Abstract
The impact of invasive species on biodiversity has attracted considerable study, but impacts of the invasion process on the invaders themselves remain less clear. Invading species encounter conditions different from those in their ancestral habitats and are subject to intense selection for rapid dispersal. The end result may be significant stress on individual organisms, with consequent health problems. Our studies on invasive cane toads in Australia reveal severe spinal arthritis in ≈10% of large adult toads, associated with the same factors (large body size, frequent movement, and relatively long legs) that have enabled toads to invade so rapidly across the Australian tropics.
Keywords: anuran, invasive species, locomotion, spondylitis, colonization
The increasing rate at which humans translocate animals around the world has resulted in a virtual epidemic of biological invasions, with taxa from many lineages flourishing in the novel environments to which they have been introduced and degrading native ecosystems (1). Although the transfer of pathogens and parasites from invasive taxa to native species has been recognized as a potential mechanism of impact (2), less attention has been paid to the health of the invasive species itself. Presumably, many introductions fail because local diseases and parasites are fatal to the invading taxon (2), but there may be another as yet unrecognized health cost to the invader: stresses imposed by the invasion process. Not only does the invader have to cope with novel challenges in its new environment, but the invasion process strongly favors individuals on the invasion front that undertake rapid dispersal that facilitates range expansion (3, 4). Such an evolved shift in individual movement patterns may impose massive challenges on the locomotor system.
Cane toads (Chaunus [Bufo] marinus) are very large (up to >2 kg), highly toxic anurans native to South and Central America. They have been intentionally translocated to more than 40 countries in attempts to control agricultural pests (5). The resultant ecological havoc has rendered the cane toad one of the world's most notorious invasive species (6). Introduced to northeastern Australia in 1935, the toads have expanded their range across more than a million square kilometers of the country and, on the invasion front, have evolved to move frequently and rapidly. Invasion-front toads average >130 m of displacement per day during the rainy season, whereas toads from old-established populations average approximately one-third this distance (4); part of this difference is due to morphological evolution [radio-tracked toads with longer legs and larger body size move further each day, and the invasion front is dominated by large toads with relatively long legs (7)] and part is due to evolved behavioral shifts [invasion-front toads move more often and further and follow straighter paths (4)].
Results
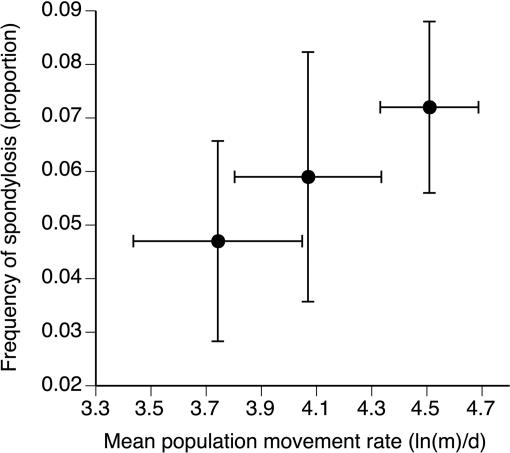
While conducting research on a cane toad invasion front in the Northern Territory (7), we noticed a high incidence of spinal abnormalities. Cane toads have nine vertebrae separated by synovial joints (not cartilaginous discs as in mammals) (8), and ≈10% of large adult toads (>110-mm snout-urostyle length) in these frontal populations had large bony growths fusing one or more of those joints (Fig. 1). The arthritic condition is absent in smaller toads (logistic regression with toad body size vs. arthritis occurrence: χ2 = 13.94; df = 1; P < 0.0002; see Fig. 2A). The frequency of spinal arthritis also is correlated with the mean rate of movement measured in toads from each of three populations spanning much of the species' invasion history in Australia (based on radio-tracking in a common garden situation: n = 3 populations; r = 0.99; P = 0.039; see Fig. 3), with frequency of spondylosis and rates of movement both highest in toads from the invasion front.
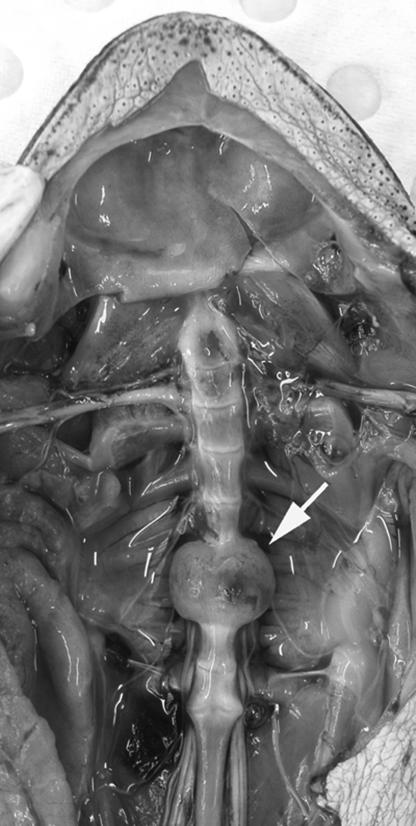
Fig. 1.
Dissection of an adult cane toad with viscera removed showing ventral view of spinal column. Note the marked enlargement of bodies of vertebrae 7 and 8 with fusion of intervertebral joint.
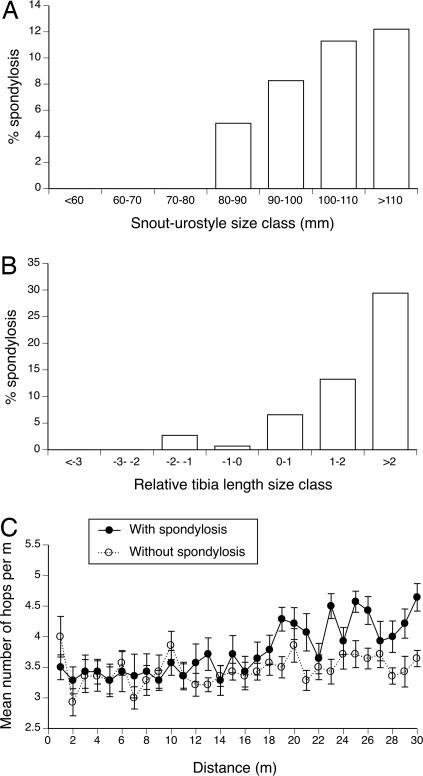
Fig. 2.
Spinal arthritis in cane toads at an invasion front in tropical Australia is more common in larger animals (A), is more common in animals with relatively long hindlimbs compared with body length (relative leg length is calculated as the residual score from the general linear regression of tibia length vs. snout-urostyle length) (B), and causes the afflicted animals to take shorter hops (C).
Fig. 3.
Relationship between prevalence of spinal arthritis and mean rates of movement (based on radio-tracking in common garden field environment) for cane toads from two long established populations [Normanton (left) and Borroloola (center)] and the invasion front [Middle Point, near Darwin (right)]. Error bars represent 1 SE. Populations characterized by more extensive movements also had a higher incidence of spinal arthritis.
Bacterial culture of affected joints yielded Ochrobactrum anthropi [99.9% certainty of identification by apiweb (bioMérieux)in seven of nine cultures vs. absent in four cultures from nonarthritic joints (df = 1; Fisher's exact test P < 0.025)]. The severity of joint impairment was highest in the most posterior joints (joint number vs. severity score: F1,367 = 17.35; P < 0.0001), as expected from the greater stresses on these joints during toad locomotion (9, 10). Further evidence suggesting a causal link between prolonged movement and joint inflammation comes from the following: (i) locomotor tests [after the first 15 m, arthritic toads covered increasingly less distance per hop than did healthy conspecifics of the same body size (see Fig. 2C; interaction distance traveled × spinal condition: F29,754 = 2.04; P < 0.0015)]; and (ii) a higher incidence of arthritis in toads with relatively longer legs (see Fig. 2B; after controlling for body size: χ2 = 17.45; df = 1; P < 0.0001), another trait associated with frequent rapid dispersal (7). Radio-tracking of free-ranging toads shows that the arthritic animals continue to move at least as far and fast under field conditions as do nonaffected conspecifics (mean distances 157 vs. 144 m per day respectively, based on 11 pairs of size-matched animals monitored over the same time periods: F1,20 = 0.06; P = 0.81). Thus, when pushed to move, spondylitic toads rapidly lose locomotor ability (presumably because of the pain associated with joint inflammation), but under field conditions spondylitic individuals move just as far as nonspondylitic individuals, despite the apparent handicap.
Discussion
Spinal infection rates in mammals increase with body size and age, suggesting a relationship with preexisting degenerative changes (11–13). The same may be true for toads with spinal arthropathy. Many toads with infected spinal joints also have other joints that show early degenerative changes with no evidence of infection (14). Published reports of O. anthropi associated with disease are limited to a few case reports in humans (15, 16). These reports commonly involve the sequelae of blood infection, including infection of the inner eye, heart valves and bone, and the sequelae are predisposed by immunosuppression (16). Invasion-front toads move consistently (every night, with no time for recovery) into novel (and often, suboptimal) habitats, a behavior that may render them more susceptible to infection by otherwise harmless bacteria (16, 17).
Our observations comprise a report of spinal arthritis in anurans and describe involvement of the ubiquitous soil bacteria O. anthropi in such a disease process. Why do Australian cane toads at the invasion front exhibit a high prevalence of this condition? Ironically, factors that have contributed to the toads' rapid spread across the continent also have rendered it susceptible to arthritis. First, cane toads are among the largest anurans. Larger body size provides advantages to a colonizer (e.g., a bigger animal can move faster, eat a wider range of prey, and withstand desiccation better and is less vulnerable to predators) but also increases susceptibility to arthritis [within lineages, larger species show a higher incidence (11)]. Second, the process of invasion appears to have selected for larger toad body sizes on the invasion front, so an already large anuran has been selected for larger size. Third, behavioral adaptations to the invasion process itself [notably, the shift to a highly active lifestyle (4, 7)] place more stress on the locomotor system, including the spine. And fourth, biomechanical stresses are exacerbated by the evolution of longer hindlimbs, which deliver greater propulsive force with each hop (10). Immunosuppression may play a role in many amphibian infections (18), and immunosuppression predisposes to spinal infections in other species (19, 20). Evidence is accumulating that show that invasive taxa invest less in their immune system, instead favoring “r-selected” life history strategies (21). An invader's immune system also may suffer from the reduced genetic variance associated with founder effects and genetic drift.
Hence, the invasion of northern Australia has modified cane toads in ways that have facilitated their rapid and successful spread across the continent but at the same time may have placed great challenges on the health of these animals. An anuran skeletal system that functions well for small, infrequently moving animals has been unable to tolerate the persistent activity of large, highly active, invasion-front toads. The major spinal deformations of these animals testify to the great stress that invading species may place upon themselves, as well as upon the ecosystems that they imperil. More generally, our study (demonstrating such stress) highlights not only the utility of invasive species for detecting responses to novel selective forces but also the value of incorporating wildlife health perspectives to fully understand the process of biological invasion.
Materials and Methods
Prevalence and Correlates of the Occurrence of Spondylosis.
We examined 495 preserved cane toads from three populations: 128 from Normanton, Queensland, Australia (17°S, 141°E, collected September 20, 2006), 102 from Borroloola, Northern Territory, Australia (16°S, 136°E, collected October 21, 2006), and 265 from Middle Point, Northern Territory, Australia (12°S, 131°E, collected from March 2005–2007). At the time of collection, toads had been present in these sites for 38, 17, and 0–2 years, respectively.
Effects of Spondylosis on Locomotor Performance of Toads.
We accumulated 14 toads (six female and eight male) with spondylosis (diagnosed by palpation of live toads). We matched each of these individuals with a toad of the same size and sex but with normal spines (later confirmed by postmortem examination). To test their locomotor performance, toads were raced down a 10-m runway three times in immediate succession, on a single night, in random order (without the investigator knowing their spinal condition).
Telemetry.
We fitted 11 pairs of toads matched by size and sex but differing in spinal condition (determined by palpation) with radio transmitter belts. These individuals were sampled from the frontal populations only. These pairs of toads were released on the same night at the same place, and located daily over 5 days by using a global positioning system receiver. In another set of trials, data on the mean movement rate of toads from Normanton, Borroloola, and Darwin were calculated from individuals collected at each of these locations and radio-tracked contemporaneously at the same location (Darwin) (see ref. 4 for detailed methods). Movement rate was expressed as the probability of moving per day multiplied by the average distance moved per day, a metric that accurately predicts long-term movement rates in this species (4).
Bacteriology.
Aseptic technique was used to obtain swabs from intervertebral joints of nine toads with spinal arthritis and four with normal spines. Swabs were inoculated onto tryptone soya agar with sheep blood and MacConkey agar (Oxoid, Thebarton, Australia). Media plates incubated at 30°C were checked for growth at 24 and 48 h. Bacteria were identified with the phenotypic systems AP 20 NE (bioMérieux, Baulkham Hills, Australia).
Ethics Approval.
All research procedures were approved by the Animal Ethics Committee of the University of Sydney, Australia.
Acknowledgments
We thank Haley Bowcock, Matt Greenlees, John Llewelyn, and James Smith for assistance in the collection of toads. This work was supported by the Australian Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17561.
References
- 1.Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E. Bioscience. 1998;48:607–615. [Google Scholar]
- 2.Lafferty KD, Smith KF, Torchin ME, Dobson AP, Kuris AM. In: Species Invasions: Insights Into Ecology, Evolution and Biogeography. Sax DF, Stachowicz JJ, Gaines SD, editors. Sunderland, MA: Sinauer; 2005. pp. 111–134. [Google Scholar]
- 3.Travis JMJ, Dytham C. Evol Ecol Res. 2002;4:1119–1129. [Google Scholar]
- 4.Phillips BL, Brown GP, Travis JMJ, Shine R. Am Nat. 2007 in press. [Google Scholar]
- 5.Lever C. The Cane Toad: The History and Ecology of a Successful Colonist. Otley, West Yorkshire: Westbury Academic and Scientific Publishing; 2001. [Google Scholar]
- 6.Global Invasive Species Database. [Accessed April 13, 2007]; www.issg.org/database/species/search.asp.
- 7.Phillips BL, Brown GP, Webb JK, Shine R. Nature. 2006;439:803. doi: 10.1038/439803a. [DOI] [PubMed] [Google Scholar]
- 8.Duellmann WE, Trueb L. Biology of Amphibians. Baltimore: The Johns Hopkins Univ Press; 1986. [Google Scholar]
- 9.Emerson SB, De Jongh HJ. J Morphol. 1980;166:129–144. doi: 10.1002/jmor.1051660202. [DOI] [PubMed] [Google Scholar]
- 10.Choi IN, Shim JH, Lee YS, Ricklefs RE. J Herpetol. 2000;34:222–227. [Google Scholar]
- 11.Nunn CL, Rothschild B, Gittleman JL. J Evol Biol. 2007;20:460–470. doi: 10.1111/j.1420-9101.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 12.Burkert BA, Kerwin WC, Hosgood GL, Pechman RD, Ponti Fontenelle J. J Am Vet Med Assoc. 2005;227:268–275. doi: 10.2460/javma.2005.227.268. [DOI] [PubMed] [Google Scholar]
- 13.Lucio E, Adesokan A, Hadjipavlou AG, Crow WN, Adegboyega PA. Arch Pathol Lab Med. 2000;124:712–716. doi: 10.5858/2000-124-0712-PS. [DOI] [PubMed] [Google Scholar]
- 14.Shilton C, Brown GP, Benedict S, Shine R. Vet Pathol. 2007 doi: 10.1354/vp.45-1-85. in press. [DOI] [PubMed] [Google Scholar]
- 15.Vaidya SA, Citron DM, Fine MB, Murakami G, Goldstein EJC. J Clin Microbiol. 2006;44:1184–1186. doi: 10.1128/JCM.44.3.1184-1186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollins-Smith LA, Cohen N. In: Amphibian Biology. Heatwole H, editor. Vol 6. Chipping Norton, Australia: Surrey Beatty and Sons; 2003. pp. 2377–2391. [Google Scholar]
- 17.Atkinson G, Davenne D. Physiol Behav. 2007;90:229–235. doi: 10.1016/j.physbeh.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor SK, Green DE, Wright KM, Whitaker BR. In: Amphibian Medicine and Captive Husbandry. Wright KM, Whitaker BR, editors. Melbourne, FL: Krieger; 2001. pp. 159–179. [Google Scholar]
- 19.Thomas WB. Vet Clin North Am Small Anim Prac. 2000;30:169–182. doi: 10.1016/s0195-5616(00)50008-4. [DOI] [PubMed] [Google Scholar]
- 20.Khan IA, Vaccaro AR, Zlotolow DA. Orthopedics. 1999;22:758–765. doi: 10.3928/0147-7447-19990801-07. [DOI] [PubMed] [Google Scholar]
- 21.Lee KA, Klasing KC. Tree. 2004;19:523–529. doi: 10.1016/j.tree.2004.07.012. [DOI] [PubMed] [Google Scholar]