Abstract
An 851-residue open reading frame (ORF) called SpaE has been discovered in the subtilin (spa) operon. Interruption of this ORF with a chloramphenicol acetyltransferase gene destroys the ability of Bacillus subtilis LH45 delta c (a derivative of B. subtilis 168) to produce subtilin, which is an antimicrobial peptide belonging to the class of ribosomally synthesized peptide antibiotics called lantibiotics. SpaE shows strong homology to NisB, which is in the nisin (nis) operon in Lactococcus lactis ATCC 11454. Despite the strong sequence homology between SpaE and NisB, the spaE and nisB genes occupy very different locations in their respective operons, indicating that they have been evolving separately for a long time. Primer extension analysis was employed to identify a promoter upstream from the spaE gene, which appears to define the 5' end of the spa operon, which contains four other ORFs (Y. J. Chung, M. T. Steen, and J. N. Hansen, J. Bacteriol. 174:1417-1422, 1992).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee S., Hansen J. N. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J Biol Chem. 1988 Jul 5;263(19):9508–9514. [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
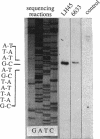
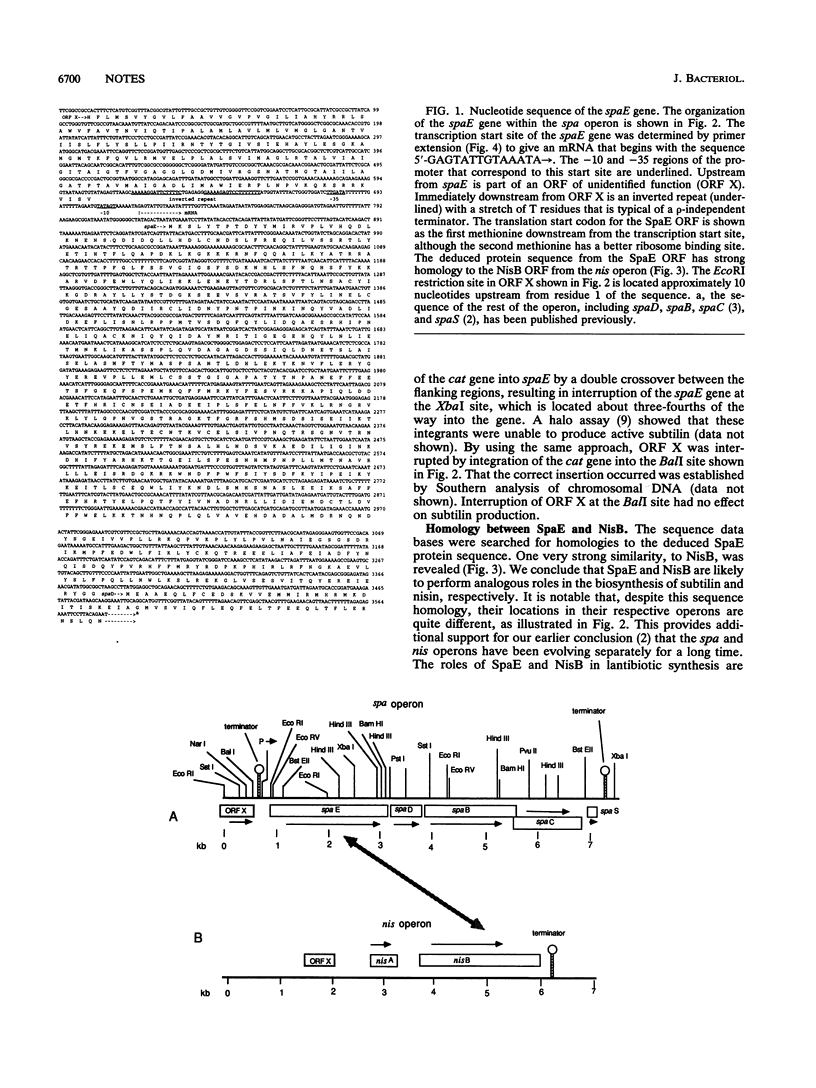
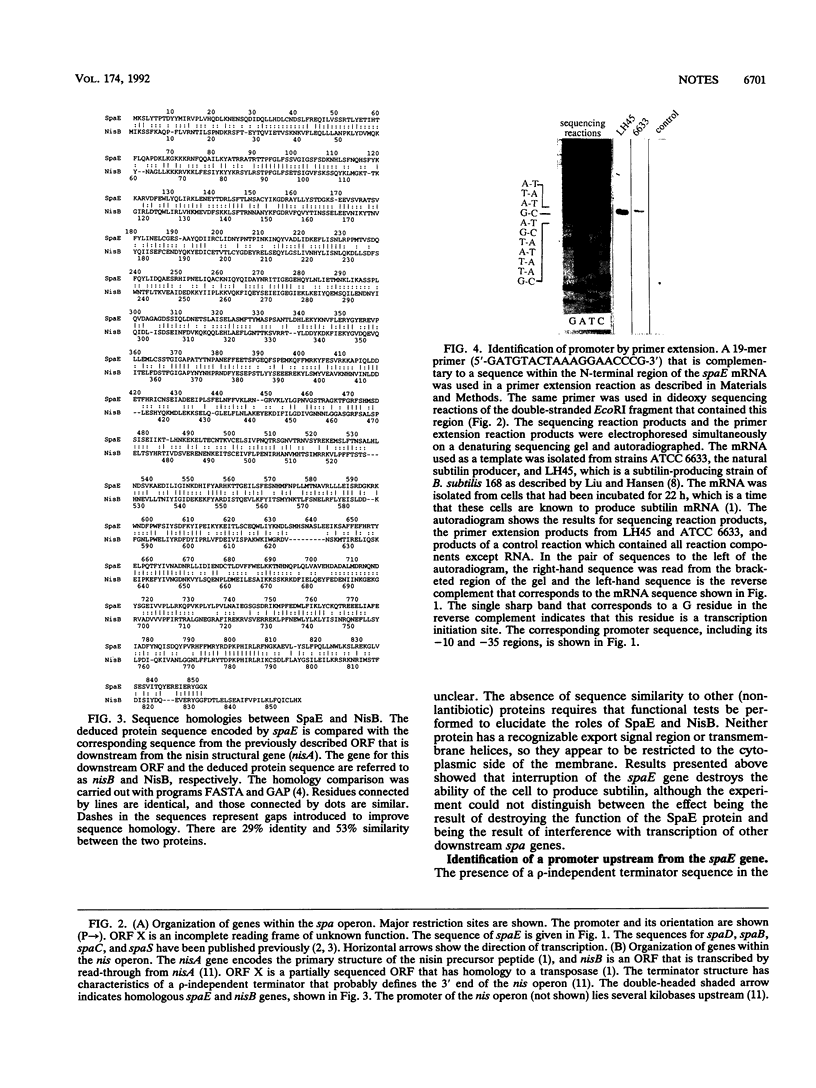
- Chung Y. J., Steen M. T., Hansen J. N. The subtilin gene of Bacillus subtilis ATCC 6633 is encoded in an operon that contains a homolog of the hemolysin B transport protein. J Bacteriol. 1992 Feb;174(4):1417–1422. doi: 10.1128/jb.174.4.1417-1422.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Kiltz H. H., Nebelin E. Subtilin, VI. Die Struktur des Subtilins. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):810–812. [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- Klein C., Kaletta C., Schnell N., Entian K. D. Analysis of genes involved in biosynthesis of the lantibiotic subtilin. Appl Environ Microbiol. 1992 Jan;58(1):132–142. doi: 10.1128/aem.58.1.132-142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Conversion of Bacillus subtilis 168 to a subtilin producer by competence transformation. J Bacteriol. 1991 Nov;173(22):7387–7390. doi: 10.1128/jb.173.22.7387-7390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M. T., Chung Y. J., Hansen J. N. Characterization of the nisin gene as part of a polycistronic operon in the chromosome of Lactococcus lactis ATCC 11454. Appl Environ Microbiol. 1991 Apr;57(4):1181–1188. doi: 10.1128/aem.57.4.1181-1188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Lundström K., Lehtovaara P., Sarvas M., Ruohonen M., Palva I. Transcription and translation of foreign genes in Bacillus subtilis by the aid of a secretion vector. J Bacteriol. 1985 Apr;162(1):176–182. doi: 10.1128/jb.162.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]