Abstract
The release of mitochondrial proapoptotic proteins into the cytosol is the key event in apoptosis signaling, leading to the activation of caspases. Once in the cytosol, cytochrome c triggers the formation of a caspase-activating protein complex called the apoptosome, whereas Smac/Diablo and Omi/htra2 antagonize the caspase inhibitory effect of inhibitor of apoptosis proteins (IAPs). Here, we identify diarylurea compounds as effective inhibitors of the cytochrome c-induced formation of the active, approximately 700-kDa apoptosome complex and caspase activation. Using diarylureas to inhibit the formation of the apoptosome complex, we demonstrated that cytochrome c, rather than IAP antagonists, is the major mitochondrial caspase activation factor in tumor cells treated with tumor necrosis factor. Thus, we have identified a novel class of compounds that inhibits apoptosis by blocking the activation of the initiator caspase 9 by directly inhibiting the formation of the apoptosome complex. This mechanism of action is different from that employed by the widely used tetrapeptide inhibitors of caspases or known endogenous apoptosis inhibitors, such as Bcl-2 and IAPs. Thus, these compounds provide a novel specific tool to investigate the role of the apoptosome in mitochondrion-dependent death paradigms.
Apoptosis is a cell suicide mechanism that enables multicellular organisms to maintain tissue homeostasis and to eliminate cells that threaten the survival of the animal. Deregulation of apoptosis is observed in various diseases, such as neurodegenerative diseases where excess cell death is pronounced, and cancer, where apoptosis is prevented (20, 22, 25). Apoptosis can be triggered by a variety of stimuli, including activation of cell surface death receptors, anticancer agents, irradiation, lack of survival factors, and ischemia (for reviews, see references 7, 11, and 30). Even though the initial signaling pathways induced by various stimuli can be very different, the signaling cascades induced by most stimuli finally converge into a common apoptotic pathway characterized by the activation of a family of cysteine proteases, the caspases. Caspases then cleave a subset of cellular proteins, and the cumulative effect of these cleavage events accounts for most of the morphological and biochemical characteristics of an apoptotic cell (31).
Caspases exist in cells as inactive zymogens that can be rapidly activated by proteolytic processing or by binding to a cofactor (31). As the activation of a few so-called initiator caspases (e.g., caspases 8 and 9) can trigger the full caspase cascade, this step must be meticulously controlled. One of the best-studied checkpoints for caspase activation resides at the outer mitochondrial membrane. Diverse signaling pathways can promote mitochondrial outer membrane permeabilization (MOMP) and the release of caspase-activating factors, in particular, cytochrome c, Smac/Diablo, and Omi/htra2, from the mitochondria to the cytosol (7, 11, 20). Even though the exact mechanism of the MOMP is still under debate, it is apparent that members of the Bcl-2 protein family are major controllers of this event. Once released into the cytosol, cytochrome c activates apoptotic protease-activating factor 1 (Apaf-1), which together with procaspase 9 forms an active ∼700-kDa holoenzyme complex termed the apoptosome (3, 16). Apoptosome-associated caspase 9 can then activate effector caspases provided that cytosolic inhibitor of apoptosis proteins (IAPs) are removed from the complex by Smac/Diablo or Omi/htra2.
The caspase cascade can also be initiated at the plasma membrane by the ligand-mediated activation of death receptors of the tumor necrosis factor (TNF) receptor family (for reviews, see references 7, 11, and 30). Upon ligand binding, death receptors cluster and form death-inducing signaling complexes consisting of adaptor proteins and several procaspase 8 molecules that activate each other as a result of juxtaposition of caspase 8 molecules (1, 23). Caspase 8 can then activate caspase 3 either directly in so-called type I cells or indirectly via the cleavage of the proapoptotic Bcl-2 family member Bid and the subsequent MOMP in so-called type II cells (15, 19, 27, 29). The cytochrome c-triggered formation of the apoptosome was originally thought to be the essential mitochondrion-dependent and Bcl-2-controlled event in the death receptor-triggered apoptosis of type II cells (27). Recent data suggest, however, that another crucial postmitochondrial event in death receptor-induced apoptosis is the release of IAP antagonists (Smac/Diablo or Omi/htra2) that allow the direct activation of caspase 3 by caspase 8 (6).
The in vivo activation of the apoptosome can be mimicked in vitro by adding cytochrome c and dATP into a cytosolic extract (16, 18). Studies employing such an experimental system suggest a model for a stepwise series of caspase activation events in response to cytochrome c release. Once activated in the apoptosome complex, caspase 9 initiates the processing of caspase 3 and caspase 7 (24, 28). Activated caspase 3 in turn activates caspase 2 and caspase 6, and it also appears capable of processing and activating caspase 9, suggesting a positive-feedback loop. The aim of this study was to identify potential drug candidates for the treatment of pathologies characterized by excessive apoptosis. For this purpose, we employed an in vitro apoptosome activation system to screen for small molecules that interfere with the formation or activity of the apoptosome. The identified compounds were further analyzed for their ability to inhibit apoptosis in vivo and to study the role of the apoptosome in various death paradigms.
MATERIALS AND METHODS
Cell lines.
The HeLa human cervix carcinoma cell line was kindly provided by J. Lukas (Danish Cancer Society, Copenhagen, Denmark). The MCF-casp3 cell line is a caspase 3-expressing pool of MCF-7S1 human breast cancer cells (21). The WEHI-S cell line is a highly TNF-sensitive subclone of WEHI-164 murine fibrosarcoma cells (10). The ME-180as (ME-ashsp2) cell line is an antisense Hsp70-expressing subclone of ME-180 human cervix carcinoma cells (10). SKW6.4 cells originate from Burkitt's B-cell lymphoma, and Neuro2 is a cell line producing Fas ligand (FasL) (26). Cells were propagated as described previously (10).
Compounds.
Recombinant human TNF alpha (TNF-α) was provided by Anthony Cerami (Kenneth Warren Laboratories, Tarrytown, N.Y.), staurosporine was from Sigma Chemical Co. (St. Louis, Mo.), and the protease inhibitors zVAD-fmk and DEVD-cmk (fmk and cmk, fluoro- and chloromethylketone, respectively) were from Bachem (Bubendorf, Switzerland), and DEVD-CHO was from Biomol (Plymouth Meeting, Pa.). dATP (ICN Biomedicals, Inc., Aurora, Ohio) was dissolved in double-distilled H2O and adjusted to pH 7.0. The small-molecule library compounds were dissolved in dimethyl sulfoxide at 10 mM (NeuroSearch A/S, Ballerup, Denmark). To obtain supernatant containing FasL, confluent Neuro2 cells (26) were provided with fresh serum-free medium, and after 24 h at 37°C, the supernatant was collected, centrifuged at 600 × g for 10 min, and stored in aliquots at −80°C.
In vitro apoptosome assay and caspase activity measurements.
Subconfluent cultures of HeLa cells were harvested by scraping on ice, washed in ice-cold phosphate-buffered saline (PBS), and resuspended in equal volume of ice-cold isotonic lysis buffer (20 mM HEPES-KOH [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 1 mM dithiothreitol [DTT], 10 μg of aprotinin per ml, 1 μg of leupeptin per ml, 1 μg of pepstatin A per ml, 100 μg of pefabloc per ml). After 30-min incubation on ice, the cells were lysed by 30 strokes of a Dounce homogenizer and centrifuged at 750 × g for 10 min. The supernatant obtained was further centrifuged at 10,000 × g for 10 min and at 20,000 × g for 30 min. The clarified supernatant was stored in aliquots at −80°C and used at protein concentrations ranging from 5 to 10 mg/ml. The apoptosome was activated by the addition of 1 mM dATP and 1 μM horse heart cytochrome c (Sigma Chemical Co.) to the cytosolic HeLa cell extract (protein concentration, 5 to 10 mg/ml) containing 100 μM DEVD-7-amino-4-(trimethyl-fluoromethyl) coumarin (AFC) (Biomol). When screening the molecular library, the compounds were added at a concentration of 100 μM prior to the addition of cytochrome c and dATP. After 30-min incubation at 37°C, the Vmax of the liberation of AFC (excitation wavelength, 400 nm; emission wavelength, 489 nm) was measured for 30 to 45 min at 37°C with a Spectramax Gemini fluorometer (Molecular Devices).
To measure the total cellular DEVDase activity, cells were treated as indicated in the figure legends, scraped on ice, washed in PBS, and resuspended in ice-cold caspase lysis buffer (25 mM HEPES, 5 mM MgCl2, 1 mM EGTA, 0,5% Triton X-100, 5 mM DTT, 10 μg of aprotinin per ml, 1 μg of leupeptin per ml, 1 μg of pepstatin A per ml, 1 μM pefabloc [pH 7.5]) and left on ice for 30 min. Lysates were then centrifuged at 20,000 × g for 10 min, and the supernatant was analyzed for protein content by using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, Calif.). The enzyme activities were estimated by adding cell extracts (10 μl) to caspase reaction buffer (40 μl) (100 mM HEPES, 20% glycerol, 0.5 mM EDTA, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 5 mM DTT, 1 mM pefabloc [pH 7.5]) in the presence of 100 μM DEVD-AFC. The Vmax of liberation of AFC was measured as described above and corrected for the protein concentration of the untreated sample.
The activities of recombinant caspases were measured as described above by mixing the indicated compounds with recombinant caspase 3 (60 ng/ml; R&D Systems Europe, Ltd., Abingdon, United Kingdom) or caspase 9 (12 μg/ml; Calbiochem) in 25 μl of caspase lysis buffer and adding 200 μM DEVD-AFC or LEHD-AFC (Biomol), respectively, in 25 μl of caspase reaction buffer. The fluorogenic chromophore AFC (Calbiochem) served as a control.
To measure DEVDase activity in SKW6.4 cells, 300,000 cells/1.5 ml of complete medium were incubated with 25% FasL supernatant for 16 h. Cells were pelleted and permeabilized for 15 min on ice in an extraction buffer (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM EDTA) containing 1 mM pefabloc and 200 μg of digitonin per ml. Cells were then pelleted, and DEVDase activity, and lactate dehydrogenase (LDH) activity was measured in 50 μl of supernatant. DEVDase activity was measured as described above, and LDH activity was measured according to the manufacturer's instructions.
Immunoblot analysis.
After 2 h at 37°C, the samples from the in vitro apoptosome assay were mixed with 0.25 volume of 4× Laemmli sample buffer, and the immunodetection of proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously employing enhanced chemiluminescence Western blotting reagents from Amersham (8). The primary antibodies used were anti-glyceraldehyde-3-phosphate dehydrogenase (0.4 μg/ml; Biogenesis, Poole, United Kingdom), anti-caspase 3 (1:1,000; BD PharMingen), anti-caspase 7 (1:1,000; Cell Signaling Technology), anti-caspase 9 (1:1,000; BD PharMingen), anti-poly(ADP)ribose polymerase (PARP); clone C2-10; 1:15,000; G. G. Poirier, Laval University, Québec, Québec, Canada) (1:100; Oncogene), anti-Apaf-1 (1 μg/ml; R&D Systems Europe, Ltd.), and anti-DNA fragmentation factor 45 (DFF45)/inhibitor of caspase-activated DNase (ICAD; 1:500; Transduction Laboratories), and the appropriate secondary antibodies were from Dako (Glostrup, Denmark).
Immunoprecipitation.
The intrinsic cell death pathway was induced in HeLa cytosolic extracts (protein concentration, 5 mg/ml) as described above in the presence or absence of inhibitors for 1 h. The anti-caspase 9 antibody immobilized on protein G-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden) was added, and the samples were incubated for 3 h at 4°C before extensive washing in isotonic lysis buffer. Precipitated proteins were analyzed by SDS-PAGE and Western blotting as described above.
Survival assay.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium assay was used to measure cell survival as described previously (9).
Immunocytochemistry.
Cells were fixed in 4% formaldehyde, incubated with blocking buffer (PBS, 10% fetal calf serum, 1% bovine serum albumin, 0.3% Triton X-100) and stained with anti-cytochrome c antibody (1:300; BD PharMingen) and AlexaFluor donkey anti-mouse immunoglobulin G (IgG) (heavy and light chains) conjugate (1:2,000; Molecular Probes, Leiden, The Netherlands). Cells with or without cytochrome c release were visualized by using an Olympus BX60 microscope, and approximately 800 cells were counted for each treatment.
Detection of the formation of the apoptosome complex in THP.1 cell lysates.
Cell lysates (supernatants after centrifugation at 100,000 × g) from THP.1 human monocytic tumor cells were prepared and stored at −80°C as described previously (4). Apoptosome formation and caspase activation were initiated in cell lysates (10 mg of protein per ml) resuspended in DEVDase assay buffer (0.1% CHAPS, 10 mM DTT, 100 mM HEPES, 10% sucrose [pH 7.0]) and incubated with 2 mM dATP-MgCl2 for 30 min at 37°C. After caspase activation, aliquots were assayed for caspase 3 and caspase 7 (DEVDase) cleavage activity and caspase 9 and caspase 3 processing.
Assembly of the apoptosome was detected as described previously by fractionating lysates (2 mg) on an analytical Superose 6 HR 10/30 column (Amersham Pharmacia Biotech, Hemel Hempstead, Herts, United Kingdom) (3). The column was eluted at 4°C and a flow rate of 0.4 ml/min with a solution consisting of 5% (wt/vol) sucrose, 0.1% (wt/vol) CHAPS, 50 mM NaCl, 20 mM HEPES-NaOH, and 5 mM DTT, pH 7.0. Fractions (0.5 ml) were collected and analyzed by Western blotting for Apaf-1 and caspase 9. The column was calibrated with protein standards (Amersham Pharmacia Biotech), including blue dextran, thyroglobulin, ferritin, catalase, aldolase, bovine serum albumin, ovalbumin, and bovine heart cytochrome c (Sigma). DEVDase activity in cell lysates or column fractions (i.e., primarily caspases 3 and 7) was measured fluorimetrically in a Wallac Victor 1420 Multilabel counter as described previously (3). Aliquots were assayed at 37°C in 96-well plates in 200 μl of assay buffer with 20 μM Z-DEVD-AFC substrate. Samples were assayed for 10 cycles, and cleavage rates were determined by linear regression and expressed as either picomoles per minute per milligram of protein or picomoles per minute per fraction.
SDS-PAGE and Western blotting for Apaf-1 and caspases 9 and 3 were performed on 10% Tris-glycine Criterion gels (Bio-Rad, Hemel Hempstead, United Kingdom) essentially as described previously (3). A monoclonal anti-human Apaf-1 antibody (MAb 868) from R&D Systems Europe, Ltd., and was used at a 1:2,000 dilution. A monoclonal antibody to the N-terminal region of caspase 9 (MO54-3) was from Medical and Biological Laboratories Co., Ltd., Nagoya, Japan, and was used at a 1:1,000 dilution. All other antibodies and reagents were obtained as previously described (3).
RESULTS
Diarylurea compounds inhibit cytochrome c- and dATP-induced activation of apoptosome.
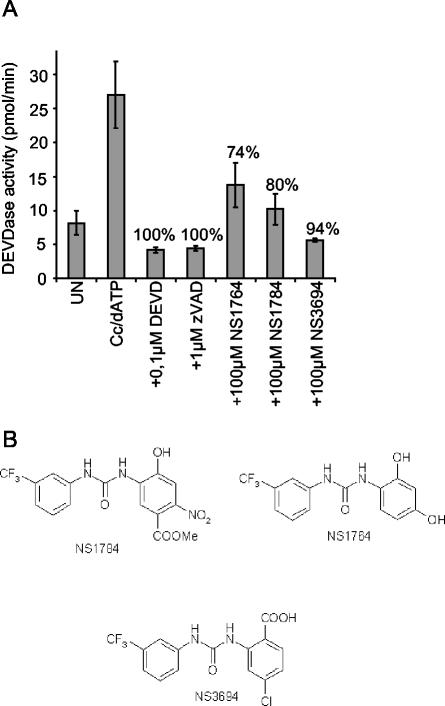
We established an in vitro apoptosome assay using cytosolic extracts obtained from HeLa cervix carcinoma cells and used it to screen a representative selection of 392 compounds of a molecular library of 5,000 nonpeptide small molecules (NeuroSearch A/S). Eleven compounds inhibited the cytochrome c- and dATP-induced activation of caspase 3-like activity (DEVDase) by more than 50%. Three of these compounds, NS3694, NS1764, and NS1784, were well-tolerated by MCF-7S1 breast cancer cells at concentrations up to 100, 50, and 25 μM, respectively, and were therefore selected for further analysis (Fig. 1 and data not shown). All three selected compounds belong to the chemical class of diarylureas (Fig. 1B).
FIG. 1.
Identification of diarylurea compounds as inhibitors of the apoptosome complex. (A) Cytosolic extract prepared from HeLa cells was left untreated (UN) or incubated with cytochrome c (Cc; 1 μM) and dATP (1 mM) in the presence (+) or absence of 100 μM concentrations of library compounds, 1 μM zVAD-fmk (zVAD), or 0.1 μM DEVD-CHO (DEVD). Caspase 3-like activity was assessed by spectrofluorometric quantification of DEVD-AFC cleavage as described in Materials and Methods. Percent inhibition is indicated above the bars. The results of three of the most potent compounds (NS1764, NS1784, and NS3694) are presented. (B) Chemical structures of NS1764, NS1784, and NS3694.
Diarylurea compounds do not inhibit caspases directly.
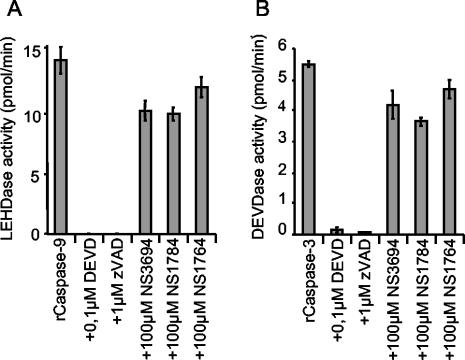
The inhibition of DEVDase activity in the in vitro caspase activation system could be due to a direct inhibition of the caspases involved or to inhibition of the formation of the apoptosome complex. To test whether NS3694, NS1784, and NS1764 were specific caspase inhibitors, they were incubated with recombinant caspase 3 or caspase 9, and caspase activity was measured using DEVD-AFC and LEHD-AFC as specific substrates for caspase 3 and caspase 9, respectively. All three compounds failed to inhibit recombinant caspase 9 and caspase 3 at concentrations ranging from 25 to 100 μM, whereas the specific tetrapeptide caspase inhibitors DEVD-CHO and zVAD-fmk produced practically complete inhibition (Fig. 2 and data not shown).
FIG. 2.
Diarylurea compounds do not inhibit the activity of caspase 9 or caspase 3. The enzymatic activity of recombinant caspase 9 (rCaspase-9) (A) and recombinant caspase 3 (rCaspase-3) (B) was assessed by spectrofluorometric quantification of LEHD-AFC or DEVD-AFC cleavage, respectively, in the presence (+) or absence of 100 μM NS3694, NS1784, NS1764, DEVD-CHO (DEVD), or zVAD-fmk (zVAD). Enzymatic activity is presented as picomoles per minute, and the means ± standard deviations (error bars) for three samples are shown. Data are representative of the results from three independent assays.
Diarylurea compounds inhibit apoptosome function upstream of caspase 9 activation.
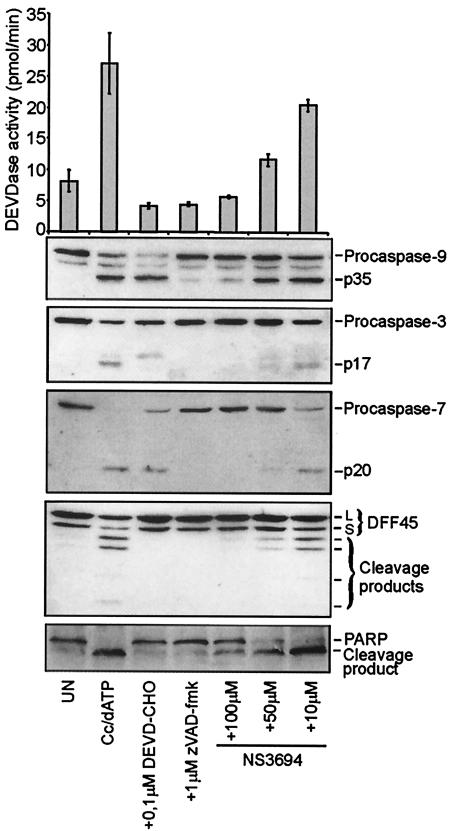
Next, the effects of the compounds on the processing of caspases and caspase substrates in the in vitro model system was analyzed by immunoblotting employing antibodies specific for caspases and their substrates. As shown in Fig. 3, the addition of cytochrome c and dATP into the cytosolic extract triggered the processing of caspase 9, caspase 3, and caspase 7 into 35-kDa (p35), 17-kDa (p17), and 20-kDa (p20) active fragments, respectively. Accordingly, cytochrome c and dATP induced a threefold increase in the DEVDase activity and the cleavage of caspase 3 substrates DFF45/ICAD and PARP into the caspase-specific cleavage fragments. When cell extracts were stimulated with cytochrome c and dATP in the presence of NS3694, a dose-dependent inhibition of caspase 9, 3, and 7 processing, DFF45/ICAD and PARP cleavage, and DEVDase activity was observed (Fig. 3). Similar results were obtained with NS1784 and NS1764 (data not shown). These results suggest that diarylurea compounds act upstream of the activation of caspase 9.
FIG. 3.
NS3694 inhibits cytochrome c- and dATP-induced DEVDase activation and processing of caspases and caspase substrates. Cytosolic extracts prepared from HeLa cells were left untreated (UN) or incubated at 37°C for 120 min with cytochrome c (Cc; 1 μM) and dATP (1 mM) in the presence (+) or absence of NS3694 (10 to 100 μM), DEVD-CHO (0.1 μM), and zVAD-fmk (1 μM). Caspase 3-like activity was assessed by spectrofluorometric quantification of DEVD-AFC cleavage and presented as picomoles per minute. After measurement of the DEVDase activity, the same samples were analyzed by immunoblotting using antibodies against the indicated proteins. The means ± standard deviations (error bars) of three independent experiments are shown, and the immunoblot analysis was repeated twice with essentially identical results.
NS3694 inhibits the coimmunoprecipitation of caspase 9 and Apaf-1 from HeLa cell cytosol stimulated with cytochrome c and dATP.
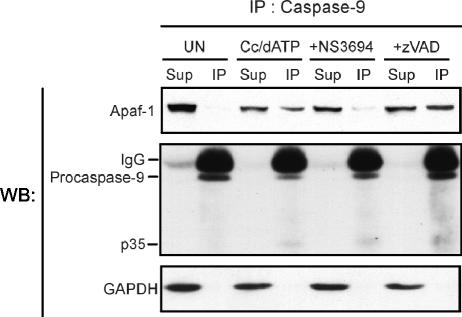
Next, we tested whether NS3694 interferes with the cytochrome c- and dATP-induced formation of the apoptosome complex. Cytosolic extracts of HeLa cells were incubated with cytochrome c and dATP in the presence or absence of 100 μM NS3694 or 1 μM zVAD-fmk for 1 h prior to the immunoprecipitation of caspase 9. Caspase 9 antibody precipitated practically all caspase 9 present in both unactivated and cytochrome c- and dATP-activated extracts. As predicted, Apaf-1 coimmunoprecipitated only with caspase 9 in activated extracts, and this was also the case in the presence of zVAD-fmk (Fig. 4). Thus, the caspase 9 antibody immunoprecipitates Apaf-1 only under conditions when the apoptosome holoenzyme complex is formed. NS3694 did not affect the ability of the antibody to bind caspase 9, but it significantly inhibited the coimmunoprecipitation of Apaf-1, indicating that NS3694 alters the cytochrome c- and dATP-triggered association between Apaf-1 and caspase 9. A reverse immunoprecipitation assay using an Apaf-1 antibody was also performed, but unfortunately, activation of the apoptosome by cytochrome c and dATP inhibited the immunoprecipitation of Apaf-1, suggesting that the epitope recognized by the anti-Apaf-1 antibody was covered by the apoptosome complex (data not shown).
FIG. 4.
NS3694 inhibits the association of caspase 9 to Apaf-1. Cytosolic extracts prepared from HeLa cells were left untreated (UN) or incubated with cytochrome c (Cc; 1 μM) and dATP (1 mM), in the presence (+) or absence of 100 μM NS3694 or 2 μM zVAD-fmk (zVAD) at 37°C. After 1-h incubation, caspase 9 was immunoprecipitated with anti-caspase 9 antibody precoupled to Sepharose beads. The supernatants (Sup) and immunoprecipitates (IP) were separated by SDS-PAGE followed by immunoblot analysis using antibodies against the indicated proteins. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a control for the specificity of the immunoprecipitation and was detected only in the supernatants. Bands corresponding to the heavy chain (IgG) of the immunoprecipitating antibody are also visible. The experiment was repeated once with essentially identical results. WB, Western blotting.
NS3694 inhibits the formation of the active ∼700-kDa apoptosome complex.
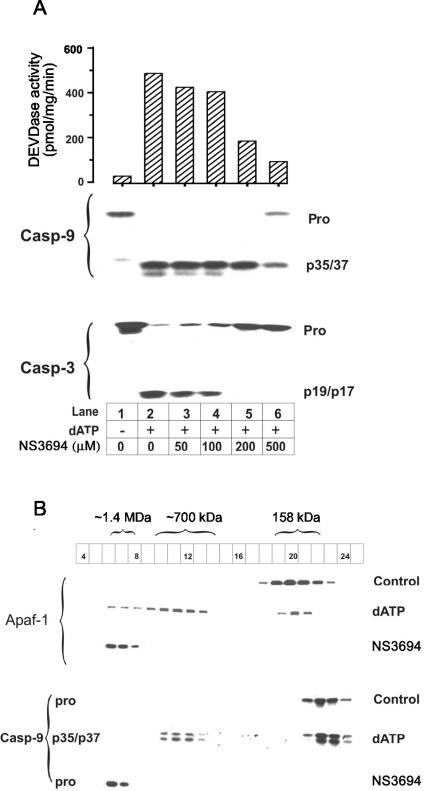
We wished to determine how NS3694 disrupted the formation of the apoptosome complex, and for this purpose, we investigated the effects of the inhibitor in dATP-stimulated THP.1 cell lysates (whole-cell lysates that contain mitochondrial proteins including cytochrome c). Apoptosome formation has been well-characterized in this model system, and NS3694 also inhibited the activation and processing of caspases in a concentration-dependent manner (Fig. 5A). To analyze whether NS3694 interfered with the assembly of the apoptosome complex, we fractionated control THP.1 cell lysates and THP.1 cell lysates stimulated for 30 min with dATP in the absence or presence of NS3694 on an analytical Superose 6 HR 10/30 column. In control lysates, Apaf-1 eluted as a monomer, whereas in dATP-stimulated lysates, it was mainly oligomerized to the active ∼700-kDa complex and to a lesser extent to the inactive 1.4-MDa complex (Fig. 5B). In the presence of NS3694, dATP failed to trigger the formation of the 700-kDa complex, and all Apaf-1 was found in the 1.4-MDa complex. In control lysates, caspase 9 eluted as a monomeric proform, whereas in dATP-stimulated lysates, it was processed to the 35- or 37-kDa form and distributed between the 700-kDa complex and the free form. In the presence of NS3694, dATP-induced processing of procaspase 9 to the 35- or 37-kDa form was blocked, and it eluted with the 1.4-MDa complex.
FIG. 5.
NS3694 blocks the formation of the ∼700-kDa apoptosome complex. (A) Increasing concentrations of NS3694 were coincubated with THP.1 cell lysates (10 mg) in the presence (+) and absence (−) of 2 mM dATP-MgCl2 for 30 min at 37°C before assaying for DEVDase activity (essentially caspases 3 and 7) and caspase 9 (Casp-9) and caspase 3 (Casp-3) processing as described in Materials and Methods. The positions of the procaspases (Pro) and processed forms (p35 and p37 [p35/37] and p19 and p17 [p19/p17]) are indicated to the right of the blots. NS3694 was added in a dimethyl sulfoxide solution, and control samples were incubated with equivalent amounts of the solvent, which did not inhibit caspase activation. (B) Two-milligram aliquots of control THP.1 cell lysates and dATP-activated THP.1 cell lysates in the presence and absence of NS3694 (500 μM) were fractionated on Superose 6 columns, and the fractions were analyzed for Apaf-1 and caspase 9 (Casp-9). The sizes of the various peaks were determined by using gel filtration marker proteins, and the elution positions of the ∼700-kDa and ∼1.4-MDa apoptosome complexes and aldolase (158 kDa) are indicated above the blots. Processed caspase 9 was not detected in the control samples and Ns3694-treated samples. In the dATP-activated sample, procaspase 9 (pro) was totally processed to its active subunits. The results shown are from a representative experiment, and similar data were obtained in a repeated experiment. Numbers in boxes, fraction numbers.
Apoptosome activation is essential for TNF-induced caspase activation in tumor cells.
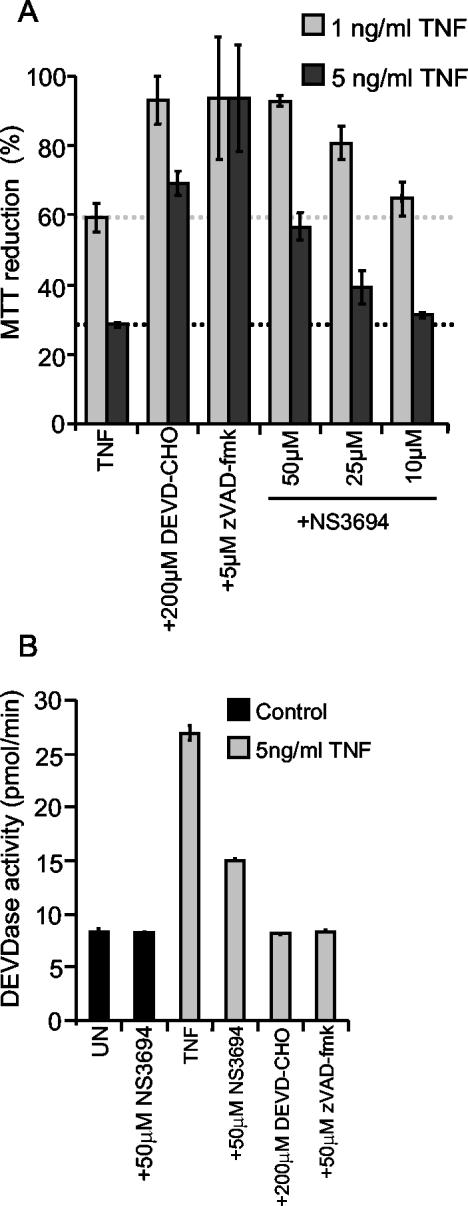
Ectopic expression of Bcl-2 or Bcl-XL effectively inhibits TNF-induced caspase activation and death in MCF-7 breast carcinoma and ME-180 cervix carcinoma cells, suggesting that MOMP is essential for death signaling in these cells (9, 27). Since MOMP can lead to caspase activation and death via either cytochrome c-induced apoptosome formation or IAP antagonism mediated by Smac/Diablo or Omi/htra2 (6, 12), we next used NS3694 to investigate the role of the apoptosome in this process. Supporting the requirement of cytochrome c release for this death pathway, the decreased viability of MCF-casp3 cells induced by 1 ng of TNF per ml was completely blocked by pretreatment of cells with 50 μM NS3694, and the protection was still evident following treatment with 5 ng of TNF per ml (Fig. 6A). The level of protection was comparable to that observed by the inhibition of effector caspases by 200 μM DEVD-CHO but was somewhat weaker than that conferred by 5 μM zVAD-fmk, which inhibits the activity of all known caspases, including the TNF-activated initiator caspase, caspase 8 (Fig. 6A).
FIG. 6.
NS3694 inhibits TNF-induced effector caspase activation and apoptosis in MCF-casp3 cells. MCF-casp3 cells were left untreated (UN) or pretreated with the indicated concentrations of DEVD-CHO or zVAD-fmk for 1 h before the indicated concentrations of TNF were added. NS3694 was added at 10 to 100 μM at the same time as TNF. (A) The survival of the cells was analyzed by the MTT assay after 48 h of TNF treatment. The means ± standard deviations (error bars) of three independent experiments are shown. (B) After 17 h of treatment, the cells were lysed, and the DEVDase activity in lysates was assessed by spectrofluorometric quantification of DEVD-AFC cleavage and presented as picomoles per minute. The means ± standard deviations (error bars) for three samples in a representative experiment are shown. The experiments were repeated several times with similar results.
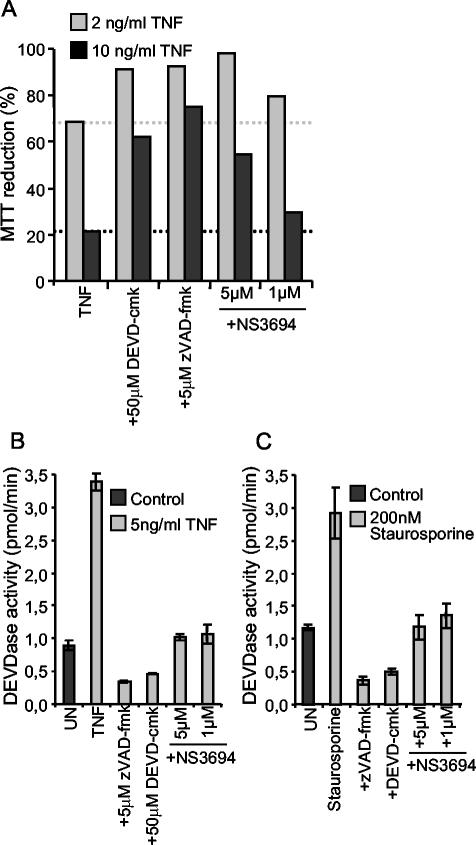
To ensure that the protective effect of NS3694 was due to its ability to inhibit the formation of the apoptosome complex, we next analyzed its effect on the TNF-induced release of cytochrome c and activation of effector caspases in MCF-casp3 cells by immunofluorescence microscopy and DEVDase enzyme assay, respectively. Approximately 11% of MCF-7 cells treated with 1 ng of TNF per ml for 16 h showed a diffuse pattern of cytochrome c staining indicative of its release from the mitochondria (data not shown). The pretreatment of cells with 50 μM NS3694 had no effect on TNF-induced cytochrome c release, whereas 5 μM zVAD-fmk inhibited it completely (data not shown). TNF triggered a 3.3-fold increase in the cellular DEVDase activity, which was almost completely inhibited by NS3694, indicating that in living cells, NS3694 functions downstream of cytochrome c release but upstream of effector caspase activation (Fig. 6B). Furthermore, these data suggest that cytochrome c rather than IAP antagonists play a major role in TNF-induced effector caspase activation in these cells. Akin to MCF-casp3 cells, complete inhibition of TNF-induced effector caspase activation and partial inhibition of cell death were obtained by cotreatment of ME-180as cells with NS3694 (Fig. 7A and B). Also, staurosporine-induced effector caspase activation was completely inhibited by the treatment of ME-180as cells with NS3694 (Fig. 7C). Caspase inhibition by either NS3694 or zVAD-fmk had, however, no effect on the survival of staurosporine-treated ME-180as cells (data not shown).
FIG. 7.
NS3694 inhibits TNF- and staurosporine-induced effector caspase activation and TNF-induced apoptosis in ME-180as cells. ME-180as cells were left untreated (UN) or pretreated with 50 μM DEVD-cmk or 5 μM zVAD-fmk for 1 h before the addition of the indicated concentrations of TNF (A and B) or 200 nM staurosporine (C). NS3694 was added at a concentration of 1 to 5 μM at the same time as TNF or staurosporine. (A) The survival of the cells was analyzed by the MTT assay after 48 h of TNF treatment. (B and C) ME-180as cells were lysed after 17 h of treatment with TNF or staurosporine, and the DEVDase activity in lysates was assessed by spectrofluorometric quantification of DEVD-AFC cleavage and presented as picomoles per minute. The means ± standard deviations (error bars) for three samples in a representative experiment are shown. The experiments were repeated twice with similar results.
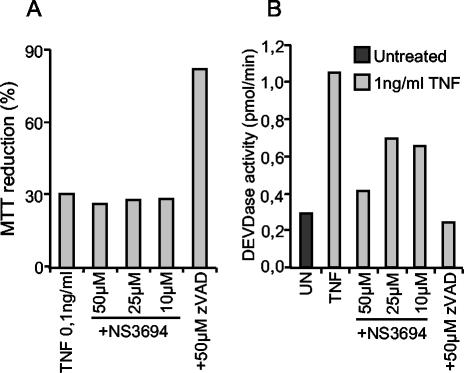
NS3694 has no effect on TNF-induced caspase-independent death of WEHI-S cells.
TNF triggers caspase activation and cell death in WEHI-S murine fibrosarcoma cells (8). The activated caspases are, however, not responsible for cell death, as the complete inhibition of activated caspases by 1 to 10 μM zVAD-fmk results in increased TNF-induced death (8). Accordingly, NS3694 (10 to 50 μM) treatment effectively inhibited TNF-induced caspase activation without affecting TNF-induced cell death (Fig. 8). As we have shown earlier, high concentrations of zVAD-fmk (>30 μM) that also inhibit other cysteine proteases (i.e., cathepsins and calpains [8, 20]) confer complete protection in this model system (Fig. 8).
FIG. 8.
NS3694 inhibits TNF-induced DEVDase activity but not TNF-induced cytotoxicity in WEHI-S cells. WEHI-S cells were left untreated (UN) or pretreated with indicated concentrations of zVAD-fmk (zVAD) and NS3694 for 1 h before treatment with 0.1 (A) or 1 (B) ng of TNF per ml. (A) The survival of the cells was analyzed by the MTT assay after 14-h treatment. The values are means ± standard deviations (error bars) for three samples in a representative experiment. (B) The cells were lysed after a 5-h treatment, and the DEVDase activity in lysates was assessed by spectrofluorometric quantification of DEVD-AFC cleavage and presented as picomoles per minute. The means ± standard deviations (error bars) for three samples in a representative experiment are shown. The experiment was repeated twice with similar results.
NS3694 does not inhibit FasL-induced caspase activation or death in type I cells.
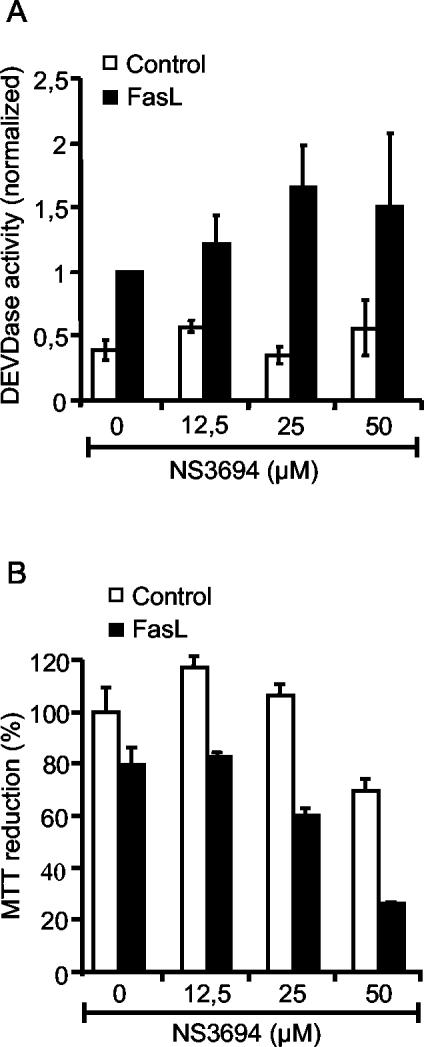
In SKW6.4 cells, FasL triggers the extrinsic pathway mediated by the direct activation of caspase 3 by caspase 8 (27). To test the ability of NS3694 to interfere with the extrinsic death pathway, we treated SKW6.4 cells with NS3694 at concentrations up to 50 μM prior to treatment with FasL and measured cell viability and effector caspase activity (DEVDase) after 16 h. NS3694 failed to inhibit FasL-induced cell death and caspase activation in SKW6.4 cells (Fig. 9).
FIG. 9.
NS3694 does not inhibit FasL-induced DEVDase activity or FasL-induced cytotoxicity in SKW6.4 cells. SKW6.4 cells were left untreated or pretreated with indicated concentrations of NS3694 for 1 h before treatment with 25% FasL supernatant. (A) The cells were permeabilized in an extraction buffer containing 200 μg of digitonin per ml after 16 h of treatment. DEVDase activity in lysates was assessed by spectrofluorometric quantification of DEVD-AFC cleavage, and LDH activity was measured using the LDH cytotoxicity kit (Roche). DEVDase activity was normalized to the LDH activity, and the values are means ± standard deviations (error bars) of at least three experiments normalized to 16 h of treatment with FasL supernatant in the absence of NS3694. (B) The survival of the cells was analyzed by the MTT assay after 14 h of treatment. The means ± standard deviations (error bars) for three samples in a representative experiment are shown.
DISCUSSION
With the aim to identify new potential drugs for the treatment of diseases characterized by excessive apoptosis, a nonpeptide small-molecule library was screened employing an apoptosome-dependent caspase activation assay. This assay is based on the finding that the in vivo induction of the intrinsic cell death pathway can be mimicked in vitro by adding cytochrome c and dATP to cytosolic extracts (16). Such an assay should allow not only the identification of direct caspase inhibitors but also putative inhibitors of the formation of the apoptosome complex. Screening 392 selected compounds representing a nonpeptide molecular library of 5,000 compounds resulted in 11 hits that conferred more than 50% inhibition of cytochrome c- and dATP-induced DEVDase activity. Most of the 11 compounds were highly toxic to cells; therefore, the further studies were performed with three of the compounds best tolerated by cells (i.e., NS3694, NS1784, and NS1764). All three compounds belonged to the chemical class of diarylureas and fulfilled the Lipinski rules for bioavailability, i.e., molecular weight under 500, less than five hydrogen bond donors, less than 10 hydrogen bond acceptors, and calculated log P value under 5 (17). This may explain the similar levels of inhibition and toxicities for the three compounds.
The in vitro studies on the mechanism of action of NS3694 revealed that it inhibited the formation of the active apoptosome complex rather than the activity of caspases. In the cytosolic extracts of HeLa cells, NS3694 inhibited the cytochrome c- and dATP-induced coimmunoprecipitation of caspase 9 and Apaf-1 and the processing of caspase 9 and all its downstream targets tested. In the whole-cell lysates of THP.1 cells, NS3694 inhibited the dATP-induced formation of the active 700-kDa apoptosome complex and the processing and activation of caspase 9 and caspase 3. However, NS3694 failed to inhibit the dATP-induced formation of the 1.4-MDa apoptosome complex in THP.1 lysates. Although this complex contains processed caspase 9, it does not cleave and activate the effector caspases (3), and in the presence of NS3694, all of the caspase 9 that eluted in this fraction was present as the inactive proform. Thus, the results obtained from dATP-stimulated THP.1 lysates suggest that NS3694 inhibits only the formation of the active apoptosome complex without interfering with the formation of the inactive complex of Apaf-1 and procaspase 9 (Fig. 5). Interestingly, in the coimmunoprecipitation experiments with HeLa cell lysates (Fig. 4), the anti-caspase 9 antibody did not precipitate all of the Apaf-1. Therefore, it may be that the interaction between the ∼1.4-MDa complex and caspase 9 is fairly weak due to the inappropriate oligomerization of Apaf-1 (2), and as a result, the Apaf-1 in this complex cannot be immunoprecipitated. Alternatively, it could be possible that the 1.4-MDa complex is not readily formed in HeLa cytosol. However, we have obtained essentially similar results with coimmunoprecipitation studies in dATP-activated THP.1 cell lysates (K. Cain and D. Brown, unpublished results). This indicates that the anti-caspase 9 antibody immunoprecipitates Apaf-1 only when caspase 9 is correctly incorporated in an active apoptosome holoenzyme complex, whose formation is inhibited by NS3694.
Supporting the data obtained from the in vitro model systems, NS3694-mediated protection from cell death in living cells occurred downstream of cytochrome c release but upstream of caspase activation. Furthermore, NS3694 inhibited death receptor-induced caspase activation in type II cells that require mitochondrial involvement for the effective effector caspase activation but not in type I cells, where caspase 8 can directly activate effector caspases. The precise mechanism by which diarylureas inhibit the formation of the active apoptosome remains to be studied. Since the formation of the apoptosome is triggered by cytochrome c and dATP, it is possible that NS3694 prevents the association of either cytochrome c or dATP to their respective domains on Apaf-1. Another possibility is that NS3694 interferes with the caspase recruitment domain (CARD)-CARD interaction between caspase 9 and Apaf-1. Diarylurea compounds are a new class of apoptosis inhibitors acting at a different step of the apoptosis signaling process than other available pharmacological inhibitors.
In contrast to most intrinsic death pathways that depend on the formation of the apoptosome complex, the role of the apoptosome in the postmitochondrial death signaling induced by death receptors is still controversial (5, 6, 13). Recently, Deng and coworkers suggested that the release of Smac/Diablo, but not the release of cytochrome c, from the mitochondria of TNF-related apoptosis-inducing ligand (TRAIL)-treated cells is required and sufficient to induce effector caspase activation and apoptosis (6). The ability of NS3694 to specifically inhibit the formation of the apoptosome complex allowed us to study the contribution of the apoptosome in caspase activation and death of TNF-treated tumor cells. In all three cell types studied, NS3694 almost completely inhibited the effector caspase activation, indicating that in these cells, Smac/Diablo-mediated inhibition of IAPs is not sufficient for the effective activation of downstream caspases. This conclusion is further supported by our preliminary data showing that the small interference RNA-mediated depletion of Apaf-1 in MCF-7 cells confers a level of protection against TNF similar to that of NS3694. Our data also demonstrate that even though the apoptosome is clearly required for caspase activation in the models used here, it is not necessarily required for cell death. For example, in TNF-treated WEHI-S cells and staurosporine-treated ME-180as cells, NS3694 almost completely inhibited caspase activation without any effect on cell viability. Thus, the death of these cells is likely to be mediated by caspase-independent signaling pathways (14).
As demonstrated above, NS3694 offers a novel tool to study the role of the apoptosome in various apoptosis paradigms. Meticulous in vivo studies on different disease models will, however, be required to evaluate the potential of NS3694 or related molecules as drug candidates for the treatment of apoptosis-associated diseases.
Acknowledgments
This work was supported in part by grants from the Danish Medical Research Council, the Danish Cancer Society, and the Novo Foundation.
We thank Birgit Poulsen for excellent technical assistance.
REFERENCES
- 1.Boldin, M. P., T. M. Goncharov, Y. V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85:803-815. [DOI] [PubMed] [Google Scholar]
- 2.Bratton, S. B., G. Walker, D. L. Roberts, K. Cain, and G. M. Cohen. 2001. Caspase-3 cleaves Apaf-1 into an approximately 30-kDa fragment that associates with an inappropriately oligomerized and biologically inactive approximately 1.4 MDa apoptosome complex. Cell Death Differ. 8:425-433. [DOI] [PubMed] [Google Scholar]
- 3.Cain, K., S. B. Bratton, C. Langlais, G. Walker, D. G. Brown, X. M. Sun, and G. M. Cohen. 2000. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J. Biol. Chem. 275:6067-6070. [DOI] [PubMed] [Google Scholar]
- 4.Cain, K., D. G. Brown, C. Langlais, and G. M. Cohen. 1999. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem. 274:22686-22692. [DOI] [PubMed] [Google Scholar]
- 5.Cecconi, F., G. Alvarez-Bolando, B. I. Meyer, K. A. Roth, and P. Gruss. 1998. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94:727-737. [DOI] [PubMed] [Google Scholar]
- 6.Deng, Y., Y. Lin, and X. Wu. 2002. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferri, K. R., and G. Kroemer. 2001. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3:E255-E263. [DOI] [PubMed] [Google Scholar]
- 8.Foghsgaard, L., D. Wissing, D. Mauch, U. Lademann, L. Bastholm, M. Boes, F. Elling, M. Leist, and M. Jäättelä. 2001. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153:999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jäättelä, M., M. Benedict, M. Tewari, J. A. Shayman, and V. M. Dixit. 1995. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 10:2297-2305. [PubMed] [Google Scholar]
- 10.Jäättelä, M., D. Wissing, K. Kokholm, T. Kallunki, and M. Egeblad. 1998. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17:6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann, S. H., and M. O. Hengartner. 2001. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 11:526-534. [DOI] [PubMed] [Google Scholar]
- 12.Kluck, R. M., E. Bossy-Wetzel, D. R. Green, and D. D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132-1136. [DOI] [PubMed] [Google Scholar]
- 13.Kuida, K., T. F. Haydar, C.-Y. Kuan, Y. Gu, C. Taya, H. Karasuyama, M. S.-S. Su, P. Rakic, and R. A. Flavell. 1998. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325-337. [DOI] [PubMed] [Google Scholar]
- 14.Leist, M., and M. Jäättelä. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell Biol. 2:589-598. [DOI] [PubMed] [Google Scholar]
- 15.Li, H., H. Zhu, C.-J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 16.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemeri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski, C. A., F. Lombardo, B. W. Dominy, and P. J. Feeney. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3-26. [DOI] [PubMed] [Google Scholar]
- 18.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147-157. [DOI] [PubMed] [Google Scholar]
- 19.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a BclII interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 20.Mathiasen, I. S., and M. Jäättelä. 2002. Triggering caspase-independent cell death to combat cancer. Trends Mol. Med. 8:212-220. [DOI] [PubMed] [Google Scholar]
- 21.Mathiasen, I. S., U. Lademann, and M. Jäättelä. 1999. Apoptosis induced by vitamin D compounds in breast cancer cells is inhibited by Bcl-2 but does not involve known caspases or p53. Cancer Res. 59:4848-4856. [PubMed] [Google Scholar]
- 22.Mattson, M. P. 2000. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 1:120-129. [DOI] [PubMed] [Google Scholar]
- 23.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 24.Pan, G., E. W. Humke, and V. M. Dixit. 1998. Activation of caspases triggered by cytochrome c in vitro. FEMS Lett. 426:151-154. [DOI] [PubMed] [Google Scholar]
- 25.Reed, J. C. 2001. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol. Med. 7:314-319. [DOI] [PubMed] [Google Scholar]
- 26.Rensing-Ehl, A., K. Frei, R. Flury, B. Matiba, S. M. Mariani, M. Weller, P. Aebischer, P. H. Krammer, and A. Fontana. 1995. Local Fas/APO-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur. J. Immunol. 25:2253-2258. [DOI] [PubMed] [Google Scholar]
- 27.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slee, E. A., M. T. Harte, R. M. Kluck, B. B. Wolf, C. A. Casiano, D. D. Newmeyer, H. G. Wang, J. C. Reed, D. W. Nicholson, E. S. Alnemri, D. R. Green, and S. J. Martin. 1999. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 144:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stennicke, H. R., J. M. Jurgensmeier, H. Shin, Q. Deveraux, B. B. Wolf, X. Yang, Q. Zhou, H. M. Ellerby, L. M. Ellerby, D. Bredesen, D. R. Green, J. C. Reed, C. J. Froelich, and G. S. Salvesen. 1998. Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 273:27084-27090. [DOI] [PubMed] [Google Scholar]
- 30.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 31.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]