Abstract
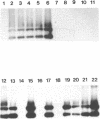
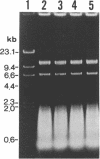
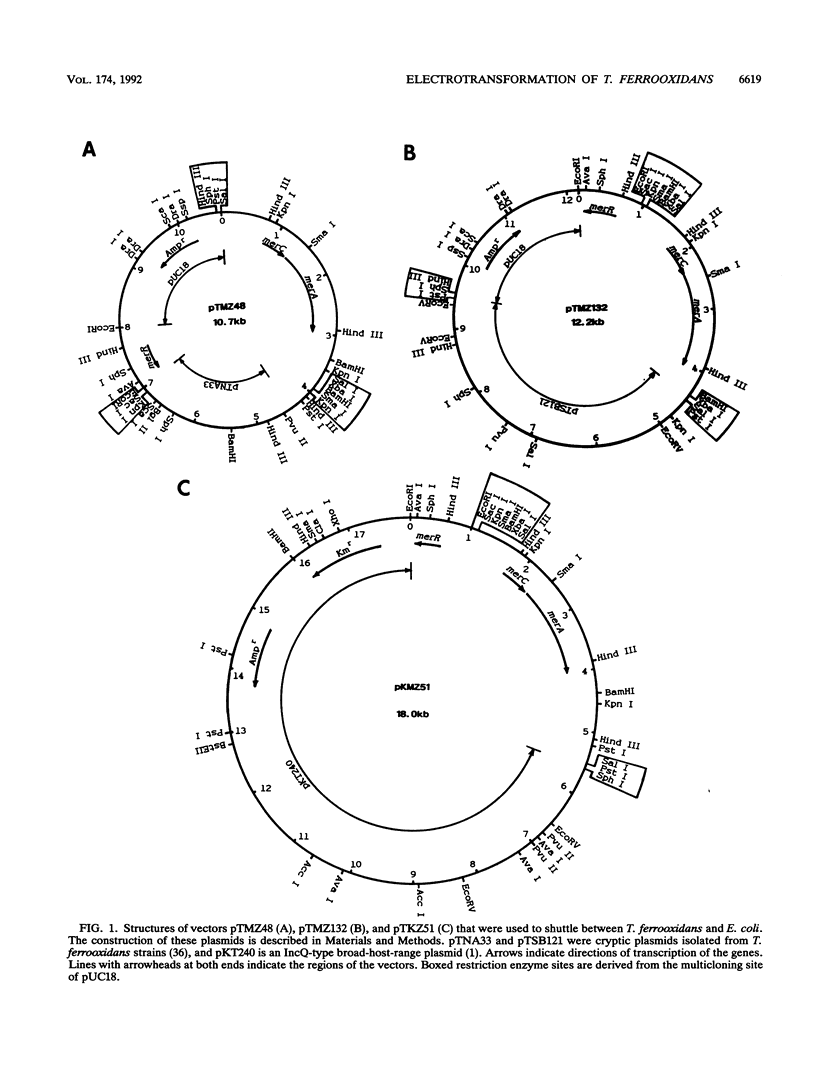
The mer operon from a strain of Thiobacillus ferrooxidans (C. Inoue, K. Sugawara, and T. Kusano, Mol. Microbiol. 5:2707-2718, 1991) consists of the regulatory gene merR and an operator-promoter region followed by merC and merA structural genes and differs from other known gram-negative mer operons. We have constructed four potential shuttle plasmids composed of a T. ferrooxidans-borne cryptic plasmid, a pUC18 plasmid, and the above-mentioned mer determinant as a selectable marker. Mercury ion-sensitive T. ferrooxidans strains were electroporated with constructed plasmids, and one strain, Y4-3 (of 30 independent strains tested), was found to have a transformation efficiency of 120 to 200 mercury-resistant colonies per microgram of plasmid DNA. This recipient strain was confirmed to be T. ferrooxidans by physiological, morphological, and chemotaxonomical data. The transformants carried a plasmid with no physical rearrangements through 25 passages under no selective pressure. Cell extracts showed mercury ion-dependent NADPH oxidation activity.
Full text
PDF


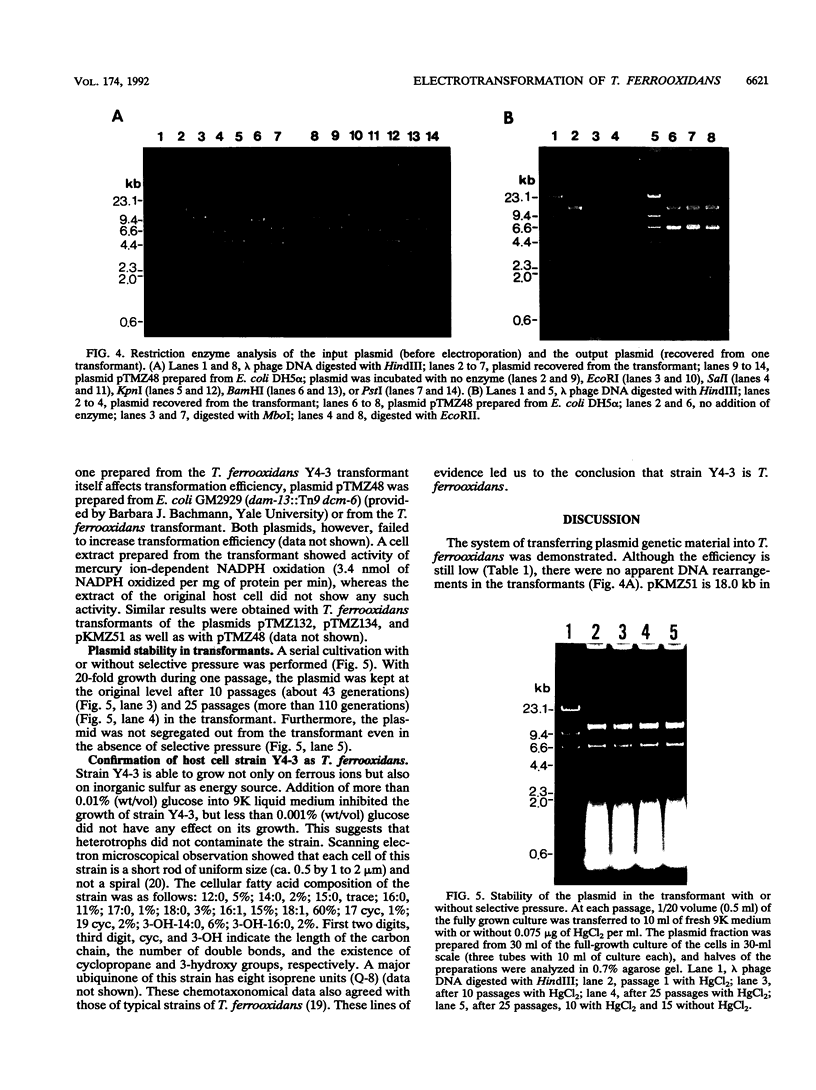


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M. M., Amann E., Lurz R., Rückert B., Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983 Dec;26(2-3):273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Belliveau B. H., Trevors J. T. Transformation of Bacillus cereus vegetative cells by electroporation. Appl Environ Microbiol. 1989 Jun;55(6):1649–1652. doi: 10.1128/aem.55.6.1649-1652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin N. M., Hanawalt P. C. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988 Jun;170(6):2796–2801. doi: 10.1128/jb.170.6.2796-2801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. S., Summers A. O. Wide-host-range plasmids function in the genus thiobacillus. Appl Environ Microbiol. 1983 Sep;46(3):565–572. doi: 10.1128/aem.46.3.565-572.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington R. A., Bardien S., Rawlings D. E. The broad-host-range plasmid pTF-FC2 requires a primase-like protein for autonomous replication in Escherichia coli. Gene. 1991 Dec 1;108(1):7–14. doi: 10.1016/0378-1119(91)90481-p. [DOI] [PubMed] [Google Scholar]
- Dorrington R. A., Rawlings D. E. Characterization of the minimum replicon of the broad-host-range plasmid pTF-FC2 and similarity between pTF-FC2 and the IncQ plasmids. J Bacteriol. 1990 Oct;172(10):5697–5705. doi: 10.1128/jb.172.10.5697-5705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Tanaka T., Ohara K., Morioka H., Uesugi S., Ikehara M., Nishikawa S. Secretion of recombinant ribonuclease T1 into the periplasmic space of Escherichia coli with the aid of the signal peptide of alkaline phosphatase. FEBS Lett. 1990 Jun 4;265(1-2):71–74. doi: 10.1016/0014-5793(90)80886-n. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Lobos J. H., Bopp L. H., Welch G. C. Cloning of a Thiobacillus ferrooxidans plasmid in Escherichia coli. J Bacteriol. 1984 Jan;157(1):324–326. doi: 10.1128/jb.157.1.324-326.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Kusano T. The merR regulatory gene in Thiobacillus ferrooxidans is spaced apart from the mer structural genes. Mol Microbiol. 1991 Nov;5(11):2707–2718. doi: 10.1111/j.1365-2958.1991.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Kusano T. Thiobacillus ferrooxidans mer operon: sequence analysis of the promoter and adjacent genes. Gene. 1990 Nov 30;96(1):115–120. doi: 10.1016/0378-1119(90)90349-v. [DOI] [PubMed] [Google Scholar]
- Inoue C., Sugawara K., Shiratori T., Kusano T., Kitagawa Y. Nucleotide sequence of the Thiobacillus ferrooxidans chromosomal gene encoding mercuric reductase. Gene. 1989 Dec 7;84(1):47–54. doi: 10.1016/0378-1119(89)90138-8. [DOI] [PubMed] [Google Scholar]
- Jin S. M., Yan W. M., Wang Z. N. Transfer of IncP Plasmids to Extremely Acidophilic Thiobacillus thiooxidans. Appl Environ Microbiol. 1992 Jan;58(1):429–430. doi: 10.1128/aem.58.1.429-430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulpa C. F., Roskey M. T., Travis M. T. Transfer of plasmid RP1 into chemolithotrophic Thiobacillus neapolitanus. J Bacteriol. 1983 Oct;156(1):434–436. doi: 10.1128/jb.156.1.434-436.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Ji G. Y., Inoue C., Silver S. Constitutive synthesis of a transport function encoded by the Thiobacillus ferrooxidans merC gene cloned in Escherichia coli. J Bacteriol. 1990 May;172(5):2688–2692. doi: 10.1128/jb.172.5.2688-2692.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Takeshima T., Inoue C., Sugawara K. Evidence for two sets of structural genes coding for ribulose bisphosphate carboxylase in Thiobacillus ferrooxidans. J Bacteriol. 1991 Nov;173(22):7313–7323. doi: 10.1128/jb.173.22.7313-7323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Takeshima T., Sugawara K., Inoue C., Shiratori T., Yano T., Fukumori Y., Yamanaka T. Molecular cloning of the gene encoding Thiobacillus ferrooxidans Fe(II) oxidase. High homology of the gene product with HiPIP. J Biol Chem. 1992 Jun 5;267(16):11242–11247. [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. A., Dugan P. R., Tuovinen O. H. Plasmid DNA in acidophilic, chemolithotrophic thiobacilli. Can J Microbiol. 1981 Aug;27(8):850–853. doi: 10.1139/m81-133. [DOI] [PubMed] [Google Scholar]
- Rawlings D. E., Pretorius I., Woods D. R. Expression of a Thiobacillus ferrooxidans origin of replication in Escherichia coli. J Bacteriol. 1984 May;158(2):737–738. doi: 10.1128/jb.158.2.737-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D. E., Woods D. R. Mobilization of Thiobacillus ferrooxidans plasmids among Escherichia coli strains. Appl Environ Microbiol. 1985 May;49(5):1323–1325. doi: 10.1128/aem.49.5.1323-1325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T., Inoue C., Sugawara K., Kusano T., Kitagawa Y. Cloning and expression of Thiobacillus ferrooxidans mercury ion resistance genes in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3458–3464. doi: 10.1128/jb.171.6.3458-3464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992 Mar;56(1):195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R., McEntee K. A rapid and efficient procedure for transformation of intact Saccharomyces cerevisiae by electroporation. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1157–1164. doi: 10.1016/0006-291x(89)91790-7. [DOI] [PubMed] [Google Scholar]
- Wirth R., Friesenegger A., Fiedler S. Transformation of various species of gram-negative bacteria belonging to 11 different genera by electroporation. Mol Gen Genet. 1989 Mar;216(1):175–177. doi: 10.1007/BF00332248. [DOI] [PubMed] [Google Scholar]
- Yankofsky S. A., Gurevich R., Grimland N., Stark A. A. Genetic transformation of obligately chemolithotrophic thiobacilli. J Bacteriol. 1983 Feb;153(2):652–657. doi: 10.1128/jb.153.2.652-657.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]