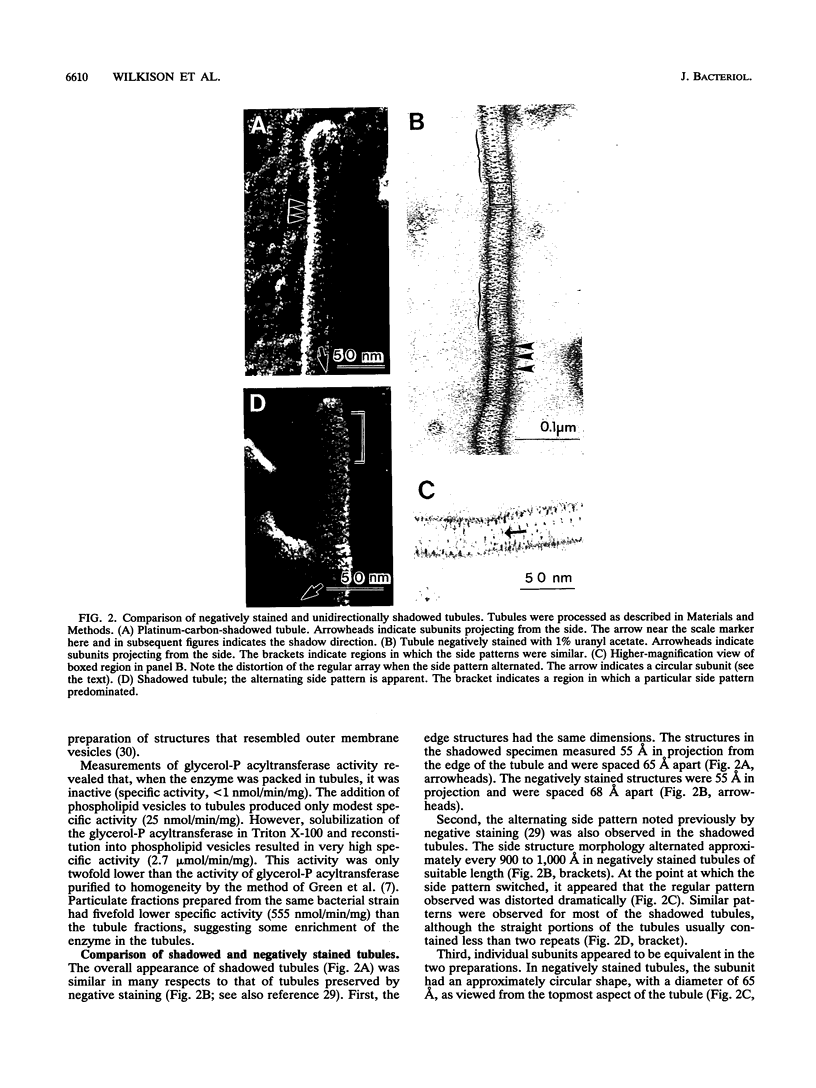
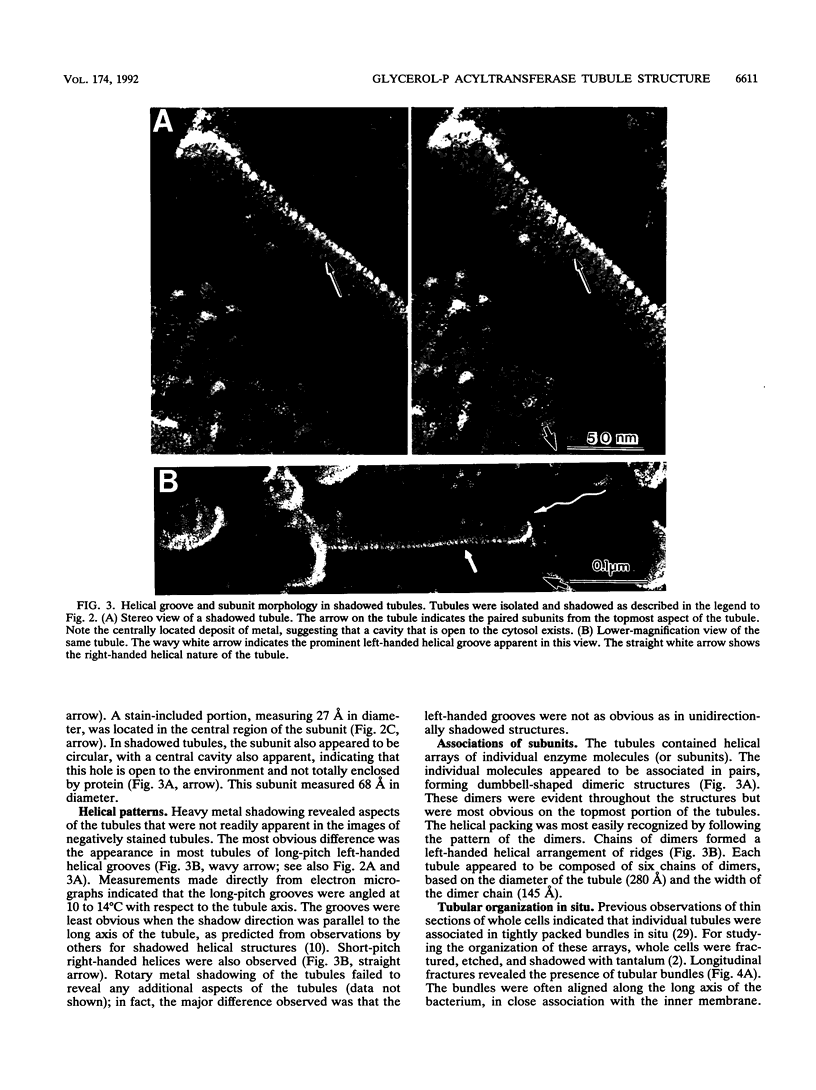
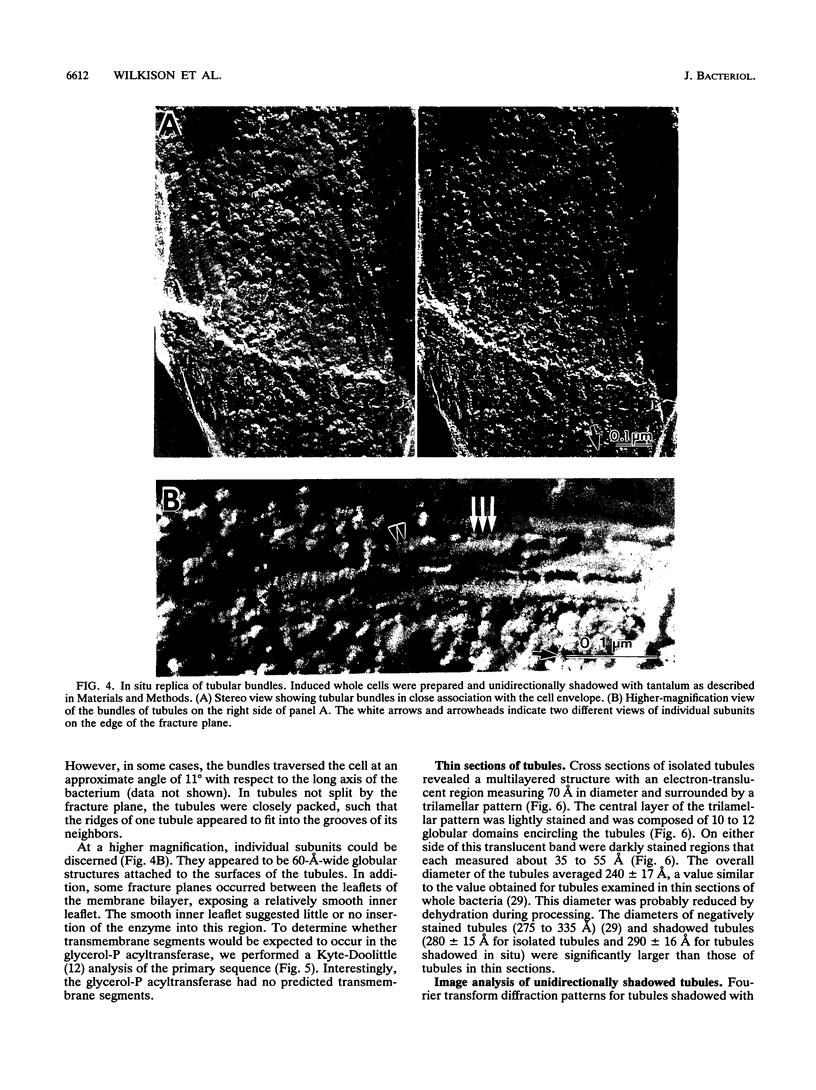
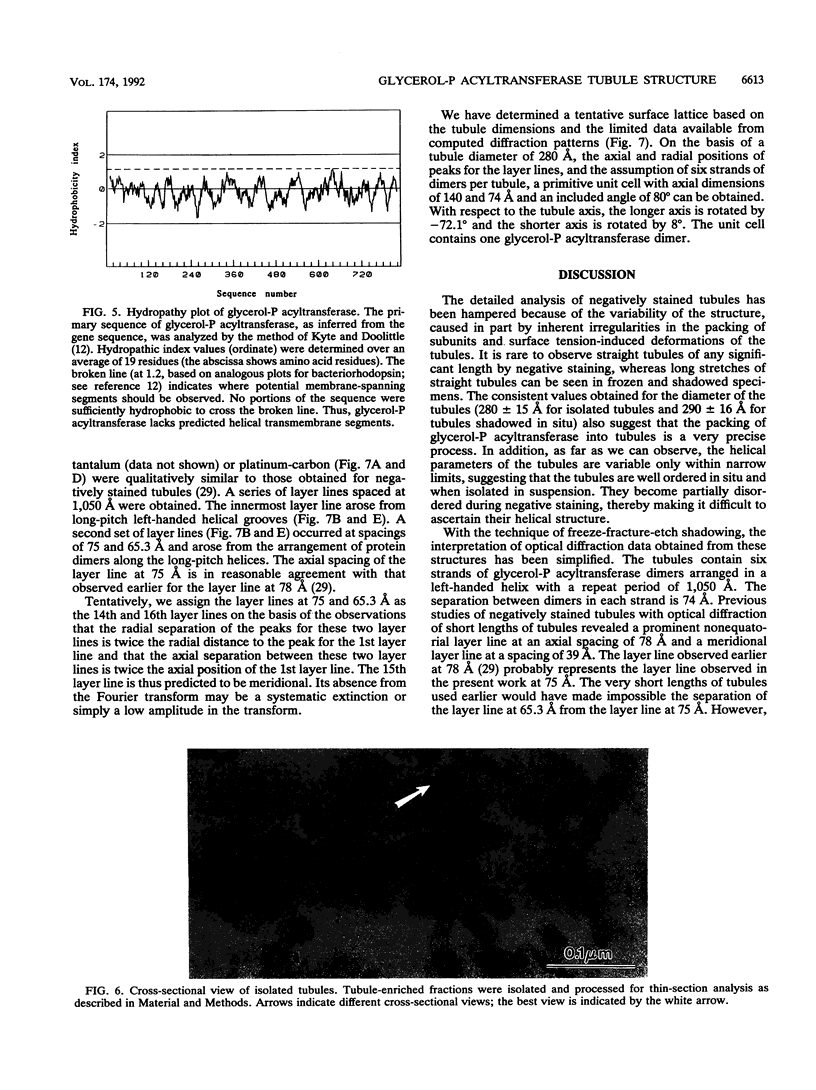
Abstract
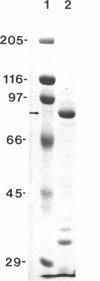
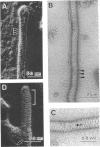
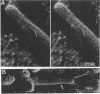
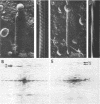
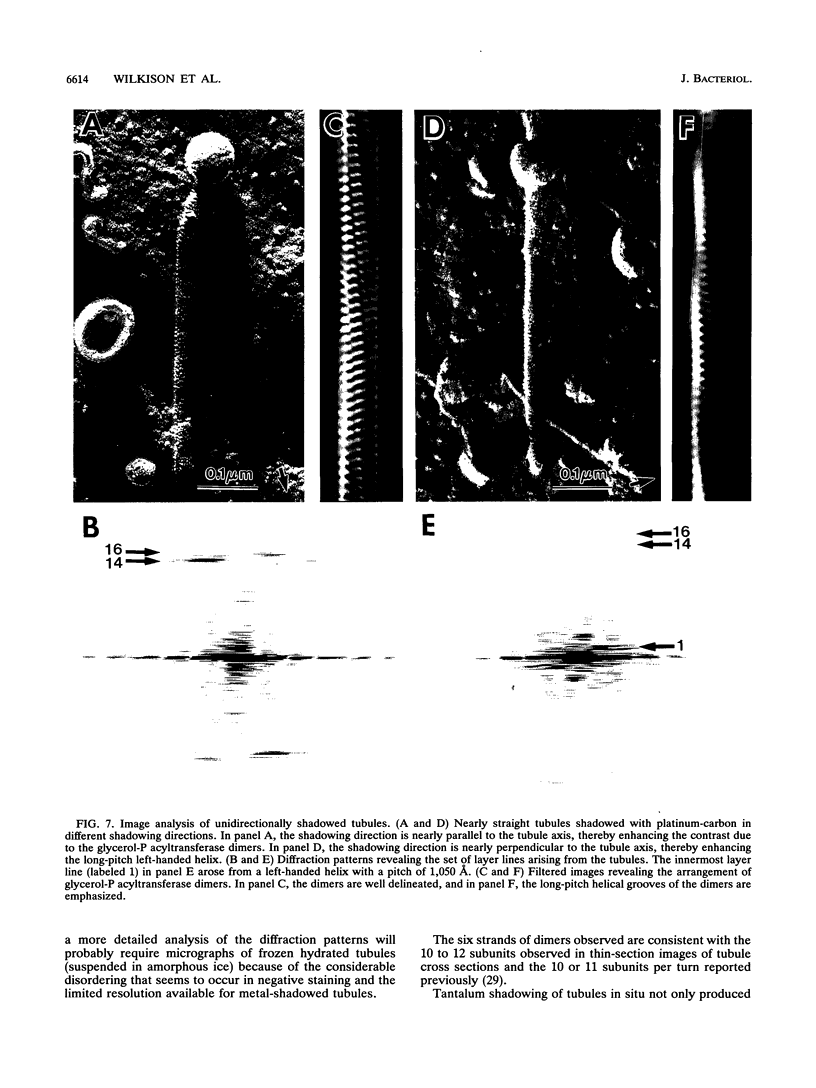
Overproduction of the sn-glycerol-3-phosphate acyltransferase in Escherichia coli leads to incorporation of this integral membrane protein into ordered tubular arrays within the cell. Freeze-fracture-etch shadowing was performed on suspensions of partially purified tubules and whole bacteria. This procedure revealed the presence of ridges and grooves defining a set of long-pitch left-handed helical ridges. The long-pitch helices represented chains of acyltransferase dimers. Tubules observed within the cell were often closely packed, with an apparent alignment of grooves and ridges in adjacent tubules. Fracture planes passing through the tubules indicated the presence of a bilayer structure, with some portion of the enzyme being associated with the membrane. The major portion of the enzyme extended from the hydrophilic surface, forming a large globular structure that, in favorable views, displayed a central cavity facing the cytoplasm. Computer analysis of shadowed tubules revealed that the left-handed helices were six stranded, with a pitch of 1,050 A (105.0 nm) and a spacing of 75 A (7.5 nm) between acyltransferase dimers along the chains. Analysis of the predicted secondary structure failed to reveal obvious transmembrane segments, suggesting that very little of the protein was inserted into the bilayer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costello M. J., Escaig J. Preparation of thin, fine-grained, tantalum metal replicas for freeze-fracture electron microscopy. Scanning Microsc Suppl. 1989;3:189–200. [PubMed] [Google Scholar]
- Costello M. J., Fetter R. D. Freeze-fracture methods: preparation of complementary replicas for evaluating intracellular ice damage in ultrarapidly cooled specimens. Methods Enzymol. 1986;127:704–718. doi: 10.1016/0076-6879(86)27055-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., Kusy A. R., DeLong S. S. Structural aberrations in T-even bacteriophage. II. Characterization of the proteins contained in aberrant heads. Virology. 1971 May;44(2):425–442. doi: 10.1016/0042-6822(71)90273-x. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Green P. R., Bell R. M. Asymmetric reconstitution of homogeneous Escherichia coli sn-glycerol-3-phosphate acyltransferase into phospholipid vesicles. J Biol Chem. 1984 Dec 10;259(23):14688–14694. [PubMed] [Google Scholar]
- Green P. R., Merrill A. H., Jr, Bell R. M. Membrane phospholipid synthesis in Escherichia coli. Purification, reconstitution, and characterization of sn-glycerol-3-phosphate acyltransferase. J Biol Chem. 1981 Nov 10;256(21):11151–11159. [PubMed] [Google Scholar]
- Heuser J. Preparing biological samples for stereomicroscopy by the quick-freeze, deep-etch, rotary-replication technique. Methods Cell Biol. 1981;22:97–122. doi: 10.1016/s0091-679x(08)61872-5. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. Simple methods for "staining with lead" at high pH in electron microscopy. J Biophys Biochem Cytol. 1961 Dec;11:729–732. doi: 10.1083/jcb.11.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Levine R. J. Determination of the handedness of the crossbridge helix of Limulus thick filaments. J Muscle Res Cell Motil. 1982 Sep;3(3):349–361. doi: 10.1007/BF00713042. [DOI] [PubMed] [Google Scholar]
- Kessels J. M., Van den Bosch H. Characterization of reconstituted partially purified glycerophosphate acyltansferase from Escherichia coli. Biochim Biophys Acta. 1982 Dec 13;713(3):570–580. doi: 10.1016/0005-2760(82)90317-4. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Beguin F., Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970 Jan 14;47(1):69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Lightner V. A., Green P. R., Modrich P., Bell R. M. Membrane phospholipid synthesis in Escherichia coli. Identification of the sn-glycerol-3-phosphate acyltransferase polypeptide as the plsB gene product. J Biol Chem. 1980 Oct 10;255(19):9421–9426. [PubMed] [Google Scholar]
- Lemire B. D., Robinson J. J., Bradley R. D., Scraba D. G., Weiner J. H. Structure of fumarate reductase on the cytoplasmic membrane of Escherichia coli. J Bacteriol. 1983 Jul;155(1):391–397. doi: 10.1128/jb.155.1.391-397.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner V. A., Bell R. M., Modrich P. The DNA sequences encoding plsB and dgk loci of Escherichia coli. J Biol Chem. 1983 Sep 25;258(18):10856–10861. [PubMed] [Google Scholar]
- Lightner V. A., Larson T. J., Tailleur P., Kantor G. D., Raetz C. R., Bell R. M., Modrich P. Membrane phospholipid synthesis in Escherichia coli. Cloning of a structural gene (plsB) of the sn-glycerol-3-phosphate acyl/transferase. J Biol Chem. 1980 Oct 10;255(19):9413–9420. [PubMed] [Google Scholar]
- Liscum L., Finer-Moore J., Stroud R. M., Luskey K. L., Brown M. S., Goldstein J. L. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J Biol Chem. 1985 Jan 10;260(1):522–530. [PubMed] [Google Scholar]
- Lopez J., Webster R. E. Morphogenesis of filamentous bacteriophage f1: orientation of extrusion and production of polyphage. Virology. 1983 May;127(1):177–193. doi: 10.1016/0042-6822(83)90382-3. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of phage lambda. V. Form-determining function of the genes required for the assembly of the head. Virology. 1972 Jun;48(3):824–835. doi: 10.1016/0042-6822(72)90163-8. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Ho M. H., Martonosi A. Image analysis of the Ca2+-ATPase from sarcoplasmic reticulum. Ann N Y Acad Sci. 1986;483:31–43. doi: 10.1111/j.1749-6632.1986.tb34493.x. [DOI] [PubMed] [Google Scholar]
- Unwin N., Toyoshima C., Kubalek E. Arrangement of the acetylcholine receptor subunits in the resting and desensitized states, determined by cryoelectron microscopy of crystallized Torpedo postsynaptic membranes. J Cell Biol. 1988 Sep;107(3):1123–1138. doi: 10.1083/jcb.107.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkison W. O., Bell R. M. sn-glycerol-3-phosphate acyltransferase tubule formation is dependent upon heat shock proteins (htpR). J Biol Chem. 1988 Oct 5;263(28):14505–14510. [PubMed] [Google Scholar]
- Wilkison W. O., Walsh J. P., Corless J. M., Bell R. M. Crystalline arrays of the Escherichia coli sn-glycerol-3-phosphate acyltransferase, an integral membrane protein. J Biol Chem. 1986 Jul 25;261(21):9951–9958. [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]
- Zampighi G., Simon S. A., Kyte J., Kreman M. One-dimensional crystals of (Na+ + K+)-ATPase dimers. Biochim Biophys Acta. 1986 Jan 16;854(1):45–57. doi: 10.1016/0005-2736(86)90063-5. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]