Abstract
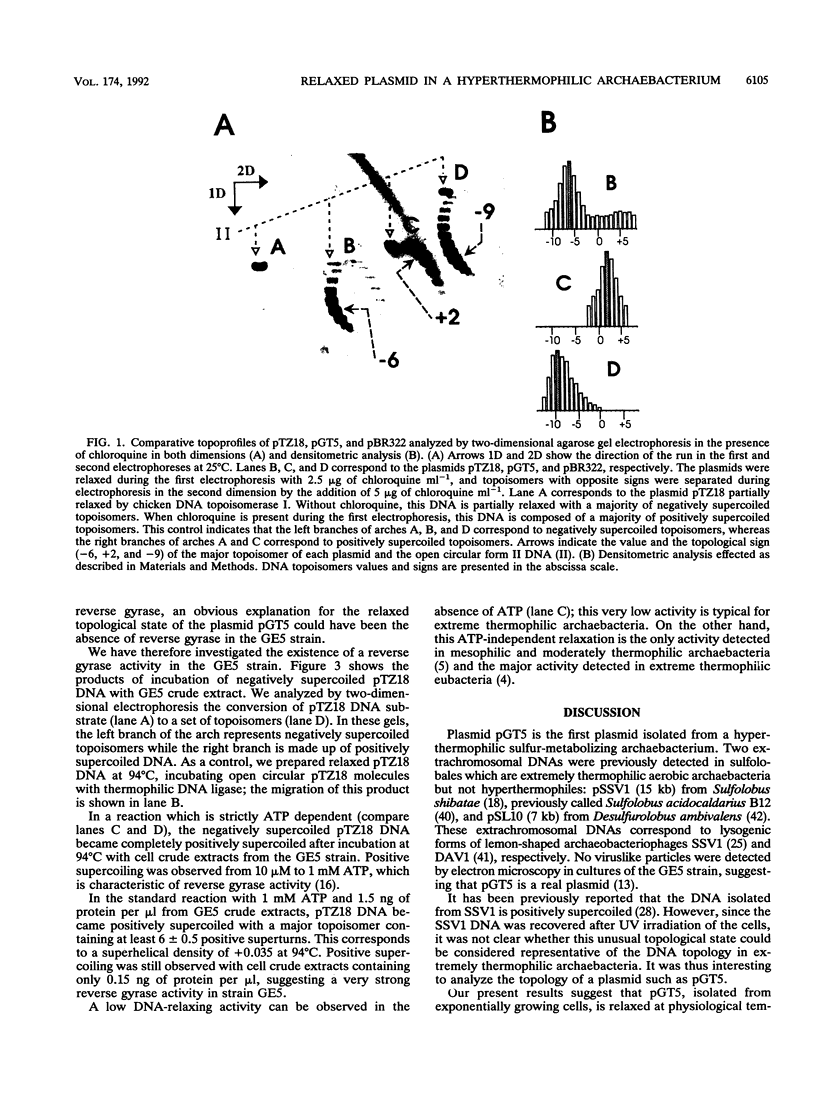
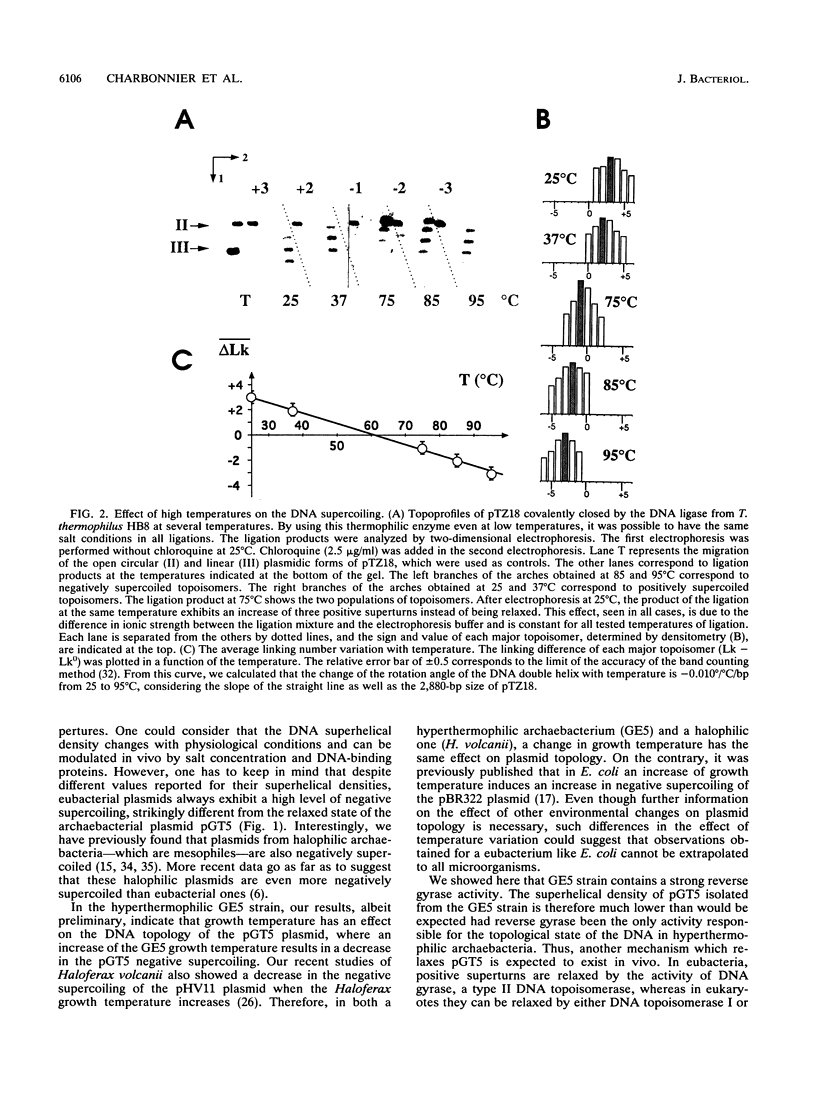
A plasmid of 3.45 kb (pGT5) was recently discovered in a strain of hyperthermophilic archaebacterium which was isolated from samples collected in a deep-sea hydrothermal vent. This strain (GE5) grows within a temperature range of 68 to 101.5 degrees C, and we show here that it contains a strong ATP-dependent reverse gyrase activity (positive DNA supercoiling). By comparison with eubacterial plasmids of known superhelical densities, we estimated the superhelical density of the archaebacterial plasmid pGT5 to be -0.026 at 25 degrees C. The equation which relates the change of the rotation angle of the DNA double helix with temperature was validated at 95 degrees C, the optimal growth temperature of the GE5 strain. Considering these new data, the superhelical density of plasmid pGT5 was calculated to be -0.006 at the physiological temperature of 95 degrees C, which is close to the relaxed state. This finding shows that the DNA topology of a plasmid isolated from a hyperthermophilic archaebacterium containing reverse gyrase activity is strikingly different from that of typical eubacterial plasmids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilai R. SV40 DNA: quantitative conversion of closed circular to open circular form by an ethidium bromide-restricted endonuclease. J Mol Biol. 1973 Mar 15;74(4):739–742. doi: 10.1016/0022-2836(73)90062-4. [DOI] [PubMed] [Google Scholar]
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Bouthier de la Tour C., Portemer C., Huber R., Forterre P., Duguet M. Reverse gyrase in thermophilic eubacteria. J Bacteriol. 1991 Jun;173(12):3921–3923. doi: 10.1128/jb.173.12.3921-3923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthier de la Tour C., Portemer C., Nadal M., Stetter K. O., Forterre P., Duguet M. Reverse gyrase, a hallmark of the hyperthermophilic archaebacteria. J Bacteriol. 1990 Dec;172(12):6803–6808. doi: 10.1128/jb.172.12.6803-6808.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Linking numbers and nucleosomes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2639–2643. doi: 10.1073/pnas.73.8.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Control of bacterial DNA supercoiling. Mol Microbiol. 1992 Feb;6(4):425–433. doi: 10.1111/j.1365-2958.1992.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Forterre P., Mirambeau G., Jaxel C., Nadal M., Duguet M. High positive supercoiling in vitro catalyzed by an ATP and polyethylene glycol-stimulated topoisomerase from Sulfolobus acidocaldarius. EMBO J. 1985 Aug;4(8):2123–2128. doi: 10.1002/j.1460-2075.1985.tb03902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Drlica K. Regulation of bacterial DNA supercoiling: plasmid linking numbers vary with growth temperature. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4046–4050. doi: 10.1073/pnas.81.13.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan D., Palm P., Zillig W. Isolate B12, which harbours a virus-like element, represents a new species of the archaebacterial genus Sulfolobus, Sulfolobus shibatae, sp. nov. Arch Microbiol. 1990;154(6):594–599. doi: 10.1007/BF00248842. [DOI] [PubMed] [Google Scholar]
- Jaxel C., Nadal M., Mirambeau G., Forterre P., Takahashi M., Duguet M. Reverse gyrase binding to DNA alters the double helix structure and produces single-strand cleavage in the absence of ATP. EMBO J. 1989 Oct;8(10):3135–3139. doi: 10.1002/j.1460-2075.1989.tb08466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A., Asai K. Reverse gyrase--a topoisomerase which introduces positive superhelical turns into DNA. Nature. 1984 Jun 21;309(5970):677–681. doi: 10.1038/309677a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Yeats S., Janekovic D., Reiter W. D., Aicher W., Zillig W. SAV 1, a temperate u.v.-inducible DNA virus-like particle from the archaebacterium Sulfolobus acidocaldarius isolate B12. EMBO J. 1984 Sep;3(9):2165–2168. doi: 10.1002/j.1460-2075.1984.tb02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal M., Jaxel C., Portemer C., Forterre P., Mirambeau G., Duguet M. Reverse gyrase of Sulfolobus: purification to homogeneity and characterization. Biochemistry. 1988 Dec 27;27(26):9102–9108. doi: 10.1021/bi00426a006. [DOI] [PubMed] [Google Scholar]
- Nakasu S., Kikuchi A. Reverse gyrase; ATP-dependent type I topoisomerase from Sulfolobus. EMBO J. 1985 Oct;4(10):2705–2710. doi: 10.1002/j.1460-2075.1985.tb03990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Tang D., Vinograd J., Vosberg H. P. Action of nicking-closing enzyme on supercoiled and nonsupercoiled closed circular DNA: formation of a Boltzmann distribution of topological isomers. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4280–4284. doi: 10.1073/pnas.72.11.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Vinograd J. The number of superhelical turns in native virion SV40 DNA and minicol DNA determined by the band counting method. Cell. 1976 Jun;8(2):215–226. doi: 10.1016/0092-8674(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Sioud M., Baldacci G., de Recondo A. M., Forterre P. Novobiocin induces positive supercoiling of small plasmids from halophilic archaebacteria in vivo. Nucleic Acids Res. 1988 Feb 25;16(4):1379–1391. doi: 10.1093/nar/16.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Possot O., Elie C., Sibold L., Forterre P. Coumarin and quinolone action in archaebacteria: evidence for the presence of a DNA gyrase-like enzyme. J Bacteriol. 1988 Feb;170(2):946–953. doi: 10.1128/jb.170.2.946-953.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesarev A. I. Positive supercoiling catalysed in vitro by ATP-dependent topoisomerase from Desulfurococcus amylolyticus. Eur J Biochem. 1988 Apr 15;173(2):395–399. doi: 10.1111/j.1432-1033.1988.tb14012.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yamaguchi E., Uchida T. Thermophilic DNA ligase. Purification and properties of the enzyme from Thermus thermophilus HB8. J Biol Chem. 1984 Aug 25;259(16):10041–10047. [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Yeats S., McWilliam P., Zillig W. A plasmid in the archaebacterium Sulfolobus acidocaldarius. EMBO J. 1982;1(9):1035–1038. doi: 10.1002/j.1460-2075.1982.tb01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]