Abstract
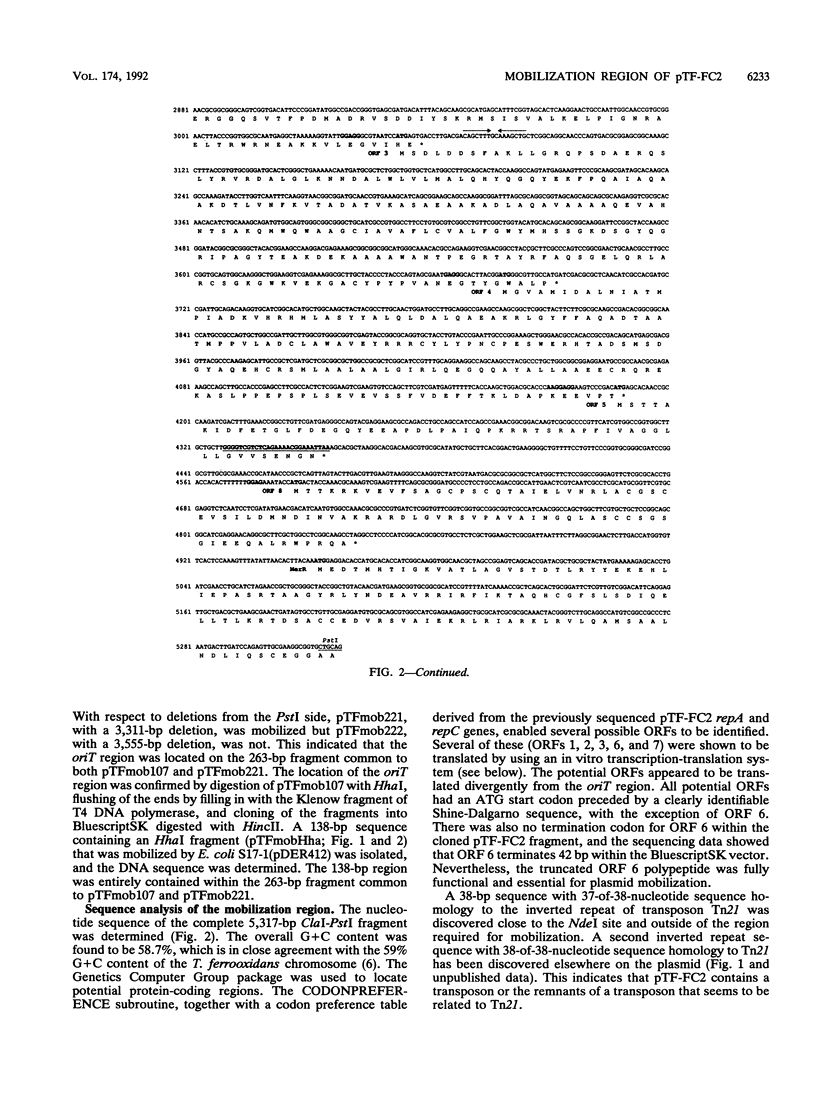
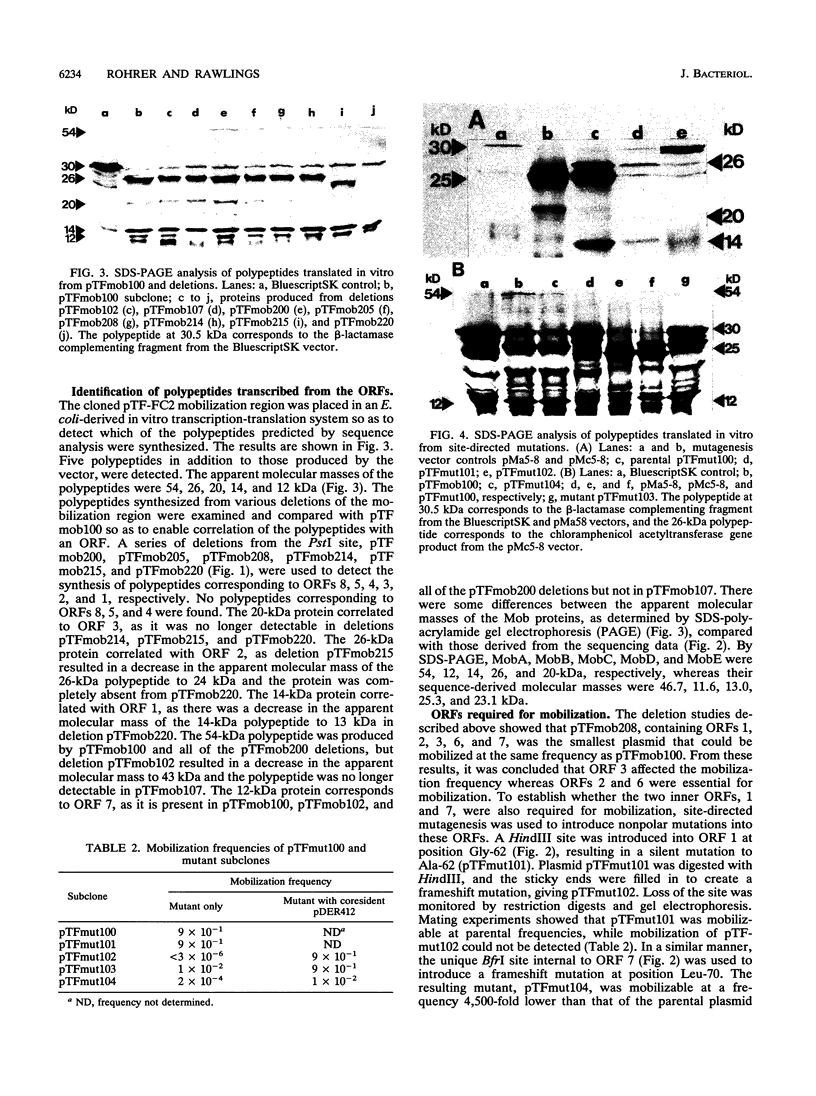
The nucleotide sequence of a 5,317-bp fragment which includes the region required for mobilization of broad-host-range plasmid pTF-FC2 was determined. A region of approximately 3.5 kb was required for plasmid mobilization, and oriT was localized on a 138-bp fragment. Polypeptides which corresponded in size and location to several of the open reading frames were detected in an in vitro transcription-translation system. Three open reading frames essential for plasmid mobilization and two which affect the mobilization frequency were identified. There was a distinct similarity in the sizes, amino acid sequences, and locations of the proteins from the mobilization region of pTF-FC2 and the Tra1 region of IncP plasmid RP4. Similarity in the structures and sequences of the oriT regions was also apparent. A sequence with 37-of-38-bp homology to the inverted repeated sequences of Tn21 and an open reading frame with strong homology to the MerR regulatory protein was identified outside of the region required for mobilization.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dorrington R. A., Bardien S., Rawlings D. E. The broad-host-range plasmid pTF-FC2 requires a primase-like protein for autonomous replication in Escherichia coli. Gene. 1991 Dec 1;108(1):7–14. doi: 10.1016/0378-1119(91)90481-p. [DOI] [PubMed] [Google Scholar]
- Dorrington R. A., Rawlings D. E. Characterization of the minimum replicon of the broad-host-range plasmid pTF-FC2 and similarity between pTF-FC2 and the IncQ plasmids. J Bacteriol. 1990 Oct;172(10):5697–5705. doi: 10.1128/jb.172.10.5697-5705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington R. A., Rawlings D. E. Identification and sequence of the basic replication region of a broad-host-range plasmid isolated from Thiobacillus ferrooxidans. J Bacteriol. 1989 May;171(5):2735–2739. doi: 10.1128/jb.171.5.2735-2739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M., Zanga P., Lau P. C. The mobilization and origin of transfer regions of a Thiobacillus ferrooxidans plasmid: relatedness to plasmids RSF1010 and pSC101. Mol Microbiol. 1990 Aug;4(8):1381–1391. doi: 10.1111/j.1365-2958.1990.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Furuya N., Komano T. Determination of the nick site at oriT of IncI1 plasmid R64: global similarity of oriT structures of IncI1 and IncP plasmids. J Bacteriol. 1991 Oct;173(20):6612–6617. doi: 10.1128/jb.173.20.6612-6617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr The acidophilic thiobacilli and other acidophilic bacteria that share their habitat. Annu Rev Microbiol. 1984;38:265–292. doi: 10.1146/annurev.mi.38.100184.001405. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Toyoshima A., Morita K., Nisioka T. Cloning and nucleotide sequence of the oriT region of the IncI1 plasmid R64. J Bacteriol. 1988 Sep;170(9):4385–4387. doi: 10.1128/jb.170.9.4385-4387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora G., Silver S., Misra T. K. Down regulation of the mercury resistance operon by the most promoter-distal gene merD. Mol Gen Genet. 1989 Dec;220(1):69–72. doi: 10.1007/BF00260858. [DOI] [PubMed] [Google Scholar]
- Pansegrau W., Balzer D., Kruft V., Lurz R., Lanka E. In vitro assembly of relaxosomes at the transfer origin of plasmid RP4. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6555–6559. doi: 10.1073/pnas.87.17.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansegrau W., Ziegelin G., Lanka E. The origin of conjugative IncP plasmid transfer: interaction with plasmid-encoded products and the nucleotide sequence at the relaxation site. Biochim Biophys Acta. 1988 Dec 20;951(2-3):365–374. doi: 10.1016/0167-4781(88)90108-x. [DOI] [PubMed] [Google Scholar]
- Rawlings D. E., Pretorius I., Woods D. R. Expression of a Thiobacillus ferrooxidans origin of replication in Escherichia coli. J Bacteriol. 1984 May;158(2):737–738. doi: 10.1128/jb.158.2.737-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D. E., Woods D. R. Mobilization of Thiobacillus ferrooxidans plasmids among Escherichia coli strains. Appl Environ Microbiol. 1985 May;49(5):1323–1325. doi: 10.1128/aem.49.5.1323-1325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz P., Haring V., Wittmann-Liebold B., Ashman K., Bagdasarian M., Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989 Feb 20;75(2):271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Stanssens P., Opsomer C., McKeown Y. M., Kramer W., Zabeau M., Fritz H. J. Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nucleic Acids Res. 1989 Jun 26;17(12):4441–4454. doi: 10.1093/nar/17.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Ziegelin G., Pansegrau W., Strack B., Balzer D., Kröger M., Kruft V., Lanka E. Nucleotide sequence and organization of genes flanking the transfer origin of promiscuous plasmid RP4. DNA Seq. 1991;1(5):303–327. doi: 10.3109/10425179109020786. [DOI] [PubMed] [Google Scholar]