Abstract
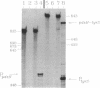
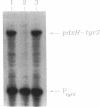
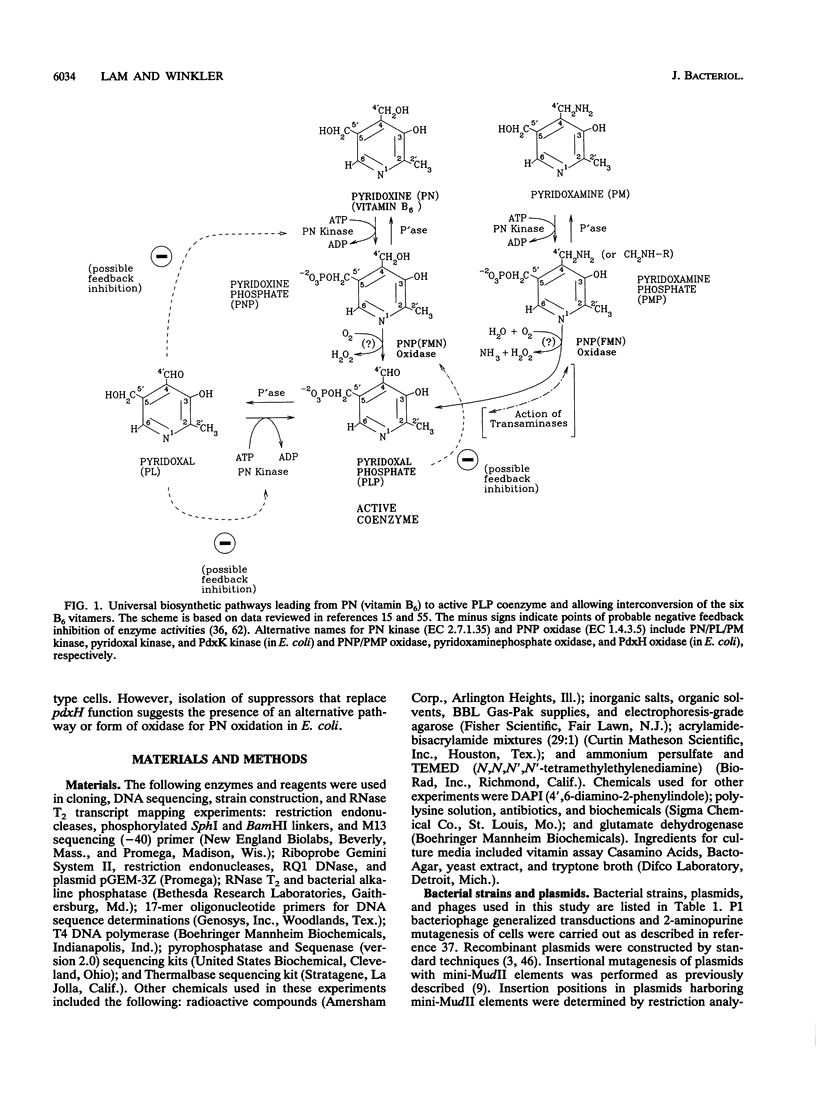
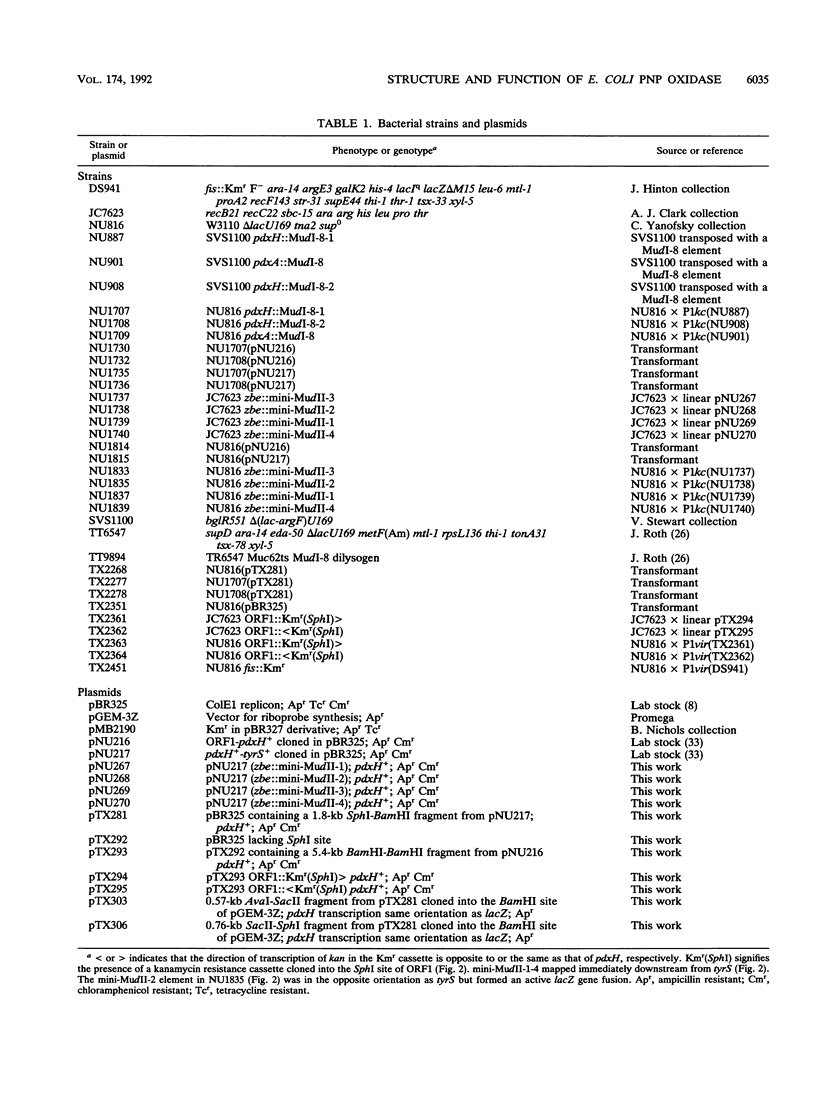
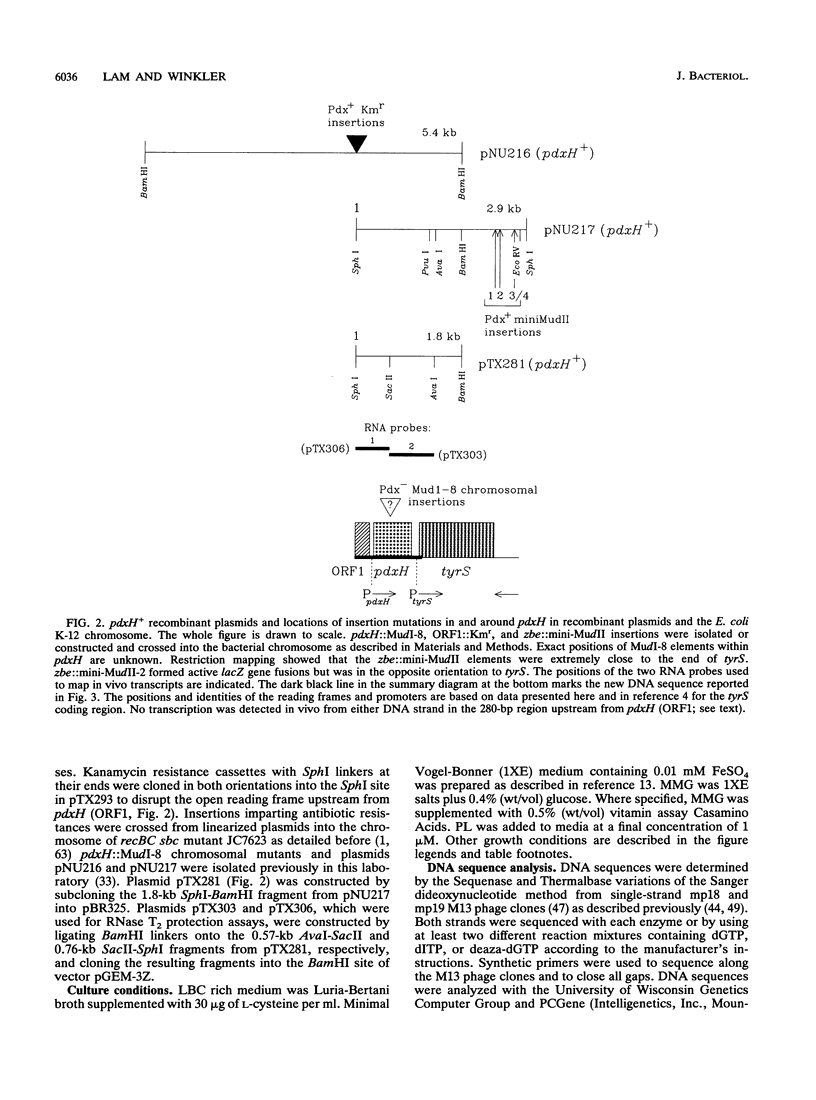
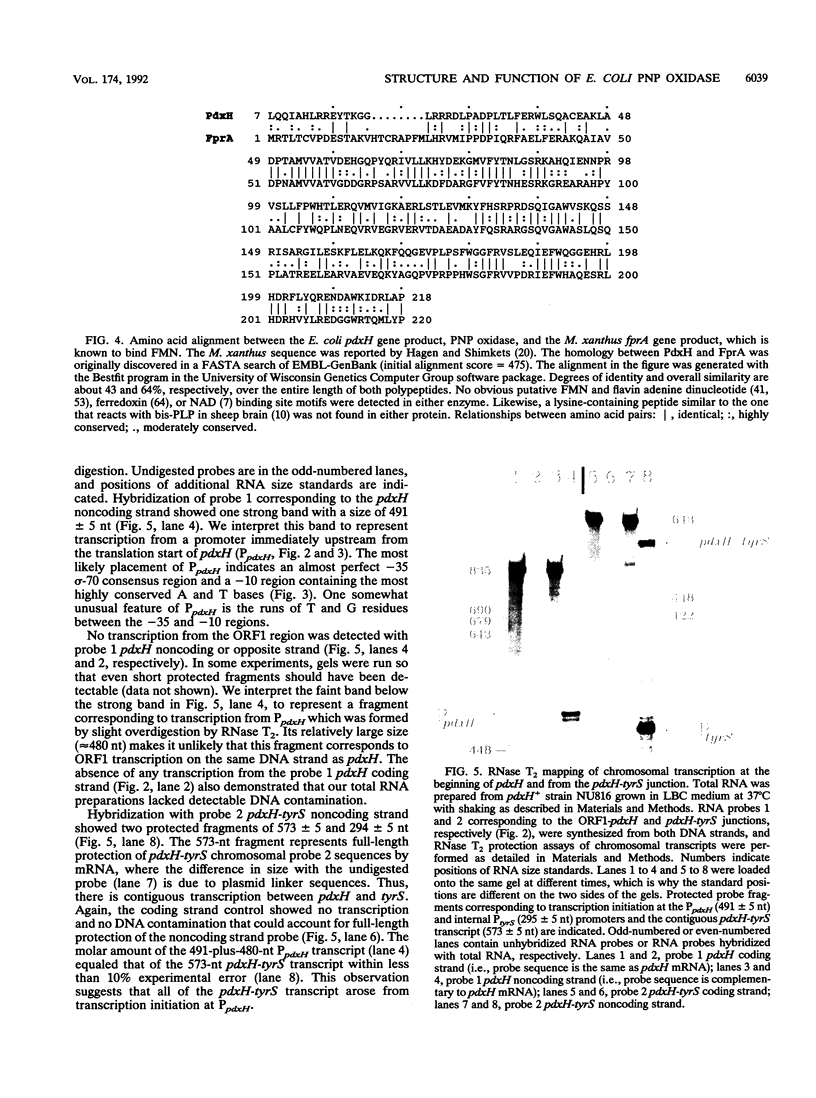
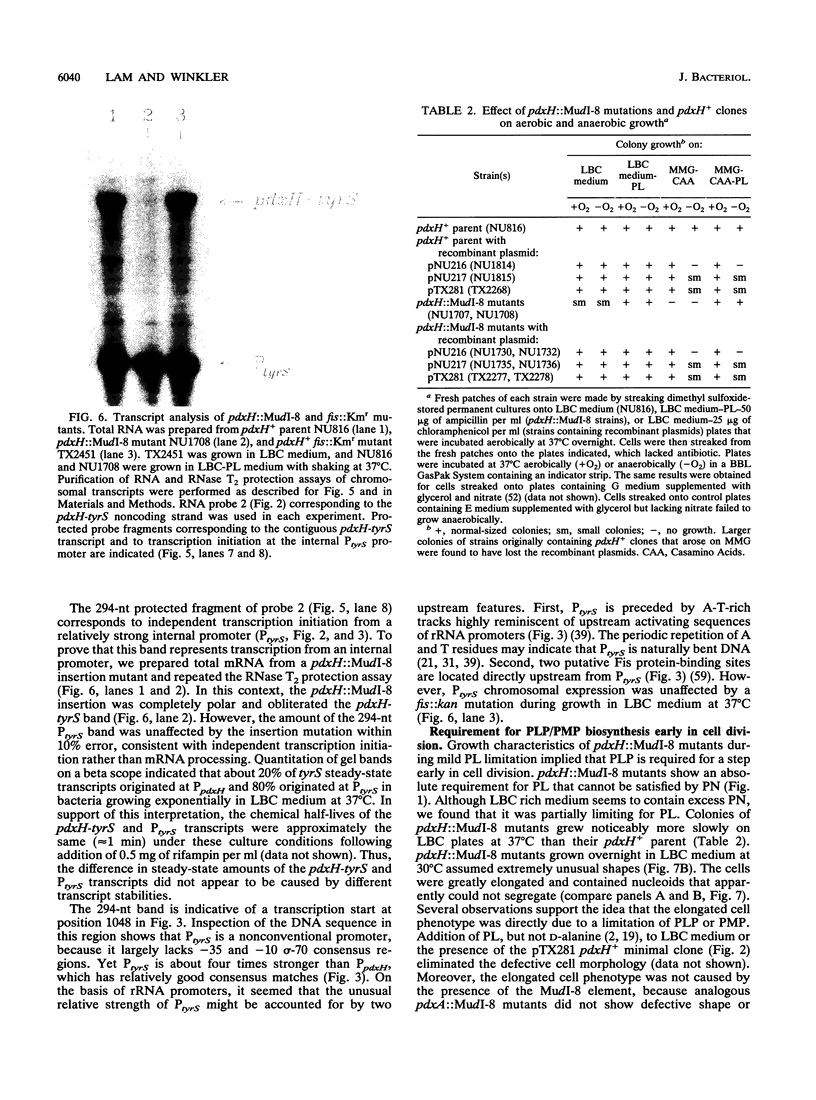
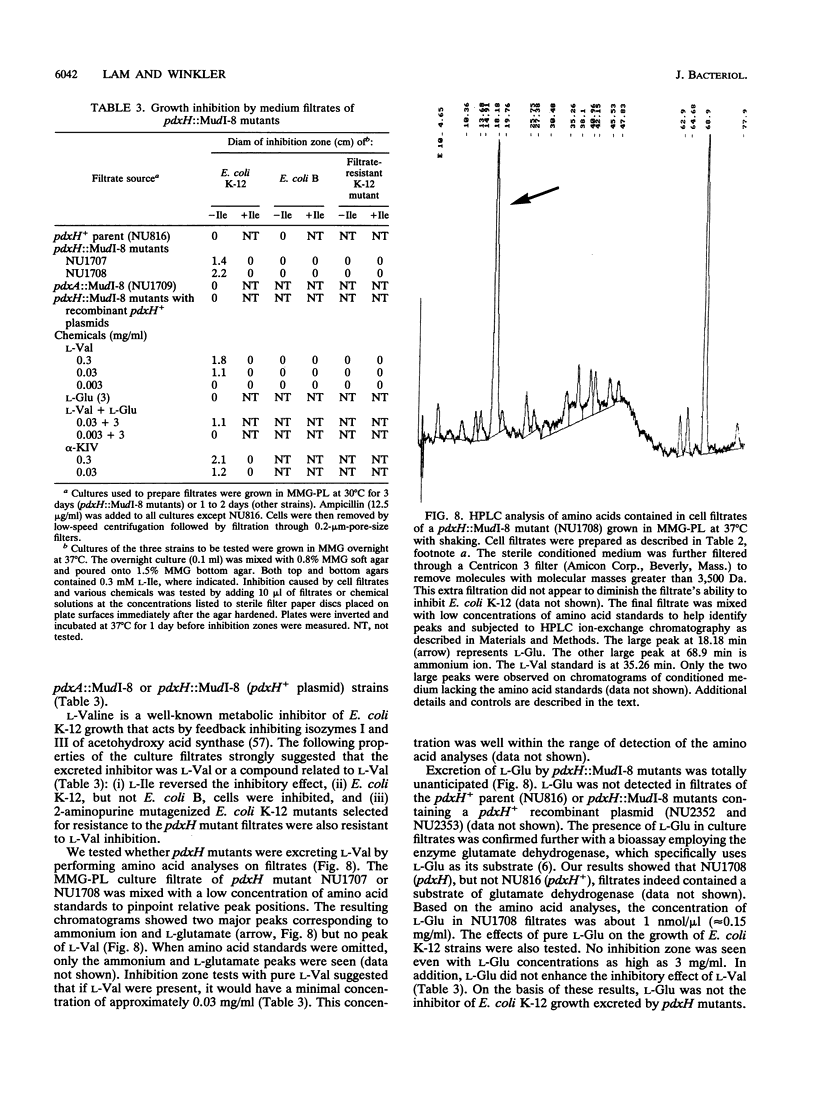
We report the first molecular genetic analysis of a pyridoxine 5'-phosphate oxidase, the PdxH gene product of Escherichia coli K-12. Chromosomal insertions in and around pdxH were generated with various transposons, and the resulting phenotypes were characterized. The DNA sequence of pdxH was determined, and the promoters of pdxH and the downstream gene tyrS, which encodes tyrosyl-tRNA synthetase, were mapped by RNase T2 protection assays of chromosomal transcripts. These combined approaches led to the following conclusions: (i) pdxH is transcribed from a sigma 70-type promoter and shares its transcript with tyrS; (ii) tyrS is additionally transcribed from a relatively strong, nonconventional internal promoter that may contain an upstream activating sequence but whose expression is unaffected by a fis mutation; (iii) PdxH oxidase is basic, has a molecular mass of 25,545 Da, and shares striking homology (greater than 40% identity) with the developmentally regulated FprA protein of Myxococcus xanthus; (iv) mild pyridoxal 5'-phosphate limitation of pdxH mutants inhibits cell division and leads to formation of unsegregated nucleoids; (v) E. coli PdxH oxidase is required aerobically and anaerobically, but second-site suppressors that replace pdxH function entirely can be isolated; and (vi) pdxH mutants excrete significant amounts of L-glutamate and a compound, probably alpha-ketoisovalerate, that triggers L-valine inhibition of E. coli K-12 strains. These findings extend earlier observations that pyridoxal 5'-phosphate biosynthetic and aminoacyl-tRNA synthetase genes are often members of complex, multifunctional operons. Our results also show that loss of pdxH function seriously disrupts cellular metabolism in unanticipated ways.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arps P. J., Winkler M. E. An unusual genetic link between vitamin B6 biosynthesis and tRNA pseudouridine modification in Escherichia coli K-12. J Bacteriol. 1987 Mar;169(3):1071–1079. doi: 10.1128/jb.169.3.1071-1079.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arps P. J., Winkler M. E. Structural analysis of the Escherichia coli K-12 hisT operon by using a kanamycin resistance cassette. J Bacteriol. 1987 Mar;169(3):1061–1070. doi: 10.1128/jb.169.3.1061-1070.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. G., Bruton C. J., Winter G. The tyrosyl-tRNA synthetase from Escherichia coli. Complete nucleotide sequence of the structural gene. FEBS Lett. 1982 Dec 27;150(2):419–423. doi: 10.1016/0014-5793(82)80781-3. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. Y., Churchich J. E., Zaiden E., Kwok F. Brain pyridoxine-5-phosphate oxidase. Modulation of its catalytic activity by reaction with pyridoxal 5-phosphate and analogs. J Biol Chem. 1987 Sep 5;262(25):12013–12017. [PubMed] [Google Scholar]
- Churchich J. E. Brain pyridoxine-5-phosphate oxidase. A dimeric enzyme containing one FMN site. Eur J Biochem. 1984 Jan 16;138(2):327–332. doi: 10.1111/j.1432-1033.1984.tb07918.x. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B. Synthesis of Pyridoxine by a Pyridoxal Auxotroph of Escherichia coli. J Bacteriol. 1966 Aug;92(2):333–337. doi: 10.1128/jb.92.2.333-337.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Coggins J. R. The serC-aro A operon of Escherichia coli. A mixed function operon encoding enzymes from two different amino acid biosynthetic pathways. Biochem J. 1986 Feb 15;234(1):49–57. doi: 10.1042/bj2340049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Vaughn V., Walter W. A., Neidhardt F. C., Gross C. A. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes Dev. 1987 Jul;1(5):419–432. doi: 10.1101/gad.1.5.419. [DOI] [PubMed] [Google Scholar]
- Grogan D. W. Temperature-sensitive murein synthesis in an Escherichia coli pdx mutant and the role of alanine racemase. Arch Microbiol. 1988;150(4):363–367. doi: 10.1007/BF00408308. [DOI] [PubMed] [Google Scholar]
- Hagen T. J., Shimkets L. J. Nucleotide sequence and transcriptional products of the csg locus of Myxococcus xanthus. J Bacteriol. 1990 Jan;172(1):15–23. doi: 10.1128/jb.172.1.15-23.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence-directed curvature of DNA. Nature. 1986 May 22;321(6068):449–450. doi: 10.1038/321449a0. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Fischer E. H. On the role of pyridoxal 5'-phosphate in phosphorylase. I. Absence of classical vitamin B6--dependent enzymatic activities in muscle glycogen phosphorylase. Biochemistry. 1965 Jul;4(7):1337–1343. doi: 10.1021/bi00883a018. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockney R. C., Scott T. A. The isolation and characterization of three types of vitamin B6 auxotrophs of Escherichia coli K12. J Gen Microbiol. 1979 Feb;110(2):275–283. doi: 10.1099/00221287-110-2-275. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Lin C. K., Regue M., Wu H. C. Characterization of the ileS-lsp operon in Escherichia coli. Identification of an open reading frame upstream of the ileS gene and potential promoter(s) for the ileS-lsp operon. J Biol Chem. 1985 May 10;260(9):5616–5620. [PubMed] [Google Scholar]
- Kawakami K., Jönsson Y. H., Björk G. R., Ikeda H., Nakamura Y. Chromosomal location and structure of the operon encoding peptide-chain-release factor 2 of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5620–5624. doi: 10.1073/pnas.85.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazarinoff M. N., McCormick D. B. Rabbit liver pyridoxamine (pyridoxine) 5'-phosphate oxidase. Purification and properties. J Biol Chem. 1975 May 10;250(9):3436–3442. [PubMed] [Google Scholar]
- Kim Y. T., Churchich J. E. Activation of a flavoprotein by proteolysis. J Biol Chem. 1989 Sep 25;264(27):15751–15753. [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Kwok F., Churchich J. E. Interaction between pyridoxal kinase and pyridoxine-5-P oxidase, two enzymes involved in the metabolism of vitamin B6. J Biol Chem. 1980 Feb 10;255(3):882–887. [PubMed] [Google Scholar]
- Lam H. M., Tancula E., Dempsey W. B., Winkler M. E. Suppression of insertions in the complex pdxJ operon of Escherichia coli K-12 by lon and other mutations. J Bacteriol. 1992 Mar;174(5):1554–1567. doi: 10.1128/jb.174.5.1554-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H. M., Winkler M. E. Metabolic relationships between pyridoxine (vitamin B6) and serine biosynthesis in Escherichia coli K-12. J Bacteriol. 1990 Nov;172(11):6518–6528. doi: 10.1128/jb.172.11.6518-6528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaux J. F., Fayat G., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Structural and transcriptional evidence for related thrS and infC expression. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6152–6156. doi: 10.1073/pnas.80.20.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neihardt F. C., Parker J., McKeever W. G. Function and regulation of aminoacyl-tRNA synthetases in prokaryotic and eukaryotic cells. Annu Rev Microbiol. 1975;29:215–250. doi: 10.1146/annurev.mi.29.100175.001243. [DOI] [PubMed] [Google Scholar]
- POGELL B. M. Enzymatic oxidation of pyridoxamine phosphate to pyridoxal phosphate in rabbit liver. J Biol Chem. 1958 Jun;232(2):761–776. [PubMed] [Google Scholar]
- Plaskon R. R., Wartell R. M. Sequence distributions associated with DNA curvature are found upstream of strong E. coli promoters. Nucleic Acids Res. 1987 Jan 26;15(2):785–796. doi: 10.1093/nar/15.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter T. D., Kasper C. B. NADPH-cytochrome P-450 oxidoreductase: flavin mononucleotide and flavin adenine dinucleotide domains evolved from different flavoproteins. Biochemistry. 1986 Apr 8;25(7):1682–1687. doi: 10.1021/bi00355a036. [DOI] [PubMed] [Google Scholar]
- Roa B. B., Connolly D. M., Winkler M. E. Overlap between pdxA and ksgA in the complex pdxA-ksgA-apaG-apaH operon of Escherichia coli K-12. J Bacteriol. 1989 Sep;171(9):4767–4777. doi: 10.1128/jb.171.9.4767-4777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNELL E. E. Chemical structure in relation to biological activities of vitamin B6. Vitam Horm. 1958;16:77–125. doi: 10.1016/s0083-6729(08)60314-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Nester E. W. Mutants of Escherichia coli with an altered tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1969 Oct;100(1):167–175. doi: 10.1128/jb.100.1.167-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenlein P. V., Roa B. B., Winkler M. E. Divergent transcription of pdxB and homology between the pdxB and serA gene products in Escherichia coli K-12. J Bacteriol. 1989 Nov;171(11):6084–6092. doi: 10.1128/jb.171.11.6084-6092.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. The Myxococcus xanthus FprA protein causes increased flavin biosynthesis in Escherichia coli. J Bacteriol. 1990 Jan;172(1):24–30. doi: 10.1128/jb.172.1.24-30.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: changes in composition associated with anaerobic growth and fumarate reductase amber mutation. J Bacteriol. 1974 Mar;117(3):954–959. doi: 10.1128/jb.117.3.954-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman B. J., Krezel A. M., Markley J. L., Leonhardt K. G., Straus N. A. Hydrogen-1, carbon-13, and nitrogen-15 NMR spectroscopy of Anabaena 7120 flavodoxin: assignment of beta-sheet and flavin binding site resonances and analysis of protein-flavin interactions. Biochemistry. 1990 Oct 16;29(41):9600–9609. doi: 10.1021/bi00493a014. [DOI] [PubMed] [Google Scholar]
- TURNER J. M., HAPPOLD F. C. Pyridoxamine phosphate-oxidase and pyridoxal phosphate-phosphatase activities in Escherichia coli. Biochem J. 1961 Feb;78:364–372. doi: 10.1042/bj0780364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiff H. E., Baker T., Copeland T., Chen S. M., Court D. L. Locating essential Escherichia coli genes by using mini-Tn10 transposons: the pdxJ operon. J Bacteriol. 1992 Mar;174(5):1544–1553. doi: 10.1128/jb.174.5.1544-1553.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K. Reaction of phosphofructokinase with maleic anhydride, succinic anhydride, and pyridoxal 5'-phosphate. Biochemistry. 1969 Jun;8(6):2366–2373. doi: 10.1021/bi00834a017. [DOI] [PubMed] [Google Scholar]
- Verbeek H., Nilsson L., Baliko G., Bosch L. Potential binding sites of the trans-activator FIS are present upstream of all rRNA operons and of many but not all tRNA operons. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):302–306. doi: 10.1016/0167-4781(90)90185-5. [DOI] [PubMed] [Google Scholar]
- WADA H., SNELL E. E. The enzymatic oxidation of pyridoxine and pyridoxamine phosphates. J Biol Chem. 1961 Jul;236:2089–2095. [PubMed] [Google Scholar]
- Waye M. M., Winter G. A transcription terminator in the 5' non-coding region of the tyrosyl tRNA synthetase gene from Bacillus stearothermophilus. Eur J Biochem. 1986 Aug 1;158(3):505–510. doi: 10.1111/j.1432-1033.1986.tb09783.x. [DOI] [PubMed] [Google Scholar]
- White R. S., Dempsey W. B. Purification and properties of vitamin B6 kinase from Escherichia coli B. Biochemistry. 1970 Oct 13;9(21):4057–4064. doi: 10.1021/bi00823a005. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias M., Göringer H. U., Wagner R. Analysis of the Fis-dependent and Fis-independent transcription activation mechanisms of the Escherichia coli ribosomal RNA P1 promoter. Biochemistry. 1992 Mar 10;31(9):2621–2628. doi: 10.1021/bi00124a024. [DOI] [PubMed] [Google Scholar]