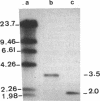
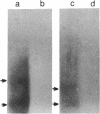
Abstract
Choleraphage phi 149 differentiates the two biotypes, classical and el tor, of Vibrio cholerae. This phage cannot replicate in V. cholerae biotype el tor cells because the concatemeric DNA intermediates produced are unstable and cannot be chased to mature phage DNA. A V. cholerae biotype el tor gene coding for a 14,000-Da inner membrane protein which destabilizes the concatemeric DNA intermediates by hindering their binding to the cell membrane has been identified. Presumably, a 22,000-Da V. cholerae biotype el tor protein might also have a role in conferring phage phi 149 resistance to cells belonging to the biotype el tor. A nucleotide sequence homologous to the 1.2-kb V. cholerae biotype el tor DNA coding for both the 14,000- and 22,000-Da proteins is present in all strains of classical vibrios but is not transcribed. The nucleotide sequence of the gene coding for the 14,000-Da protein has been determined.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHASKARAN K. Recombination of characters between mutant stocks of Vibrio cholerae, strain 162. J Gen Microbiol. 1960 Aug;23:47–54. doi: 10.1099/00221287-23-1-47. [DOI] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera T. K., Ghosh S. K., Das J. Cloning and characterization of mutL and mutS genes of Vibrio cholerae: nucleotide sequence of the mutL gene. Nucleic Acids Res. 1989 Aug 11;17(15):6241–6251. doi: 10.1093/nar/17.15.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K., Sinha V. B., Iyer S. S. Chromosome mobilization in Vibrio cholerae (biotype eltor) mediated by sex factor P. J Gen Microbiol. 1973 Sep;78(1):119–124. doi: 10.1099/00221287-78-1-119. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champness W. C., Snyder L. The gol site: a cis-acting bacteriophage T4 regulatory region that can affect expression of all the T4 late genes. J Mol Biol. 1982 Mar 15;155(4):395–407. doi: 10.1016/0022-2836(82)90478-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chowdhury R., Biswas S. K., Das J. Abortive replication of choleraphage phi 149 in Vibrio cholerae biotype el tor. J Virol. 1989 Jan;63(1):392–397. doi: 10.1128/jvi.63.1.392-397.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Das J. Infection by choleraphage phi 138: bacteriophage DNA and replicative intermediates. J Virol. 1986 Mar;57(3):960–967. doi: 10.1128/jvi.57.3.960-967.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Ray A., Ray P., Das J. Replication and packaging of choleraphage phi 149 DNA. J Virol. 1987 Dec;61(12):3999–4006. doi: 10.1128/jvi.61.12.3999-4006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citarella R. V., Colwell R. R. Polyphasic taxonomy of the genus Vibrio: polynucleotide sequence relationships among selected Vibrio species. J Bacteriol. 1970 Oct;104(1):434–442. doi: 10.1128/jb.104.1.434-442.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G., Sil K., Das J. Repair of ultraviolet-light-induced DNA damage in vibrio cholerae. Biochim Biophys Acta. 1981 Oct 27;655(3):413–420. doi: 10.1016/0005-2787(81)90053-8. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Yamagishi M., Matsuo-Kato H., Minagawa T. Purification of DNA-binding proteins of bacteriophage T3 and heir role in in vitro packaging of phage T3 DNA. Virology. 1980 Sep;105(2):480–489. doi: 10.1016/0042-6822(80)90048-3. [DOI] [PubMed] [Google Scholar]
- Ghosh S. K., Panda D. K., Das J. Lack of umuDC gene functions in Vibrio cholerae cells. Mutat Res. 1989 Jan;210(1):149–156. doi: 10.1016/0027-5107(89)90054-7. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lohia A., Majumdar S., Chatterjee A. N., Das J. Effect of changes in the osmolarity of the growth medium on Vibrio cholerae cells. J Bacteriol. 1985 Sep;163(3):1158–1166. doi: 10.1128/jb.163.3.1158-1166.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Jr, Lee M. The role of bacteriophage T7 exonuclease (gene 6) in genetic recombination and production of concatemers. J Mol Biol. 1976 Feb 25;101(2):223–234. doi: 10.1016/0022-2836(76)90374-0. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Goldberg E. B. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J Mol Biol. 1977 Nov;116(4):877–881. doi: 10.1016/0022-2836(77)90276-5. [DOI] [PubMed] [Google Scholar]
- Palit B. N., Das G., Das J. Repair of ultraviolet light-induced DNA damage in cholera bacteriophages. J Gen Virol. 1983 Aug;64(Pt 8):1749–1755. doi: 10.1099/0022-1317-64-8-1749. [DOI] [PubMed] [Google Scholar]
- Panda D. K., Dasgupta U., Das J. Transformation of Vibrio cholerae by plasmid DNA. Gene. 1991 Aug 30;105(1):107–111. doi: 10.1016/0378-1119(91)90520-l. [DOI] [PubMed] [Google Scholar]
- Paul K., Ghosh S. K., Das J. Cloning and expression in Escherichia coli of a recA-like gene from Vibrio cholerae. Mol Gen Genet. 1986 Apr;203(1):58–63. doi: 10.1007/BF00330384. [DOI] [PubMed] [Google Scholar]
- Prashad N., Hosoda J. Role of genes 46 and 47 in bacteriophage T4 reproduction. II. Formation of gaps on parental DNA of polynucleotide ligase defective mutants. J Mol Biol. 1972 Oct 14;70(3):617–635. doi: 10.1016/0022-2836(72)90562-1. [DOI] [PubMed] [Google Scholar]
- Priefer U. B., Simon R., Pühler A. Extension of the host range of Escherichia coli vectors by incorporation of RSF1010 replication and mobilization functions. J Bacteriol. 1985 Jul;163(1):324–330. doi: 10.1128/jb.163.1.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P., Sengupta A., Das J. Phosphate repression of phage protein synthesis during infection by choleraphage phi 149. Virology. 1984 Jul 15;136(1):110–124. doi: 10.1016/0042-6822(84)90252-6. [DOI] [PubMed] [Google Scholar]
- Roy N. K., Das G., Balganesh T. S., Dey S. N., Ghosh R. K., Das J. Enterotoxin production, DNA repair and alkaline phosphatase of Vibrio cholerae before and after animal passage. J Gen Microbiol. 1982 Sep;128(9):1927–1932. doi: 10.1099/00221287-128-9-1927. [DOI] [PubMed] [Google Scholar]
- Samadi A. R., Huq M. I., Shahid N., Khan M. U., Eusof A., Rahman A. S., Yunus M., Faruque A. S. Classical Vibrio cholerae biotype displaces EL tor in Bangladesh. Lancet. 1983 Apr 9;1(8328):805–807. doi: 10.1016/s0140-6736(83)91860-3. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A., Ray P., Das J. Characterization and physical map of choleraphage phi 149 DNA. Virology. 1985 Jan 30;140(2):217–229. doi: 10.1016/0042-6822(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zabrovitz S., Segev N., Cohen G. Growth of bacteriophage P1 in recombination-deficient hosts of Escherichia coli. Virology. 1977 Jul 15;80(2):233–248. doi: 10.1016/s0042-6822(77)80001-9. [DOI] [PubMed] [Google Scholar]