Abstract
Normal cell growth requires a precisely controlled balance between cell death and survival. This involves activation of different types of intracellular signaling cascades within the cell. While some types of signaling proteins regulate apoptosis, or programmed cell death, other proteins within the cell can promote survival. The serine/threonine kinase PAK4 can protect cells from apoptosis in response to several different types of stimuli. As is the case for other members of the p21-activated kinase (PAK) family, one way that PAK4 may promote cell survival is by phosphorylating and thereby inhibiting the proapoptotic protein Bad. This leads in turn to the inhibition of effector caspases such as caspase 3. Here we show that in response to cytokines which activate death domain-containing receptors, such as the tumor necrosis factor and Fas receptors, PAK4 can inhibit the death signal by a different mechanism. Under these conditions, PAK4 inhibits apoptosis early in the caspase cascade, antagonizing the activation of initiator caspase 8. This inhibition, which does not require PAK4's kinase activity, may involve inhibition of caspase 8 recruitment to the death domain receptors. This role in regulating initiator caspases is an entirely novel role for the PAK proteins and suggests a new mechanism by which these proteins promote cell survival.
The balance between apoptosis and survival in a cell is controlled by various intracellular signaling pathways. A number of different stimuli can trigger apoptosis in cells, including ligation of death domain receptors such as the Fas receptor or the tumor necrosis factor alpha (TNF-α) receptor (2, 52, 72) or deprivation of nutrients such as growth factors or serum (56). Apoptosis is generally mediated by caspase cascades that lead to cleavage or activation of molecules that are important for cell death (9, 59, 68). Cell survival pathways can be mediated by proteins which inhibit the caspase cascades at various stages.
Different types of apoptotic stimuli can trigger cell death by different mechanisms. Fas ligand and the cytokine TNF-α, for example, bind to cell surface receptors and in turn induce the activation and cleavage of the initiator caspases, such as caspase 8 and caspase 10. Once activated, caspase 8 can activate two different apoptotic pathways (27). First, it can directly cleave and activate effector caspases, such as caspases 3 and 7. Effector caspases in turn cleave a number of different target proteins that play important roles in mediating the apoptotic response (59, 68). Second, caspase 8 can activate a mitochondrial pathway which is mediated by the caspase 8 substrate Bid (29, 43, 45, 76). Once it is cleaved by caspase 8, the truncated Bid translocates to the mitochondria, where it interacts with members of the Bcl2 family to promote cytochrome c release. Release of cytochrome c from the mitochondria leads to activation of caspase 9 followed by cleavage and activation of caspase 3, leading to apoptosis (26, 27).
Signaling by cytokine receptors such as the Fas receptor and the TNF receptor (TNFR) actually starts when the receptors trimerize following binding by the ligand. The trimerized receptors recruit a number of proteins through their protein-protein interaction motifs, and these proteins in turn lead to activation of the caspase cascades (2, 72). The main docking protein of TNFR1 is the TNFR-associated death domain protein (TRADD), which binds to the TNFR via an interaction between the respective death domains (33). TRADD then recruits other death domain-containing proteins, including the Fas-associated protein with death domain (FADD) or receptor-interacting protein (RIP), via its death domain (7, 14, 32, 33, 66). Finally, FADD can recruit caspase 8 to the complex (7, 50), which in turn is cleaved and activated, triggering the apoptotic response described above. In contrast, RIP, together with its interacting protein TRAF, signals to the NF-κB pathway which can lead instead to protection from apoptosis (5, 31-33, 44, 65, 78). The major binding partner for the Fas receptor is FADD, which also binds to the receptor through its death domain (7, 14). FADD in turn binds directly to caspase 8, which is activated by oligomerization and self-cleavage (6, 49-51). The signaling network that is formed at the death receptor after stimulation is referred to as the death-inducing signaling complex (DISC) (41).
Throughout development, excess cells are eliminated by the process of apoptosis, while other cells are protected from apoptosis by different mechanisms. A number of cell survival pathways exist for protecting cells from apoptosis. For example, NF-κB can protect cells from apoptosis by inducing the expression of genes involved in cell survival (22). Another example of a protein that can protect cells from apoptosis is the lipid kinase phosphatidylinositol 3-kinase (PI 3-kinase) (12, 21). PI 3-kinase activity is stimulated by exposure to growth factors or serum. This leads to activation of the survival protein AKT. AKT phosphorylates a number of substrates, including the proapoptotic protein Bad, leading to its inactivation (18). Phosphorylation of Bad prevents activation of the mitochondrial pathway and cytochrome c release and thereby protects cells from apoptosis (20). For this reason, many cells are highly sensitive to serum deprivation and undergo apoptosis when grown under low-serum conditions.
The p21-activated kinase (PAK) serine/threonine kinases are a family of protein kinases that may also play a protective role against apoptosis. The PAK family members were originally identified as molecular targets for the Rho GTPases Rac and Cdc42 (1, 4, 8, 10, 47, 48; for reviews, see references 3, 16, and 63). There are six members of the PAK family in mammals, which fall into two categories (group A and group B) based on their amino acid sequences and functions (35). The group A PAKs consist of the closely related PAK1, -2, and -3 (4, 8, 47, 48), whereas the group B PAKs consist of PAK4, -5, and -6 (1, 15, 53, 75). All of the PAKs contain a GTPase binding domain, which binds to the Rho GTPases, and a serine/threonine kinase domain that is similar to the kinase domain of yeast STE20. The group A and B PAKs are only approximately 50% identical to each other in the GTPase binding domain and kinase domain, and they differ completely throughout the rest of their amino acid sequences (35).
Although they were first identified as proteins that play an important role in regulating cytoskeletal organization and cell morphology, the PAKs have also been shown to have important roles in regulating the apoptotic response, although their effects differ under different conditions. For example, PAK2 is cleaved during Fas-induced apoptosis in T cells, most likely by caspase 3. This was shown to lead to its activation, leading to morphological and membrane changes that occur during apoptosis (42, 58, 73). In other studies, however, PAK2 was shown to protect fibroblasts from apoptosis in response to various stimuli, including TNF-α, serum starvation, and UV irradiation (36). Likewise, PAK1, which is not cleaved during apoptosis, was reported to protect cells from apoptosis induced by either serum withdrawal in fibroblasts or interleukin-3 withdrawal in lymphoid cells (62, 67). The survival signal induced by PAK1 is apparently due to direct phosphorylation of Bad on both serines 112 and 136 (62, 67). PAK2 may also operate by phosphorylating Bad, as well as by activating mitogen-activated protein (MAP) kinase pathways (36).
We have found that PAK4 can also protect cells from apoptosis in response to a number of different stimuli (23). As is the case for PAK1, one way that PAK4 may protect cells from apoptosis is by phosphorylating the proapoptotic protein Bad, although unlike PAK1, PAK4 phosphorylates Bad specifically on serine 112 (23). Consistent with this, we have found that effector caspases such as caspase 3 are inhibited in response to several stimuli in PAK4-expressing cells (23). Here we have found, however, that in response to some types of stimuli, notably ligation of TNF or Fas receptors, PAK4 inhibits apoptosis by a mechanism that does not depend on its kinase activity. Under these conditions, we have found that PAK4 inhibits apoptosis by inhibiting the activity of the initiator caspase 8. PAK4 can thereby block the caspase cascade by acting upstream of the mitochondrial pathway and effector caspases, rather than by preventing phosphorylation of Bad and cytochrome c release. This may be mediated in part by inhibition of recruitment of caspase 8 to the death receptors.
MATERIALS AND METHODS
Cell culture and transfections.
The HeLa pLPC and PAK4 cell lines (23) were cultured at 37°C in 5% CO2 and maintained in Dulbecco's modified Eagle's medium (Life Technologies) containing 10% fetal bovine serum (Life Technologies), 4 mM glutamine (Life Technologies), and 50 U of penicillin per ml and 50 μg of streptomycin per ml (Cellgro), supplemented with 1 μg of puromycin per ml. NIH 3T3 pLPC, PAK4wt (55), and PAK4(K350 M) (PAK4KM) cells were maintained in Dulbecco's modified Eagle's medium with glutamine and antibiotics, containing 10% bovine calf serum (HyClone) and 2 μg of puromycin per ml. Before the experiments, cells were passaged and plated in medium without puromycin. HeLa cells (2.7 million per 10-cm-diameter plates) were transfected with Lipofectamine (Life Technologies) according to the manufacturer's instructions. For serum deprivation experiments, cells were washed twice in medium lacking serum and cultured for 24 h in the presence of 0.1, 0.5, or 10% (complete medium) serum. Cells were harvested, fixed, stained with propidium iodide, and analyzed by flow cytometry as described previously (23).
Generation of the PAK4K350 M HeLa and NIH 3T3 cell lines.
ΦNX and BOSC packaging cell lines were transfected by the calcium phosphate method with a retroviral vector plasmid containing a Myc-tagged PAK4K350M sequence (pLPCmycPAK4KM [pNG81]). Viruses were collected and used to infect HeLa or NIH 3T3 cells, respectively, and positive cells were selected with puromycin. Expanded colonies were tested for the expression of the Myc tagged PAK4 protein by Western blotting with Myc or PAK4 antibodies and by immunofluorescence.
Plasmids.
pNG81 (pLPCmycPAK4K350M) was obtained by subcloning the EcoRI-StuI fragment of the PAK4K350M sequence (1) in frame with the Myc epitope tag sequence into the pCDNA3 vector and subsequently inserting it as a HindIII-XhoI fragment in the pLPC vector. The chimeric receptor expression plasmid pCD120a/CD95, containing the extracellular domain of CD120a (p55TNFR) and intracellular domain of CD95 (Fas), was described previously (24). pCDNA3 was used as an empty vector or as buffering DNA for transient transfections. pEBG was used as a control for transfection efficiency for the expression of glutathione S-transferase (GST).
Reagents and antibodies.
Recombinant human TNF-α was purchased from R&D Systems; cycloheximide (CHX) and other reagents were purchased from Sigma unless otherwise stated. Monoclonal caspase 8 antibodies (clone 1C12), rabbit polyclonal cleaved caspase 3 (D175), and human Bid were from Cell Signaling; goat polyclonal TNFR1 antibodies were from R&D systems; monoclonal TNFR1 (H-5) and FLIPs/l (G11) antibodies were from Santa Cruz; monoclonal TRADD (clone 37), RIP (clone 38), and FADD (clone 1) were from BD Transduction Labs; monoclonal poly(ADP-ribose) polymerase (PARP) (clone 4C10-5) antibodies were from PharMingen; monoclonal CD95 Fas antibodies (clone CH-11) were from Immunotech; and monoclonal actin antibodies (clone AC-40) were from Sigma. Monoclonal PAK4 antibodies were described previously (55).
Western blot analysis.
Horseradish peroxidase-conjugated secondary antibodies were from Sigma. Western blot protein bands were visualized by the ECL method (Amersham). Stripping of antibodies from membranes after the ECL reaction was performed according to the manufacturer's instructions. Immobilon-P-polyvinylidene difluoride membranes were from Millipore.
Analysis of TNFR and Fas signaling.
For the analysis of endogenous proteins in whole-cell lysates after TNF-α or Fas stimulation, cells were stimulated and collected as described previously (23) with 10 ng of TNF-α per ml or 500 ng of Fas antibody per ml, with or without 10 μg of CHX per ml. Lysates were then prepared in M2 buffer supplemented with protease and phosphatase inhibitors, and equal amounts of proteins were loaded on gels for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) fractionation and Western blotting.
For the experiments involving coimmunoprecipitation of activated receptors, protocols were adapted from those described previously (38, 77). For the analysis of the endogenous TNFR1 signaling complex, 20 million cells were stimulated with TNF-α for the indicated times, rinsed in cold phosphate-buffered saline, and lysed in 1 ml of Triton lysis buffer (TLB) (25 mM Tris [pH 7.6], 150 mM NaCl, 1% Triton X-100) supplemented with 10 μg of aprotinin and leupeptin per ml, 1 mM Na3VO4, 1 mM EDTA, 30 mM NaF, 2 mM Na pyrophosphate, and 1 mM phenylmethylsulfonyl fluoride for 1 h on ice. One-fiftieth of the clarified lysates was saved and loaded as a control. The rest was added to 40 μl of protein G-agarose beads precoupled with 15 μg of goat TNFR1 antibodies or goat serum (Gibco) as a preimmune control and rotated overnight at 4°C. Immunoprecipitates were washed twice with 1 ml of lysis buffer, twice with lysis buffer containing 0.5 M NaCl, and twice again with lysis buffer. Samples were denatured in sample buffer and analyzed by SDS-PAGE and Western blotting.
For the analysis of the Fas signaling complex, cells were seeded in 10-cm-diameter plates and transfected the next day with the indicated amounts of pCD120a/CD95 DNA (24) and pCDNA plasmid, to a total of 15 μg, with Lipofectamine. At 48 h after transfection, cells were stimulated with TNF-α and lysed with 600 μl of TLB as described above. Immunoprecipitation was were performed as described above, using 5 μg of TNFR antibodies, with the following washes: two washes in lysis buffer, two washes in high-salt buffer (TLB with 1 M NaCl), one wash in lysis buffer, and two final washes in lysis buffer with the detergent omitted.
RESULTS
PAK4 kinase activity is not required for protection from TNF-α-induced apoptosis.
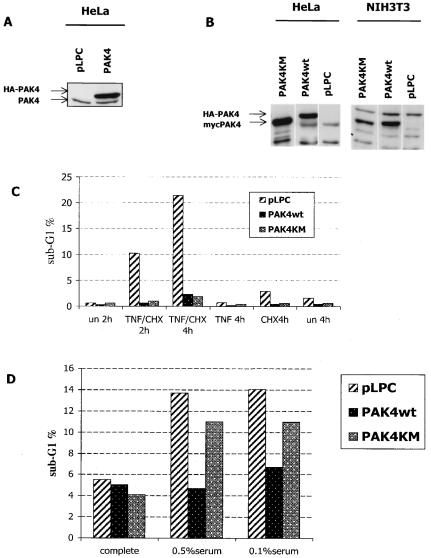
Although PAK4 is widely expressed during development, it is expressed at quite low levels in many adult cells and tissues (1; J. Qu and A. Minden, unpublished results). We have therefore been able to generate stable cell lines which express PAK4 at levels that are higher than those of endogenous PAK4 (Fig. 1). These cell lines provide a good model system for studying the function of PAK4 in apoptosis and survival. We have previously shown that overexpression of PAK4 in different cell lines provides a survival advantage over control cells following application of death-inducing stimuli, protecting cells from undergoing apoptosis. To determine whether PAK4's ability to protect cells from apoptosis depends on its kinase activity, stable cell lines containing empty vector, wild-type PAK4, or kinase-dead PAK4 [PAK4(K350M)/PAK4KM] were treated with death-inducing stimuli. As expected, after TNF-α treatment of HeLa cells or serum deprivation of NIH 3T3 cells, the expression of wild-type PAK4 inhibited apoptosis under both conditions (23) (Fig. 1). Surprisingly, however, although PAK4(K350M) did not protect cells from serum deprivation-induced apoptosis, it was as efficient as wild-type PAK4 in inhibiting TNF-α-induced apoptosis (Fig. 1). This suggests that at least in response to TNF-α stimulation, PAK4 may protect HeLa cells from undergoing apoptosis by a mechanism that does not directly involve its ability to phosphorylate substrates such as Bad.
FIG. 1.
PAK4 kinase activity is not necessary for protection against death stimuli in HeLa cells. Stably transfected PAK4 protein was overexpressed in HeLa and NIH 3T3 cell lines (A) pLPC or PAK4 (PAK4 refers to wild-type PAK4 in all of the figures) HeLa cell extracts were probed with PAK4 antibodies to detect endogenous (PAK4) or stably transfected (hemagglutinin [HA]-PAK4) levels. (B) Expression of HA-tagged wild-type PAK4 (HA-PAK4), Myc-tagged wild type PAK4, Myc-tagged PAK4KM (kinase dead) in HeLa or NIH 3T3 cell lines was detected as described above. (C) Kinase-dead PAK4 cell lines are protected from TNF-α induced apoptosis. HeLa control cells (pLPC) or HeLa cells expressing wild-type (PAK4) or kinase-dead (PAK4KM) PAK4 were left unstimulated (un) or stimulated with 10 ng of TNF-α per ml and 10 μg of CHX per ml for the indicated time in hours (TNF/CHX). Cells were collected, fixed, and stained with propidium iodide, and DNA content was analyzed by flow cytometry. The percentage of cells displaying DNA content lower than the G1 peak (sub-G1%) is shown in the graph. (D) PAK4 kinase activity is required for protection from serum deprivation-induced apoptosis in NIH 3T3 cell lines. NIH 3T3 control cells (pLPC) or cells expressing wild type (PAK4) or kinase-dead (PAK4KM) PAK4 were cultured for 24 h in medium containing 10% (complete), 0.5%, or 0.1% serum. Cells were collected, and apoptosis (sub-G1%) was analyzed by flow cytometry as described above. Data for one representative experiment of at least three independent ones are shown. (NIH 3T3 cells were used in these experiments because HeLa cells were resistant to serum deprivation-induced apoptosis.)
TNF-α-induced activation of initiator caspase 8 and effector caspase 3 is inhibited in PAK4-expressing cell lines.
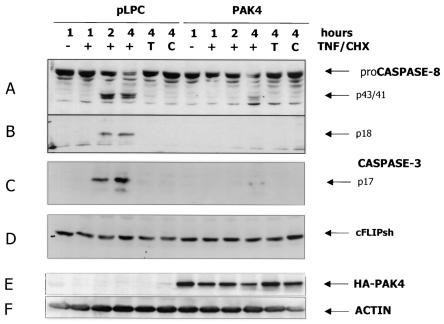
Since PAK4 protects cells from TNF-induced apoptosis independently of its kinase activity, and therefore presumably independently of Bad phosphorylation, we were interested in determining what steps of the TNF-induced death pathway were blocked by PAK4. One possibility is that PAK4 can affect initiator caspases and thereby function upstream of, or independently of, the mitochondrial pathway itself. To test this, cleavage of the initiator caspase 8 and the effector caspase 3 was observed following TNF-α treatment of the different stable HeLa cell lines. Normally, engaging TNFR1 (also called p55 TNFR or CD120a) with TNF-α in the presence of CHX causes initiator pro-caspase 8 (a and b forms [p57/55]) to be processed and cleaved into intermediate and active fragments (p43/41, p20, p18, and p10). Active caspase 8 in turn can either activate the mitochondrial pathway or cleave effector caspases such as caspase 3. As expected, after 2 h of stimulation, considerable amounts of intermediate and active caspase 8 fragments (p43/41 and p18) could be detected in the control cells. By 4 h most of the proenzyme had undergone processing, as detectable by the decreased amount of full-length protein (Fig. 2). Effector caspase 3 activation followed a similar time course, as judged by the appearance of the active p17 fragment. In contrast, in cells overexpressing PAK4, cleavage of both caspase 8 and caspase 3 was dramatically reduced and delayed (Fig. 2). As previously observed (23), the terminal apoptotic process is actually delayed rather than completely blocked in PAK4 cells, since caspase activation (see also Fig. 4) and DNA fragmentation (not shown) eventually occur at the latest time points but remain substantially lower than in control cells, indicating that the onset of the apoptotic program is delayed. Thus, following TNF-α stimulation, not only was effector caspase 3 activation inhibited, but initiator caspase 8 was also affected. This did not appear to be due to changes in the levels of cellular Fas-associated death domain-like interleukin-1-converting-enzyme-like inhibitory protein (cFLIP), which has been shown to have a role in inhibiting caspase 8 activation in T cells (34, 40, 61) (Fig. 2). These results suggest that the role of PAK4 in inhibiting apoptosis by TNF-α resides at the level of initiator caspases.
FIG. 2.
Activation of both effector and initiator caspase is affected in PAK4 cell lines. HeLa control (pLPC) or PAK4 cells were left untreated (−) or stimulated with 10 ng of TNF-α per ml and 10 μg of CHX per ml (+), with TNF-α alone (T), or with CHX alone (C) for the indicated times in hours. Cells were harvested, and equal amounts of protein lysates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with antibodies against caspase 8 (A and B), cleaved caspase 3 (C), or cFLIP (D). PAK4 expression levels and equal loading of the samples were detected by probing the extracts with hemagglutinin (HA) (E) and actin (F) antibodies. Specific bands of caspase 8 a and b proenzymes (proCaspase 8) and proteolytic products (p43/41), active p18 fragment, caspase 3 active p17 fragment, cFLIPshort, HA-PAK4, and actin are indicated by arrows.
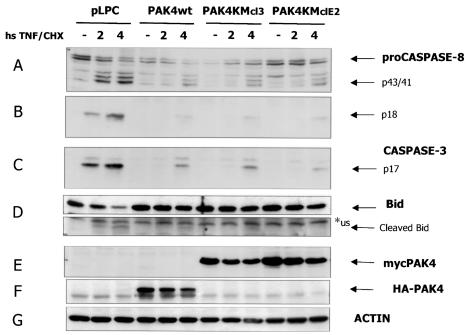
FIG. 4.
Cleavage of initiator caspase substrate Bid is affected in wild-type and kinase-dead PAK4 cell lines. HeLa control cells (pLPC) or cells expressing wild-type PAK4 (PAK4) or two independent kinase-dead cell lines (PAK4KMcl3 and PAK4KMclE2) were left unstimulated (−) or stimulated with 10 ng of TNF-α per ml and 10 μg of CHX per ml for the indicated times in hours (hs TNF/CHX). Cells were harvested, and equal amounts of protein lysates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with antibodies against caspase 8 (A and B), cleaved caspase 3 (B), or Bid (D). PAK4 expression levels and equal loading of the samples were detected by probing the extracts with Myc (E), hemagglutinin (HA) (F), and actin (G) antibodies. Specific bands of caspase 8 a and b proenzymes (proCaspase 8) and proteolytic products (p43/41), active p18 fragment, caspase 3 active p17 fragment, or Bid full-length protein or p15 active fragment (cleaved Bid), wild-type HA-PAK4 or kinase-dead myc-PAK4, and actin are indicated by arrows. An unspecific reactive band (us) migrating near the p15 Bid fragment is indicated by an asterisk.
PAK4-expressing cells are resistant to anti-FAS-induced apoptosis.
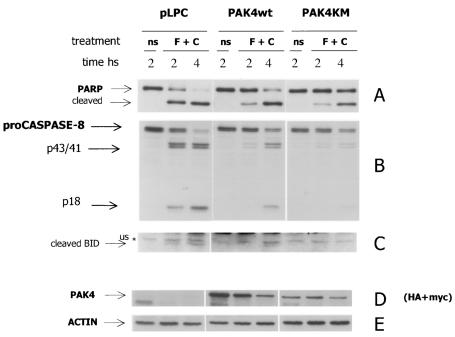
To determine whether PAK4 affects other death receptor cascades in addition to the TNF-α cascade, stable HeLa cell lines were treated with anti-Fas receptor antibodies. Apoptosis was then detected by observing cleavage of the effector caspase substrate PARP (Fig. 3). In control cells, effector caspase activation (as judged by the appearance of the p85 PARP cleavage product) was detected as early as 2 h after anti-FAS stimulation in the presence of CHX, when more than 50% of the PARP protein had been cleaved, as most of the cells had started the apoptotic program. By 4 h the full-length p116 PARP protein was detectable only at very low levels, as most of the protein had been cleaved. Conversely, when PAK4 cells were treated with the same stimuli, caspase activation was significantly delayed. After 2 h only a small portion of PARP had been cleaved in the PAK4 cells, and at 4 h the full-length p116 was still detectable. (Fig. 3). A similar delay in the activation of caspases was observed in kinase-dead PAK4 cells. Importantly, as in the TNF-α-treated cells, caspase 8 cleavage was also significantly impaired in the PAK4-containing Fas-treated cells (Fig. 3). Thus, as with TNF-α-treated cells, Fas-induced apoptosis and caspase 8 activation were reduced in the presence of both wild-type and kinase-dead PAK4.
FIG. 3.
Stable cell lines expressing PAK4 are resistant to anti-FAS-induced apoptosis. HeLa control (pLPC) and wild-type (PAK4) or kinase-dead (PAK4KM) PAK4 cells were left unstimulated (ns) or treated with 500 ng of anti-Fas per ml and 10 μg of CHX per ml (F + C), for the indicated time in hours. Cells were harvested, and equal amounts of protein lysates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with antibodies against PARP (A), caspase 8 (B), or Bid (C). PAK4 expression levels and equal loading of the samples were detected by probing the extracts with hemagglutinin (HA) and Myc antibodies (D) and actin antibodies (E). (Wild-type PAK4 is HA tagged, and PAK4KM is Myc tagged). Specific bands of PARP full-length p116 protein or cleaved p85 product, caspase 8 a and b proenzymes (proCaspase 8) and proteolytic products (p43/41), active p18 fragment, active Bid p15 fragment (cleaved Bid), wild type HA-PAK4 or kinase dead myc-PAK4 (PAK4), and actin are indicated by arrows. An unspecific reactive band (us) migrating near to the p15 Bid fragment is indicated by an asterisk.
Suppression of Bid cleavage parallels caspase 8 inhibition in PAK4-expressing cells.
Although caspase 8 activation is necessary for death receptor-induced apoptosis (39, 71), in many cell types (also described as type II cells) the mitochondrial pathway is also required in order to induce full activation of effector caspases and cell death (60). Cleavage and activation of the proapoptotic Bcl2 family member Bid (by initiator caspase 8) was shown to be necessary for the activation of the mitochondrial pathway by death receptors (29, 43, 45, 76). In order to rule out the possibility that the small amount of caspase 8 activity still present in PAK4 cells was still sufficient to cause normal activation of this pathway, the caspase 8 substrate Bid was analyzed. When TNF-α-treated control cell lysates were analyzed (Fig. 4D), the full-length Bid protein decreased over time, and the cleavage product p15 was detectable as early as 2 h, coincident with the appearance of the active caspase 8 fragment p18 (as well as the caspase 3 fragment p17). In contrast, when PAK4-expressing cells were analyzed, the level of full-length Bid remained high at the analyzed time points. Only a small amount of p15 was detectable at the latest time point, and this coincided with a slight increase in the caspase 8 p18 fragment and the caspase 3 p17 fragment (Fig. 4). Similar results were obtained when anti-FAS-treated cells were analyzed (Fig. 3C). Most importantly, they indicate that the low level of caspase 8 seen in response to TNF-α was not sufficient to cause normal activation of the mitochondrial pathway. These results lend further support to the hypothesis that signaling by the death receptors TNFR1 and Fas is most likely compromised at the level of caspase 8 activation in PAK4-expressing cell lines.
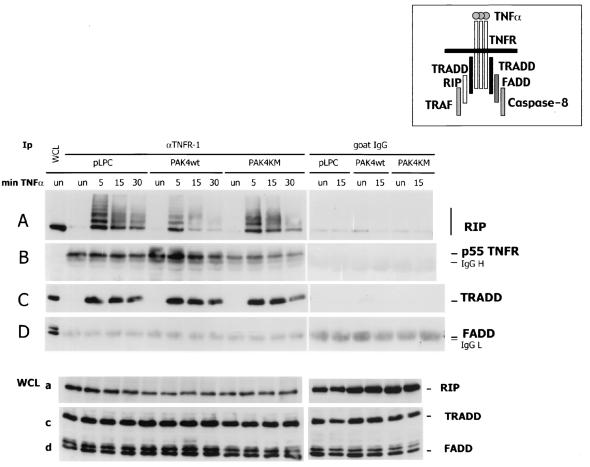
TNF-α-induced association of TRADD and RIP with the TNFR1 receptor is not inhibited by PAK4 expression.
Upon binding with their ligands, receptors of the TNF ligand family trimerize and recruit a number of proteins through their protein-protein interaction motifs (2, 72), as described in the introduction (Fig. 5, diagram). Given the defects in initiator caspase activation in PAK4-expressing cells, we were interested in determining whether there were defects in the recruitment of TNFR1-interacting proteins in the presence of PAK4. Cells containing empty vector, wild-type PAK4, or kinase-dead PAK4 were stimulated with TNF-α, and lysates were collected at different time points. After immunopurification of the TNFR1, precipitates were analyzed by Western blotting for the presence of the different interacting proteins (Fig. 5). After 5 min of stimulation, TNF-α-dependent binding of TRADD and RIP to the p55 receptor could be detected. This interaction decreased at a later time points (15 and 30 min). (Under these conditions, it was not possible to detect the association of FADD [Fig. 5D] or caspase 8 [not shown]). While in wild-type PAK4 cells a lower recruitment of RIP was observed, this defect was not detected in PAK4KM cells. Our results indicate that expression of PAK4 had no major effect on recruitment of TRADD and RIP to the TNFR.
FIG. 5.
TNF-α-induced association of TRADD with the TNFR1 receptor is not inhibited by PAK4 expression. HeLa control (pLPC) or wild-type (PAK4WT) or kinase dead (PAK4KM) PAK4 cells were left untreated (un) or stimulated with 10 ng of TNF-α per ml for the indicated times in minutes (min TNF-α.). Cells were harvested, and equal volumes of protein lysates (WCL) were analyzed by Western blotting as described below or used for immunopurification (Ip) (1/100 of total extracts) with goat antibodies against the extracellular domain of p55 TNFR (TNFR1) or preimmune goat serum (goat immunoglobulin G [IgG]). Immunopurified complexes and whole-cell lysates were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with mouse antibodies against RIP (A and a), TNFR1 (B), TRADD (C and c), or FADD (D and d). Specific bands are indicated by arrows on the right. Migration positions of IgG heavy (IgG H) and light (IgG L) chains are also indicated. A diagram of TNFR-interacting proteins is also shown.
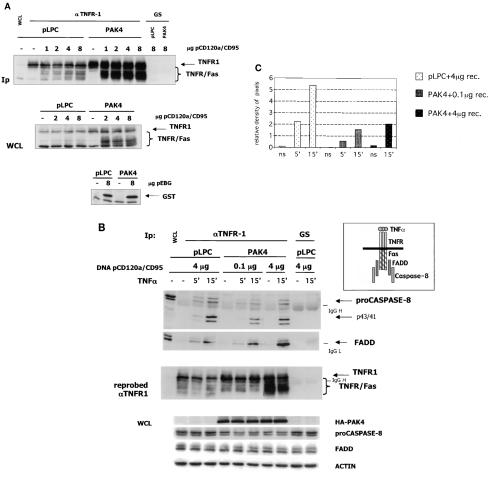
Caspase 8 binding to the Fas DISC is partially blocked in PAK4-expressing cells.
Recruitment of FADD and caspase 8 to the endogenous TNFR1 could not be detected in the experiment described above. In fact, in previous work, caspase 8 binding to TNFR1 could be detected only when the proteins were overexpressed (6). Therefore, in order to analyze the recruitment of FADD and caspase 8 to death domain receptors, we evaluated their binding after activation of a transfected Fas receptor. To visualize the Fas receptor DISC, cells were transfected with a receptor that contains the extracellular domain of CD120a (p55 TNFR1) and the intracellular domain of CD95 (Fas/Apo-1) (24) (Fig. 6A). Control cells consistently expressed only low levels of the transfected receptor, even when large amounts of DNA were transfected. This was probably due to the fact that apoptosis occurred when high levels of receptor were expressed (24). In contrast, when PAK4 cells were transfected with the same amounts of receptor DNA, high expression levels were obtained (Fig. 6A), even though both cell types had equivalent transfection efficiencies (see expression of GST, bottom panel of Fig. 6A). Therefore, in order to compare DISC formation in the two cell lines, the amount of receptor expression plasmid DNA was titrated in order to obtain similar levels of the transfected receptor in both cell types. Cells were then stimulated, and binding of the DISC proteins was observed following receptor immunoprecipitation (Fig. 6B). The amount of FADD that was recruited to the receptor appeared to be the same for both control and PAK4 cells (Fig. 6B). However, the overall recruitment of caspase 8 was diminished in PAK4-expressing cells, as illustrated by densitometry quantitation of the caspase 8 and receptor bands of the immunoprecipitates (Fig. 6C). Processing of the small amount of caspase 8 protein that was present in the complex was not inhibited, since the partially cleaved precursors were detectable (Fig. 6B). Similar results were obtained with PAK4KM cells, where high levels of transfected receptor and lower caspase 8 recruitment were observed (not shown). These data indicate that pro-caspase 8 recruitment at the Fas DISC is reduced in the presence of excess PAK4.
FIG. 6.
PAK4-expressing cells show decreased Fas DISC formation. (A) The transfected TNFR/Fas chimeric receptor in highly expressed in PAK4 cell lines. HeLa control (pLPC) or wild-type PAK4 cells were transfected with empty vector (−) or the indicated amounts (in micrograms) of TNFR/Fas chimeric receptor (pCD120a/CD95) or GST (pEBG) expression vector. After 48 h, cells were harvested and equal volumes of protein lysates (1/100 of total extracts) (WCL) were analyzed by Western blotting as described below or used for immunopurification (Ip) with goat antibodies against the extracellular domain of p55 TNFR (αTNFR1) or preimmune goat serum (GS). Immunopurified complexes and whole-cell lysates were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with mouse antibodies against TNFR1 or GST. Specific bands are indicated by arrows on the right. Migration positions of the TNFR/Fas chimera are indicated by a brace. (B) Recruitment of caspase 8 is compromised in stable PAK4 cell lines. HeLa control (pLPC) or wild-type PAK4 cells were transfected with the indicated amounts (in micrograms) of TNFR/Fas chimeric receptor (DNA pCD120a/CD95) expression vector. After 48 h, cells were left untreated (−) or stimulated with 10 ng of TNF-α per ml for the indicated time in minutes and harvested. Equal volumes of protein lysates were analyzed by Western blotting as described below or used for immunopurification (1/100 of total extracts) with goat antibodies against the extracellular domain of p55 TNFR or preimmune goat serum. Immunopurified complexes were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with mouse antibodies against caspase 8 or FADD. The blot probed with caspase 8 antibodies was stripped and reprobed with mouse antibodies against TNFR1 to verify TNFR1 and TNFR/Fas expression levels (as indicated at the left of the middle panel). Specific bands are indicated by arrows on the right. Migration positions of the TNFR/Fas chimera are indicated by a brace. Migration positions of IgG heavy and light chains are also indicated. To verify equal loading, the lysates (WCL) were analyzed by Western blotting for PAK4 (HA-PAK4), full-length caspase 8 (proCaspase8), FADD, and actin, as indicated. A diagram of the proteins interacting with the Fas intracellular domain of the TNFR/Fas chimeric receptor is also shown. (C) Quantitation of the caspase 8 signal in the DISC shows reduced caspase 8 protein per amount of receptor in PAK4 cells. The pro-caspase 8 bands of the top panel of panel B and the TNFR1 and TNFR/Fas bands (receptor) of the middle panel of panel B (pLPC and PAK4 lanes) were quantitated by densitometry (NIH Image). The densities of pixels in equal areas were measured, and backgrounds (measured in the GS Ip lanes) were subtracted. Receptor amounts for the single Ips were expressed as values relative to the amount of receptor present in the pLPC (ns) Ip lane. The relative amount of caspase 8 pixels shown in the graph was obtained by dividing the measured value of caspase 8 by its relative receptor levels (relative density of pixels). (The lowest relative value obtained [PAK4 ns lane] was similar to background levels and was set as the zero point of the y axis.)
DISCUSSION
Since inhibition of apoptosis can lead to pathologies such as cancer (28), it is extremely important to understand the molecular mechanism by which cells protect themselves from apoptosis. Our previous studies indicate that PAK4 overexpression protects cells from several different kinds of apoptotic stimuli (23). While other PAKs can also promote cell survival, PAK4 appears to be most directly involved in the oncogenic process. Unlike other PAKs, for example, PAK4 is highly transforming in fibroblasts (55). Furthermore, while it is expressed at low levels in most adult tissues, PAK4 is overexpressed in a wide array of tumor cell lines (11). Our results raise the possibility that the role of PAK4 in transformation may be mediated at least in part by PAK4's antiapoptotic role. It is therefore of great interest to understand the mechanism by which PAK4 exerts its survival effects in cells.
One way that PAK4 may protect cells from apoptosis is by phosphorylating the proapoptotic protein Bad (23), and other PAK family members may operate by a similar mechanism (36, 62, 67, 74). However, we found that although in NIH 3T3 fibroblasts PAK4 kinase activity is required for, and correlates with, the cell survival response following serum deprivation (23), it is not required for protection from death receptor-induced apoptosis in epithelial cells. This indicates that in response to death receptor signals, PAK4 can operate by a mechanism that does not depend on its kinase activity. This effect was also evident in transient-transfection experiments, where both wild-type and kinase-dead PAK4 expression plasmids conferred lower sensitivity to TNF-induced apoptosis (not shown).
Although we previously showed that effector caspases are inhibited in PAK4-expressing cells (23), here we have found that in response to death receptors, the initiator caspase 8 is also inhibited when PAK4 is overexpressed. Cleavage of the caspase 8 substrate Bid is also inhibited in PAK4-expressing cells, which further supports the idea that PAK4 functions at the level of caspase 8. In HeLa cells after TNF/CHX treatment, caspase 8 and Bid cleavage has also been shown to occur (with time courses similar to the ones we observed) under conditions where the mitochondrial pathway is inhibited and caspase 3 activation is not observed (54). In this perspective, Bid cleavage thus occurs because it is a target for caspase 8, and its cleavage cannot be explained by a possible feedback loop. Taken together, these results suggest that PAK4 can block apoptosis early in the caspase cascade.
We do not yet know how PAK4 inhibits caspase 8 activity. However, we have ruled out the possibility that PAK4 functions by causing changes in the protein levels of FLIP, which can affect the activity of caspase 8 (13, 34, 40). Receptor activation also appeared to occur normally in the presence of overexpressed PAK4. This was evident by a normal interaction between TNFR1 and its binding protein TRADD in the PAK4-expressing cells, as well as between the Fas receptor and FADD. However, recruitment of caspase 8 (which binds via FADD) to the Fas receptor was suppressed in the PAK4-expressing cells. Processing to the intermediate (inactive) fragments seemed to occur normally, but it has been shown that partial processing is not sufficient to induce apoptosis (40, 70). These data, together with the inefficient formation of the active p18 fragment observed in cell lysates, indicate that the low recruitment of caspase 8 that we observe in PAK4 cells is not sufficient to induce full activation. We were not able to detect caspase 8 recruitment to the activated endogenous TNFR1 receptor in any of the cell lines. This is most likely due to the fact that this interaction, although well established, is observed only when the proteins are ectopically overexpressed (6). We expect that caspase 8 recruitment to the TNFR1 is also inhibited by PAK4, however, since this recruitment is also mediated by FADD. Furthermore, caspase 8 cleavage was similarly inhibited in both TNFR1- and Fas-stimulated cells.
The regulation of caspase 8 activity and recruitment is a totally new function for PAK4, and our results suggest a novel mechanism for PAK4 inhibition of apoptosis. Recently, caspase 8 has also been implicated as a target for several other cell survival proteins. For example, AKT, a survival protein and target of the PI 3-kinase pathway, was previously been shown to inhibit apoptosis by phosphorylating Bad as well as by activating the NF-κB pathway (19, 46, 57, 67). Recently, however, activated AKT has also been shown to inhibit caspase 8 activation, as well as its recruitment to the Fas DISC, and this is thought to contribute to its role in protecting T cells from Fas-induced apoptosis (37). PI 3-kinase, which can activate AKT, was also recently shown to protect cells from apoptosis by inhibiting caspase 8 activity. However, unlike in the previous study, caspase 8 recruitment to the DISC was found to be unaffected by PI 3-kinase. Instead, its processing seems to be impaired via a mechanism that controls lateral diffusion and aggregation of CD95 (69, 70). Other signaling proteins that have recently been shown to protect cells from Fas-induced apoptosis via inhibition of caspase 8 include protein kinase C and MAP kinase (25, 30). In the case of protein kinase C, recruitment of both FADD and caspase 8 to the receptor was inhibited, while DISC assembly was unaffected in response to MAP kinase. Thus, while Bad phosphorylation and the mitochondrial pathway have been considered to be primary targets for several cell survival pathways, caspase 8 is emerging as a new target for several important apoptosis inhibitors.
Our results indicate that PAK4 is one of the growing number of cell survival proteins recently shown to inhibit apoptosis at the level of caspase 8 activity. It will be interesting to determine the molecular mechanism by which PAK4 inhibits caspase 8 activity and, possibly, recruitment to the DISC. One possibility is that it operates by activating one of the pathways described above. However, incubation with either MAP kinase inhibitors or PI 3-kinase inhibitors did not affect PAK4's ability to inhibit caspase 8 activity and apoptosis (data not shown). Since a kinase-inactive form of PAK4 can inhibit caspase 8 activity as efficiently as wild-type PAK4 (Fig. 4), our results indicate that PAK4 can operate a mechanism that does not depend on its kinase activity. Interestingly, other members of the PAK family have been shown to regulate some cellular functions, such as cytoskeletal organization, independently of their kinase activity (17, 64). It has been suggested, therefore, that PAKs operate not only as protein kinases but also as scaffold proteins that mediate important protein-protein interactions (3, 17). This raises the possibility that PAK4 may bind to caspase 8 or other components of the DISC and thereby directly inhibit recruitment of caspase 8 to the receptor. Another possibility is that PAK4 could control the recruitment of caspase 8 inhibitors or could inhibit the dissociation of silencers of death domains.
In summary, we have found that PAK4 inhibits death domain-induced apoptosis by inhibiting the initiator caspase 8. Similarly, recruitment of caspase 8 to death domain receptors is also partially inhibited in PAK4-expressing cells. This is a novel function for PAK4, and our results indicate that in addition to phosphorylating, on serine 112, the proapoptotic protein Bad (23), PAK4 can also function by inhibiting the activities of initiator caspases.
Acknowledgments
This work was supported by a grant from the NIH (CA76342) and by an American Scientist Development grant from the American Heart Association to A.M.
REFERENCES
- 1.Abo, A., J. Qu, M. S. Cammarano, C. Dan, A. Fritsch, V. Baud, B. Belisle, and A. Minden. 1998. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 17:6527-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia, S., and R. A. Cerione. 1999. PAK to the future. Trends Cell Biol. 9:350-355. [DOI] [PubMed] [Google Scholar]
- 4.Bagrodia, S., S. J. Taylor, C. L. Creasy, J. Chernoff, and R. A. Cerione. 1995. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 270:22731-22737. [DOI] [PubMed] [Google Scholar]
- 5.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 6.Boldin, M. P., T. M. Goncharov, Y. V. Goltsev, and D. Wallach. 1996. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85:803-815. [DOI] [PubMed] [Google Scholar]
- 7.Boldin, M. P., E. E. Varfolomeev, Z. Pancer, I. L. Mett, J. H. Camonis, and D. Wallach. 1995. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 270:7795-7798. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. L., L. Stowers, M. Baer, J. Trejo, S. Coughlin, and J. Chant. 1996. Human Ste20 homologue hPAK1 links GTPases to the JNK MAP kinase pathway. Curr. Biol. 6:598-605. [DOI] [PubMed] [Google Scholar]
- 9.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 10.Burbelo, P. D., D. Drechsel, and A. Hall. 1995. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 270:29071-29074. [DOI] [PubMed] [Google Scholar]
- 11.Callow, M. G., F. Clairvoyant, S. Zhu, B. Schryver, D. B. Whyte, J. R. Bischoff, B. Jallal, and T. Smeal. 2002. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277:550-558. [DOI] [PubMed] [Google Scholar]
- 12.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. [DOI] [PubMed] [Google Scholar]
- 13.Chang, D. W., Z. Xing, Y. Pan, A. Algeciras-Schimnich, B. C. Barnhart, S. Yaish-Ohad, M. E. Peter, and X. Yang. 2002. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 21:3704-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinnaiyan, A. M., K. O'Rourke, M. Tewari, and V. M. Dixit. 1995. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81:505-512. [DOI] [PubMed] [Google Scholar]
- 15.Dan, C., N. Nath, M. Liberto, and A. Minden. 2002. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol. Cell. Biol. 22:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels, R. H., and G. M. Bokoch. 1999. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24:350-355. [DOI] [PubMed] [Google Scholar]
- 17.Daniels, R. H., P. S. Hall, and G. M. Bokoch. 1998. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 17:754-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 19.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 20.Downward, J. 1999. How BAD phosphorylation is good for survival. Nat. Cell Biol. 1:E33-E35. [DOI] [PubMed] [Google Scholar]
- 21.Downward, J. 1998. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 8:49-54. [DOI] [PubMed] [Google Scholar]
- 22.Foo, S. Y., and G. P. Nolan. 1999. NF-kappaB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 15:229-235. [DOI] [PubMed] [Google Scholar]
- 23.Gnesutta, N., J. Qu, and A. G. Minden. 2001. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J. Biol. Chem. 276:14414-14419. [DOI] [PubMed] [Google Scholar]
- 24.Goltsev, Y. V., A. V. Kovalenko, E. Arnold, E. E. Varfolomeev, V. M. Brodianskii, and D. Wallach. 1997. CASH, a novel caspase homologue with death effector domains. J. Biol. Chem. 272:19641-19644. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Angelats, M., and J. A. Cidlowski. 2001. Protein kinase C regulates FADD recruitment and death-inducing signaling complex formation in Fas/CD95-induced apoptosis. J. Biol. Chem. 276:44944-44952. [DOI] [PubMed] [Google Scholar]
- 26.Green, D., and G. Kroemer. 1998. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 8:267-271. [DOI] [PubMed] [Google Scholar]
- 27.Green, D. R. 1998. Apoptotic pathways: the roads to ruin. Cell 94:695-698. [DOI] [PubMed] [Google Scholar]
- 28.Green, D. R., and G. I. Evan. 2002. A matter of life and death. Cancer Cell 1:19-30. [DOI] [PubMed] [Google Scholar]
- 29.Gross, A., X. M. Yin, K. Wang, M. C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S. J. Korsmeyer. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156-1163. [DOI] [PubMed] [Google Scholar]
- 30.Holmstrom, T. H., I. Schmitz, T. S. Soderstrom, M. Poukkula, V. L. Johnson, S. C. Chow, P. H. Krammer, and J. E. Eriksson. 2000. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 19:5418-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu, H., J. Huang, H. B. Shu, V. Baichwal, and D. V. Goeddel. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4:387-396. [DOI] [PubMed] [Google Scholar]
- 32.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 33.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 34.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 35.Jaffer, Z. M., and J. Chernoff. 2002. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34:713-717. [DOI] [PubMed] [Google Scholar]
- 36.Jakobi, R., E. Moertl, and M. A. Koeppel. 2001. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J. Biol. Chem. 276:16624-16634. [DOI] [PubMed] [Google Scholar]
- 37.Jones, R. G., A. R. Elford, M. J. Parsons, L. Wu, C. M. Krawczyk, W. C. Yeh, R. Hakem, R. Rottapel, J. R. Woodgett, and P. S. Ohashi. 2002. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J. Exp. Med. 196:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones, S. J., E. C. Ledgerwood, J. B. Prins, J. Galbraith, D. R. Johnson, J. S. Pober, and J. R. Bradley. 1999. TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J. Immunol. 162:1042-1048. [PubMed] [Google Scholar]
- 39.Juo, P., C. J. Kuo, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 40.Kirchhoff, S., W. W. Muller, A. Krueger, I. Schmitz, and P. H. Krammer. 2000. TCR-mediated up-regulation of c-FLIPshort correlates with resistance toward CD95-mediated apoptosis by blocking death-inducing signaling complex activity. J. Immunol. 165:6293-6300. [DOI] [PubMed] [Google Scholar]
- 41.Kischkel, F. C., S. Hellbardt, I. Behrmann, M. Germer, M. Pawlita, P. H. Krammer, and M. E. Peter. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, N., H. MacDonald, C. Reinhard, R. Halenbeck, A. Roulston, T. Shi, and L. T. Williams. 1997. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc. Natl. Acad. Sci. USA 94:13642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, H., H. Zhu, C.-J. Xy, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 44.Liu, Z. G., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 45.Luo, L., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 46.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol. Cell. Biol. 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367:40-46. [DOI] [PubMed] [Google Scholar]
- 48.Martin, G. A., G. Bollag, F. McCormick, and A. Abo. 1995. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 14:1970-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 51.Muzio, M., B. R. Stockwell, H. R. Stennicke, G. S. Salvesen, and V. M. Dixit. 1998. An induced proximity model for caspase-8 activation. J. Biol. Chem. 273:2926-2930. [DOI] [PubMed] [Google Scholar]
- 52.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 53.Pandey, A., I. Dan, T. Z. Kristiansen, N. M. Watanabe, J. Voldby, E. Kajikawa, R. Khosravi-Far, B. Blagoev, and M. Mann. 2002. Cloning and characterization of PAK5, a novel member of mammalianp21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene 21:3939-3948. [DOI] [PubMed] [Google Scholar]
- 54.Perez, D., and E. White. 2000. TNF-alpha signals apoptosis through a bid-dependent conformational change in Bax that is inhibited by E1B 19K. Mol. Cell 6:53-63. [PubMed] [Google Scholar]
- 55.Qu, J., M. S. Cammarano, Q. Shi, K. C. Ha, P. de Lanerolle, and A. Minden. 2001. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol. Cell. Biol. 21:3523-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raff, M. C. 1992. Social controls on cell survival and cell death. Nature 356:397-400. [DOI] [PubMed] [Google Scholar]
- 57.Romashkova, J. A., and S. S. Makarov. 1999. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 58.Rudel, T., and G. M. Bokoch. 1997. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science 276:1571-1574. [DOI] [PubMed] [Google Scholar]
- 59.Salvesen, G. S., and V. M. Dixit. 1997. Caspases: intracellular signaling by proteolysis. Cell 91:443-446. [DOI] [PubMed] [Google Scholar]
- 60.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatin, P. H. Krammer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scaffidi, C., I. Schmitz, P. H. Krammer, and M. E. Peter. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274:1541-1548. [DOI] [PubMed] [Google Scholar]
- 62.Schurmann, A., A. F. Mooney, L. C. Sanders, M. A. Sells, H. G. Wang, J. C. Reed, and G. M. Bokoch. 2000. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol. Cell. Biol. 20:453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sells, M. A., and J. Chernoff. 1997. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 7:162-167. [DOI] [PubMed] [Google Scholar]
- 64.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7:202-210. [DOI] [PubMed] [Google Scholar]
- 65.Shu, H. B., M. Takeuchi, and D. V. Goeddel. 1996. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc. Natl. Acad. Sci. USA 93:13973-13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanger, B. Z., P. Leder, T. H. Lee, E. Kim, and B. Seed. 1995. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81:513-523. [DOI] [PubMed] [Google Scholar]
- 67.Tang, Y., H. Zhou, A. Chen, R. N. Pittman, and J. Field. 2000. The Akt proto-oncogene links Ras to Pak and cell survival signals. J. Biol. Chem. 275:9106-9109. [DOI] [PubMed] [Google Scholar]
- 68.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 69.Varadhachary, A. S., M. Edidin, A. M. Hanlon, M. E. Peter, P. H. Krammer, and P. Salgame. 2001. Phosphatidylinositol 3′-kinase blocks CD95 aggregation and caspase-8 cleavage at the death-inducing signaling complex by modulating lateral diffusion of CD95. J. Immunol. 166:6564-6569. [DOI] [PubMed] [Google Scholar]
- 70.Varadhachary, A. S., M. E. Peter, S. N. Perdow, P. H. Krammer, and P. Salgame. 1999. Selective up-regulation of phosphatidylinositol 3′-kinase activity in Th2 cells inhibits caspase-8 cleavage at the death-inducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J. Immunol. 163:4772-4779. [PubMed] [Google Scholar]
- 71.Varfolomeev, E. E., M. Schuchmann, V. Luria, N. Chiannilkulchai, J. S. Beckmann, I. L. Mett, D. Rebrikov, V. M. Brodianski, O. C. Kemper, O. Kollet, T. Lapidot, D. Soffer, T. Sobe, K. B. Avraham, T. Goncharov, H. Holtmann, P. Lonai, and D. Wallach. 1998. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9:267-276. [DOI] [PubMed] [Google Scholar]
- 72.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331-367. [DOI] [PubMed] [Google Scholar]
- 73.Walter, B. N., Z. Huang, R. Jakobi, P. T. Tuazon, E. S. Alnemri, G. Litwack, and J. A. Traugh. 1998. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. J. Biol. Chem. 273:28733-28739. [DOI] [PubMed] [Google Scholar]
- 74.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 75.Yang, F., X. Li, M. Sharma, M. Zarnegar, B. Lim, and Z. Sun. 2001. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J. Biol. Chem. 276:15345-15353. [DOI] [PubMed] [Google Scholar]
- 76.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, S. Q., A. Kovalenko, G. Cantarella, and D. Wallach. 2000. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity 12:301-311. [DOI] [PubMed] [Google Scholar]