Scholar]
- 16.Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33-42. [DOI] [PubMed] [Google Scholar]
- 17.Erhardt, P., E. J. Schremser, and G. M. Cooper. 1999. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 19:5308-5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulda, S., W. Wick, M. Weller, and K. M. Debatin. 2002. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat. Med. 8:808-815. [DOI] [PubMed] [Google Scholar]
- 19.Hegde, R., S. M. Srinivasula, Z. Zhang, R. Wassell, R. Mukattash, L. Cilenti, G. DuBois, Y. Lazebnik, A. S. Zervos, T. Fernandes-Alnemri, and E. S. Alnemri. 2002. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 277:432-438. [DOI] [PubMed] [Google Scholar]
- 20.Hu, S., and X. Yang. 2003. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J. Biol. Chem. 278:10055-10060. [DOI] [PubMed] [Google Scholar]
- 21.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 22.Laupeze, B., L. Amiot, B. Drenou, M. Bernard, B. Branger, J. M. Grosset, T. Lamy, R. Fauchet, and O. Fardel. 2002. High multidrug resistance protein activity in acute myeloid leukaemias is associated with poor response to chemotherapy and reduced patient survival. Br. J. Haematol. 116:834-838. [DOI] [PubMed] [Google Scholar]
- 23.Lawlor, M. A., and D. R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903-2910. [DOI] [PubMed] [Google Scholar]
- 24.Liu, J. R., A. W. Opipari, L. Tan, Y. Jiang, Y. Zhang, H. Tang, and G. Nunez. 2002. Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res. 62:924-931. [PubMed] [Google Scholar]
- 25.MacFarlane, M., W. Merrison, S. B. Bratton, and G. M. Cohen. 2002. Proteasome-mediated degradation of Smac during apoptosis: XIAP promotes Smac ubiquitination in vitro. J. Biol. Chem. 277:36611-36616. [DOI] [PubMed] [Google Scholar]
- 26.Martins, L. M., I. Iaccarino, T. Tenev, S. Gschmeissner, N. F. Totty, N. R. Lemoine, J. Savopoulos, C. W. Gray, C. L. Creasy, C. Dingwall, and J. Downward. 2002. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J. Biol. Chem. 277:439-444. [DOI] [PubMed] [Google Scholar]
- 27.Miyake, H., I. Hara, K. Gohji, K. Yoshimura, S. Arakawa, and S. Kamidono. 1998. Expression of basic fibroblast growth factor is associated with resistance to cisplatin in a human bladder cancer cell line. Cancer Lett. 123:121-126. [DOI] [PubMed] [Google Scholar]
- 28.Noda, K., Y. Nishiwaki, M. Kawahara, S. Negoro, T. Sugiura, A. Yokoyama, M. Fukuoka, K. Mori, K. Watanabe, T. Tamura, S. Yamamoto, and N. Saijo. 2002. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N. Engl. J. Med. 346:85-91. [DOI] [PubMed] [Google Scholar]
- 29.Okada, H., W. K. Suh, J. Jin, M. Woo, C. Du, A. Elia, G. S. Duncan, A. Wakeham, A. Itie, S. W. Lowe, X. Wang, and T. W. Mak. 2002. Generation and characterization of Smac/DIABLO-deficient mice. Mol. Cell. Biol. 22:3509-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardo, O. E., A. Arcaro, G. Salerno, S. Raguz, J. Downward, and M. J. Seckl. 2002. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway: correlation with resistance to etoposide-induced apoptosis. J. Biol. Chem. 277:12040-12046. [DOI] [PubMed] [Google Scholar]
- 31.Pardo, O. E., A. Arcaro, G. Salerno, T. D. Tetley, T. Valovka, I. Gout, and M. J. Seckl. 2001. Novel cross talk between MEK and S6K2 in FGF-2 induced proliferation of SCLC cells. Oncogene 20:7658-7667. [DOI] [PubMed] [Google Scholar]
- 32.Salvesen, G. S., and M. Renatus. 2002. Apoptosome: the seven-spoked death machine. Dev. Cell 2:256-257. [DOI] [PubMed] [Google Scholar]
- 33.Schulze, A., K. Lehmann, H. B. Jefferies, M. McMahon, and J. Downward. 2001. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 15:981-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi, Y. 2002. Apoptosome: the cellular engine for the activation of caspase-9. Structure (Cambridge) 10:285-288. [DOI] [PubMed] [Google Scholar]
- 35.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song, S., M. G. Wientjes, Y. Gan, and J. L. Au. 2000. Fibroblast growth factors: an epigenetic mechanism of broad spectrum resistance to anticancer drugs. Proc. Natl. Acad. Sci. USA 97:8658-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasula, S. M., R. Hegde, A. Saleh, P. Datta, E. Shiozaki, J. Chai, R. A. Lee, P. D. Robbins, T. Fernandes-Alnemri, Y. Shi, and E. S. Alnemri. 2001. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410:112-116. [DOI] [PubMed] [Google Scholar]
- 38.Sun, X. M., S. B. Bratton, M. Butterworth, M. MacFarlane, and G. M. Cohen. 2002. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J. Biol. Chem. 277:11345-11351. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki, Y., Y. Imai, H. Nakayama, K. Takahashi, K. Takio, and R. Takahashi. 2001. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8:613-621. [DOI] [PubMed] [Google Scholar]
- 40.Tashker, J. S., M. Olson, and S. Kornbluth. 2002. Post-cytochrome c protection from apoptosis conferred by a MAPK pathway in Xenopus egg extracts. Mol. Biol. Cell 13:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Loo, G., X. Saelens, M. van Gurp, M. MacFarlane, S. J. Martin, and P. Vandenabeele. 2002. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 9:1031-1042. [DOI] [PubMed] [Google Scholar]
- 42.Verhagen, A. M., P. G. Ekert, M. Pakusch, J. Silke, L. M. Connolly, G. E. Reid, R. L. Moritz, R. J. Simpson, and D. L. Vaux. 2000. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43-53. [DOI] [PubMed] [Google Scholar]
- 43.Verhagen, A. M., and D. L. Vaux. 2002. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis 7:163-166. [DOI] [PubMed] [Google Scholar]
- 44.Villa, P., S. H. Kaufmann, and W. C. Earnshaw. 1997. Caspases and caspase inhibitors. Trends Biochem. Sci. 22:388-393. [DOI] [PubMed] [Google Scholar]
- 45.Witherden, I., and T. Tetley. 2001. Isolation and culture of human alveolar type II pneumocytes. Methods Mol. Med. 56:137-146. [DOI] [PubMed] [Google Scholar]
- 46.Yang, J., X. Liu, K. Bhalla, C. N. Kim, A. M. Ibrado, J. Cai, T. I. Peng, D. P. Jones, and X. Wang. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129-1132. [DOI] [PubMed] [Google Scholar]