Abstract
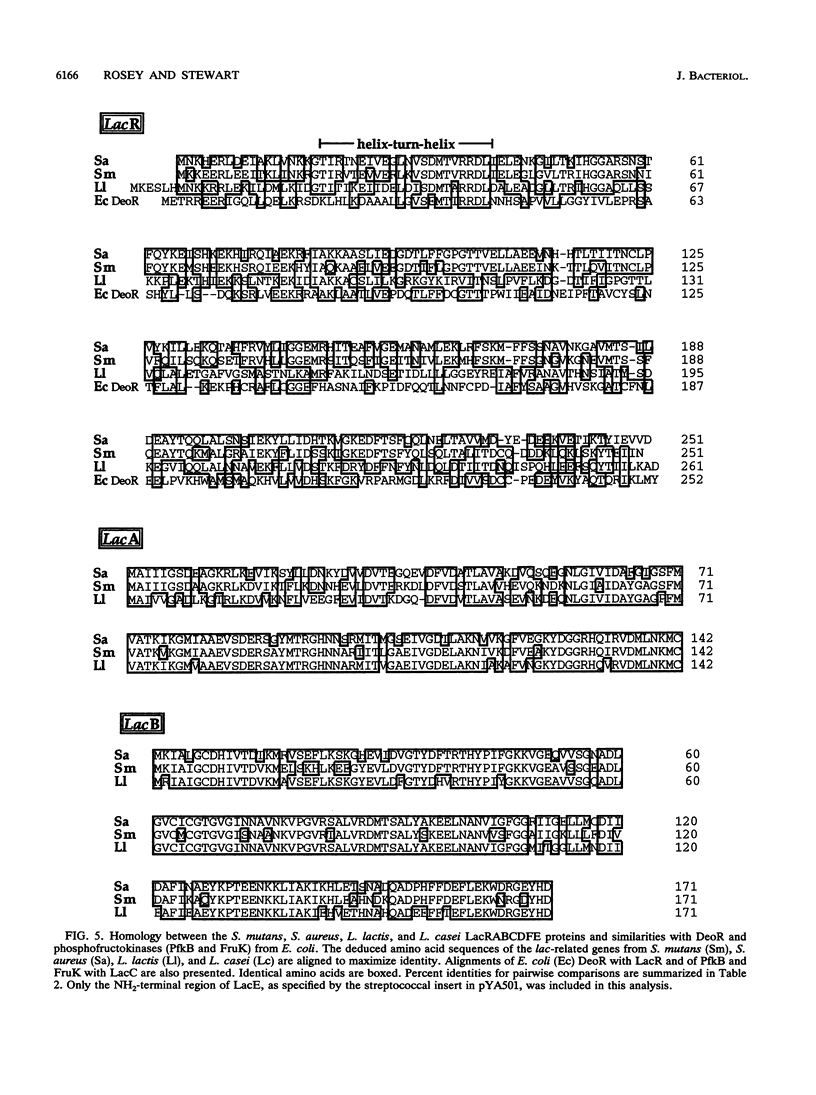
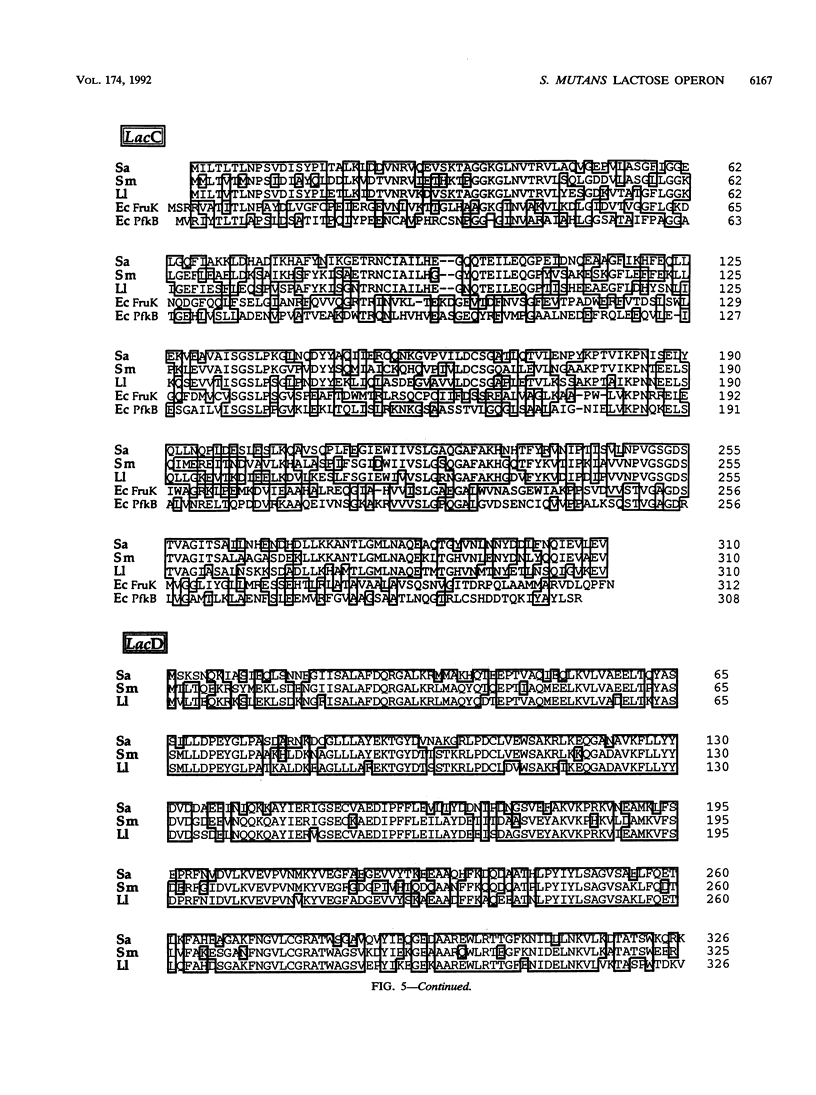
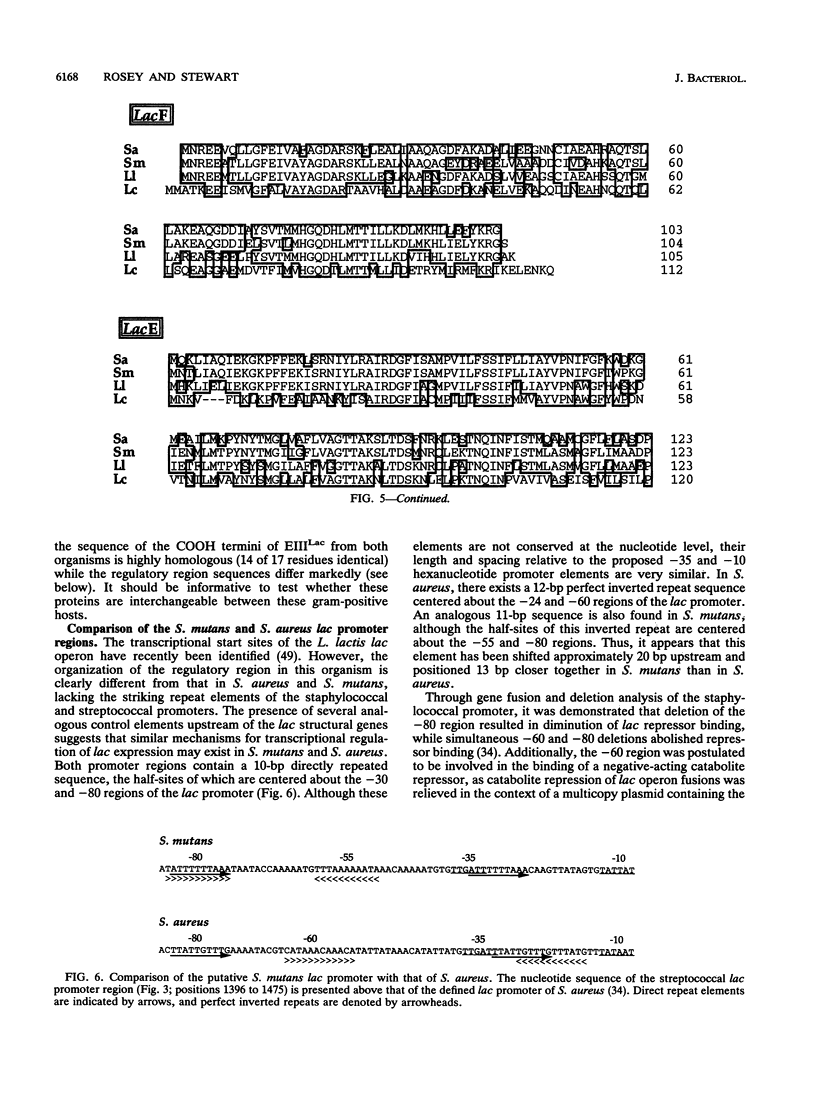
The complete nucleotide sequences of lacRABCDF and partial nucleotide sequence of lacE from the lactose operon of Streptococcus mutans are presented. Comparison of the streptococcal lac determinants with those of Staphylococcus aureus and Lactococcus lactis indicate exceptional protein and nucleotide identity. The deduced polypeptides also demonstrate significant, but lower, sequence similarity with the corresponding lactose proteins of Lactobacillus casei. Additionally, LacR has sequence homology with the repressor (DeoR) of the Escherichia coli deoxyribonucleotide operon, while LacC is similar to phosphokinases (FruK and PfkB) from E. coli. The primary translation products of the lacRABCDFE genes are polypeptides of 251 (M(r) 28,713), 142 (M(r) 15,610), 171 (M(r) 18,950), 310 (M(r) 33,368), 325 (M(r) 36,495), 104 (M(r) 11,401), and 123 (NH2-terminal) amino acids, respectively. As inferred from their direct homology to the staphylococcal lac genes, these determinants would encode the repressor of the streptococcal lactose operon (LacR), galactose-6-phosphate isomerase (LacA and LacB), tagatose-6-phosphate kinase (LacC), tagatose-1,6-bisphosphate aldolase (LacD), and the sugar-specific components enzyme III-lactose (LacF) and enzyme II-lactose (LacE) of the S. mutans phosphoenolpyruvate-dependent phosphotransferase system. The nucleotide sequence encompassing the S. mutans lac promoter appears to contain repeat elements analogous to those of S. aureus, suggesting that repression and catabolite repression of the lactose operons may be similar in these organisms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert C. A., Chassy B. M. Molecular cloning and DNA sequence of lacE, the gene encoding the lactose-specific enzyme II of the phosphotransferase system of Lactobacillus casei. Evidence that a cysteine residue is essential for sugar phosphorylation. J Biol Chem. 1990 Dec 25;265(36):22561–22568. [PubMed] [Google Scholar]
- Alpert C. A., Chassy B. M. Molecular cloning and nucleotide sequence of the factor IIIlac gene of Lactobacillus casei. Gene. 1988;62(2):277–288. doi: 10.1016/0378-1119(88)90565-3. [DOI] [PubMed] [Google Scholar]
- Beck von Bodman S., Hayman G. T., Farrand S. K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Genetic evidence for the physiological significance of the D-tagatose 6-phosphate pathway of lactose and D-galactose degradation in staphylococcus aureus. J Bacteriol. 1974 Sep;119(3):698–704. doi: 10.1128/jb.119.3.698-704.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. III. Purification and properties of D-tagatose-6-phosphate kinase. J Biol Chem. 1980 Sep 25;255(18):8745–8749. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. IV. Isolation and properties of a class I D-ketohexose-1,6-diphosphate aldolase that catalyzes the cleavage of D-tagatose 1,6-diphosphate. J Biol Chem. 1980 Sep 25;255(18):8750–8755. [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D0galactose metabolism in Staphylococcus aureus: pathway of D-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973 May 15;52(2):641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- Bissett D. L., Wenger W. C., Anderson R. L. Lactose and D-galactose metabolism in Staphylococcus aureus. II. Isomerization of D-galactose 6-phosphate to D-tagatose 6-phosphate by a specific D-galactose-6-phosphate isomerase. J Biol Chem. 1980 Sep 25;255(18):8740–8744. [PubMed] [Google Scholar]
- Breidt F., Jr, Hengstenberg W., Finkeldei U., Stewart G. C. Identification of the genes for the lactose-specific components of the phosphotransferase system in the lac operon of Staphylococcus aureus. J Biol Chem. 1987 Dec 5;262(34):16444–16449. [PubMed] [Google Scholar]
- Breidt F., Jr, Stewart G. C. Nucleotide and deduced amino acid sequences of the Staphylococcus aureus phospho-beta-galactosidase gene. Appl Environ Microbiol. 1987 May;53(5):969–973. doi: 10.1128/aem.53.5.969-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. T. The role of dietary carbohydrates in plaque formation and oral disease. Nutr Rev. 1975 Dec;33(12):353–361. doi: 10.1111/j.1753-4887.1975.tb05089.x. [DOI] [PubMed] [Google Scholar]
- Daldal F. Nucleotide sequence of gene pfkB encoding the minor phosphofructokinase of Escherichia coli K-12. Gene. 1984 Jun;28(3):337–342. doi: 10.1016/0378-1119(84)90151-3. [DOI] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., Iandolo J. J. Rapid isolation of DNA from Staphylococcus aureus. Appl Environ Microbiol. 1983 Jul;46(1):283–285. doi: 10.1128/aem.46.1.283-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostell G., Keyes P. H., Larson R. H. Effect of various sugars and sugar substitutes on dental caries in hamsters and rats. J Nutr. 1967 Sep;93(1):65–76. doi: 10.1093/jn/93.1.65. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lebtag H. Lactose metabolism by Streptococcus mutans: evidence for induction of the tagatose 6-phosphate pathway. J Bacteriol. 1979 Dec;140(3):1102–1104. doi: 10.1128/jb.140.3.1102-1104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. C., Stewart G. C. Differential utilization of Staphylococcus aureus promoter sequences by Escherichia coli and Bacillus subtilis. Gene. 1986;48(1):93–100. doi: 10.1016/0378-1119(86)90355-0. [DOI] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Hansen J. B., Crow V. L., Thomas T. D., Honeyman A. L., Curtiss R., 3rd Streptococcus mutans serotype c tagatose 6-phosphate pathway gene cluster. J Bacteriol. 1992 Oct;174(19):6152–6158. doi: 10.1128/jb.174.19.6152-6158.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Ye S. Z., Weissenborn D. L., Hoffmann H. J., Schweizer H. Purification and characterization of the repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K12. J Biol Chem. 1987 Nov 25;262(33):15869–15874. [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Lysogenic conversion of staphylococcal lipase is caused by insertion of the bacteriophage L54a genome into the lipase structural gene. J Bacteriol. 1986 May;166(2):385–391. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Orchard L. M., Kornberg H. L. Sequence similarities between the gene specifying 1-phosphofructokinase (fruK), genes specifying other kinases in Escherichia coli K12, and lacC of Staphylococcus aureus. Proc Biol Sci. 1990 Nov 22;242(1304):87–90. doi: 10.1098/rspb.1990.0108. [DOI] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Cloning and characterization of the repressor gene of the Staphylococcus aureus lactose operon. J Bacteriol. 1987 Dec;169(12):5459–5465. doi: 10.1128/jb.169.12.5459-5465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Repression and catabolite repression of the lactose operon of Staphylococcus aureus. J Bacteriol. 1990 Jul;172(7):3804–3812. doi: 10.1128/jb.172.7.3804-3812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosey E. L., Oskouian B., Stewart G. C. Lactose metabolism by Staphylococcus aureus: characterization of lacABCD, the structural genes of the tagatose 6-phosphate pathway. J Bacteriol. 1991 Oct;173(19):5992–5998. doi: 10.1128/jb.173.19.5992-5998.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosey E. L., Stewart G. C. The nucleotide sequence of the lacC and lacD genes of Staphylococcus aureus. Nucleic Acids Res. 1989 May 25;17(10):3980–3980. doi: 10.1093/nar/17.10.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Yamada M., Erni B., Suda K., Lengeler J., Ebner R., Argos P., Rak B., Schnetz K., Lee C. A. Sugar permeases of the bacterial phosphoenolpyruvate-dependent phosphotransferase system: sequence comparisons. FASEB J. 1988 Mar 1;2(3):199–208. doi: 10.1096/fasebj.2.3.2832233. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983 May-Jun;22(2-3):181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Smorawinska M., Hsu J. C., Hansen J. B., Jagusztyn-Krynicka E. K., Abiko Y., Curtiss R., 3rd Clustered genes for galactose metabolism from Streptococcus mutans cloned in Escherichia coli. J Bacteriol. 1983 Feb;153(2):1095–1097. doi: 10.1128/jb.153.2.1095-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. A., Yasbin R. E., Young F. E. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene. 1984 Jul-Aug;29(1-2):21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Valentin-Hansen P., Højrup P., Short S. The primary structure of the DeoR repressor from Escherichia coli K-12. Nucleic Acids Res. 1985 Aug 26;13(16):5927–5936. doi: 10.1093/nar/13.16.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. F., Reizer A., Reizer J., Cai B., Tomich J. M., Saier M. H., Jr Nucleotide sequence of the Rhodobacter capsulatus fruK gene, which encodes fructose-1-phosphate kinase: evidence for a kinase superfamily including both phosphofructokinases of Escherichia coli. J Bacteriol. 1991 May;173(10):3117–3127. doi: 10.1128/jb.173.10.3117-3127.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos W. M., Boerrigter I., van Rooyen R. J., Reiche B., Hengstenberg W. Characterization of the lactose-specific enzymes of the phosphotransferase system in Lactococcus lactis. J Biol Chem. 1990 Dec 25;265(36):22554–22560. [PubMed] [Google Scholar]
- van Rooijen R. J., Gasson M. J., de Vos W. M. Characterization of the Lactococcus lactis lactose operon promoter: contribution of flanking sequences and LacR repressor to promoter activity. J Bacteriol. 1992 Apr;174(7):2273–2280. doi: 10.1128/jb.174.7.2273-2280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen R. J., de Vos W. M. Molecular cloning, transcriptional analysis, and nucleotide sequence of lacR, a gene encoding the repressor of the lactose phosphotransferase system of Lactococcus lactis. J Biol Chem. 1990 Oct 25;265(30):18499–18503. [PubMed] [Google Scholar]
- van Rooijen R. J., van Schalkwijk S., de Vos W. M. Molecular cloning, characterization, and nucleotide sequence of the tagatose 6-phosphate pathway gene cluster of the lactose operon of Lactococcus lactis. J Biol Chem. 1991 Apr 15;266(11):7176–7181. [PubMed] [Google Scholar]