Abstract
Sarcolipin is a novel regulator of cardiac sarcoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) and is expressed abundantly in atria. In this study we investigated the physiological significance of sarcolipin in the heart by generating a mouse model deficient for sarcolipin. The sarcolipin-null mice do not show any developmental abnormalities or any cardiac pathology. The absence of sarcolipin does not modify the expression level of other Ca2+ handling proteins, in particular phospholamban, and its phosphorylation status. Calcium uptake studies revealed that, in the atria, ablation of sarcolipin resulted in an increase in the affinity of the SERCA pump for Ca2+ and the maximum velocity of Ca2+ uptake rates. An important finding is that ablation of sarcolipin resulted in an increase in atrial Ca2+ transient amplitudes, and this resulted in enhanced atrial contractility. Furthermore, atria from sarcolipin-null mice showed a blunted response to isoproterenol stimulation, implicating sarcolipin as a mediator of β-adrenergic responses in atria. Our study documented that sarcolipin is a key regulator of SERCA2a in atria. Importantly, our data demonstrate the existence of distinct modulators for the SERCA pump in the atria and ventricles.
Keywords: atria, calcium uptake, sarcoplasmic reticulum Ca2+ ATPase 2
Sarcolipin (SLN), a low-molecular-weight protein (31 aa), is expressed in both cardiac and skeletal muscles (1–5). It colocalizes with sarcoplasmic reticulum (SR) Ca2+ ATPase (SERCA) in the cardiac SR (3) and physically interacts with the SERCA pump (6). Amino acid composition and structural analysis have suggested that SLN and phospholamban (PLB) may belong to the same family of proteins with similar functions (1, 7–9). Consistent with the notion, in vitro studies have shown that SLN can inhibit the SERCA activity by decreasing the apparent Ca2+ affinity of the pump (7, 10). Protein expression analyses have demonstrated that within the heart there are chamber-specific differences in the expression pattern of SLN and PLB (4). SLN is predominantly expressed in the atrial compartment, whereas PLB is abundant in the ventricles. In addition to atria, SLN is expressed in skeletal muscle tissues (4). SLN expression is regulated during cardiac and skeletal muscle development (2–4). Furthermore, SLN expression levels are altered in atria during cardiac pathology both in animal models (2, 4, 11–13) and in humans (14), suggesting that SLN levels may play an important role in maintaining atrial Ca2+ homeostasis during cardiac pathophysiology.
The importance of SLN as a regulator of the cardiac SERCA pump was recently demonstrated by using adenoviral gene transfer into adult rat ventricular myocytes (3) and transgenic overexpression of SLN in the heart (15–17). These studies suggest that overexpression of SLN into ventricular myocytes resulted in decreased rates of SR Ca2+ uptake, Ca2+ transient amplitude, and myocyte contractility. Overexpression of SLN in the PLB-null heart revealed that SLN can inhibit SERCA pump activity independent of PLB and can be relieved upon treatment with isoproterenol (ISO) (17).
Based on the available data, we hypothesized that SLN is a key regulator of SERCA2a in atria and that ablation of SLN would modify atrial Ca2+ transport and contractility. To test these hypotheses and to establish its role in cardiac physiology, we generated a SLN knockout mouse model. The data obtained in this study demonstrate that SLN acts as a major regulator of SERCA2a and could mediate the β-adrenergic responses in atria.
Results
Successful Generation of SLN Knockout Mouse Model.
Given the small size of SLN cDNA, we reasoned that the best strategy to create a null mouse was to eliminate the entire SLN coding sequence. The targeting vector consisted of 7.9 kb of mouse genomic sequence in which the internal 2.1-kb HindIII fragment containing the coding exon (exon 2) of SLN gene was replaced with a neomyocin resistance cassette by homologous recombination (Fig. 1A). Details on the generation of SLN knockout mice are described in Materials and Methods. The heterozygous (sln+/−) mice appeared normal and were fertile. Heterozygous mice have been intercrossed to generate SLN homozygous knockout (sln−/−) mice. Pups lacking SLN were born at the expected Mendelian ratio and appeared normal. Furthermore, sln−/− mice were viable and reached the adult stage without any gross morphological abnormalities. The absence of SLN expression was confirmed by analyzing the SLN mRNA by RT-PCR analysis and protein expression by Western blot analysis using a SLN-specific antibody (4). As expected, SLN mRNA and protein were absent in atria and ventricles of sln−/− mice (Fig. 1 B and C). These results demonstrate that we were successful in generating a mouse model deficient in SLN.
Fig. 1.
Targeted disruption of SLN gene. (A) Schematic representation of the SLN gene knockout strategy. Exon 2 was replaced with a neomycin gene in reverse orientation. (B) RT-PCR analysis of SLN mRNA expression in the atria and ventricles of WT and homozygous SLN knockout (sln−/−) mice. (C) Western blotting analysis of SLN protein expression. SR-enriched microsomal fractions prepared from atria (A, 1 μg), and ventricles (V, 5 μg) of WT and sln−/− mice were separated on a 16% Tricine PAGE and immunoprobed with anti-rabbit SLN antibody.
Ablation of SLN Did Not Affect the Tissue Morphology and Muscle Structure.
We next examined whether the absence of SLN results in any type of structural abnormalities and cardiac pathology. The heart weight to body weight ratio of sln−/− mice is not different from that of age- and sex-matched WT controls. Hematoxylin/eosin staining of tissues, including atria, ventricles, soleus, and quadriceps, did not reveal any significant difference in tissue morphology (data not shown). Electron microscopic analyses of atria and ventricular tissues from sln−/− mice revealed no obvious changes in muscle structure and content compared with the WT controls (data not shown).
Expression Levels of SR Ca2+ Handling Proteins Are Unchanged in the SLN Knockout Heart.
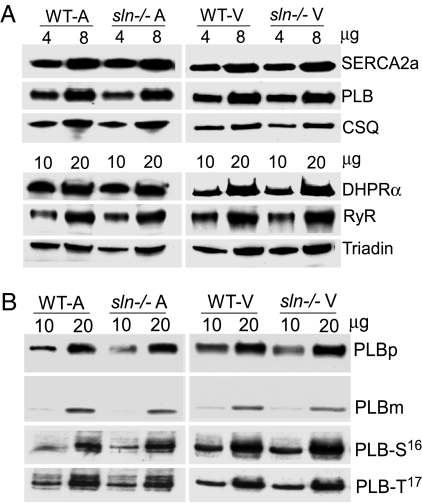
To determine whether ablation of SLN caused alterations in the expression levels of SERCA2a and PLB, quantitative Western blot analysis was carried out. Our results show that the expression levels of SERCA2a and PLB were unchanged in the sln−/− atria and ventricles (Fig. 2A). We also quantitated the expression levels of other major Ca2+ handling proteins, ryanodine receptor (RyR), the L-type Ca2+ channel subunit dihydropyridine receptor (DHPR) α2, calsequestrin (CSQ), and triadin, to determine changes in Ca2+ release and entry mechanisms. As shown in Fig. 2A, RyR, DHPRα2, CSQ, and triadin protein levels were unaffected in the sln−/− hearts.
Fig. 2.
Quantification of SR Ca2+ handling proteins. (A) Two different concentrations of total homogenates (4 and 8 μg for SERCA2a, PLB, and CSQ; 10 and 20 μg for DHPRα, RyR, and triadin) prepared from atrial and ventricular tissues of WT and sln−/− mice were separated on SDS/PAGE and immunoprobed with specific antibodies. (B) Total homogenates prepared from sln−/− atria and ventricles were unboiled and analyzed for PLB monomers (PLBm) and pentamers (PLBp) by Western blot analysis. To determine the basal phosphorylation of PLB at serine-16 (PLB-S16) and threonine-17 (PLB-T17), snap-frozen samples were processed for protein extraction and immunoprobed with S16- or T17-specific PLB antibodies. Data shown are representative of three independent experiments.
Basal Phosphorylation of PLB and Its Monomer-to-Pentamer Ratio Are Not Affected in the sln−/− Heart.
SLN was shown to affect the PLB monomer-to-pentamer ratio (10). Therefore, we tested whether the absence of SLN altered the PLB monomer-to-pentamer ratio and the basal phosphorylation of PLB by Western blot analysis. Our results (Fig. 2B) clearly show that the monomer-to-pentamer ratio of PLB was not altered in the sln−/− atria (WT = 23.93/76.07 ± 1.6; sln−/− = 25.18/74.82 ± 1.0) and ventricles (WT = 21.93/78.07 ± 0.6; sln−/− = 21.54/78.46 ± 0.9). Furthermore, the basal phosphorylation of PLB at serine-16 and threonine-17 were not different between sln−/− and WT control hearts (Fig. 2B). These results suggest that ablation of SLN did not affect PLB basal phosphorylation or its monomer-to-pentamer ratio.
Ablation of SLN Increases the Apparent Ca2+ Affinity and Rate of Ca2+ Uptake.
Previously we have shown that overexpression of SLN in the heart decreases the rate of Ca2+ uptake by the SR (16). To determine how loss of SLN affected the SR Ca2+ transport function, we measured rates of SR Ca2+ uptake in total homogenates from sln−/− atria and ventricles. Results in Fig. 3 suggest that SLN plays a more prominent role in atrial myocytes in comparison to the ventricles. Ca2+ uptake was only slightly altered in the ventricular myocytes compared with atrial myocytes in sln−/− hearts (see Fig. 3). Specifically, the rate of Ca2+-dependent Ca2+ uptake was significantly increased both in atria (Fig. 3A) and ventricles (Fig. 3B) of sln−/− mice when compared with the age- and sex-matched WT control hearts. The EC50 value for Ca2+ decreased significantly in sln−/− atria (WT = 119 ± 9 nM; sln−/− = 95 ± 7 nM; P < 0.03) and ventricles (WT = 215.0 ± 21 nM; sln−/− = 153.4 ± 10 nM; P < 0.02) when compared with WT hearts. On the other hand, the maximum velocity (Vmax) of Ca2+ uptake was significantly increased in the sln−/− atria (WT = 17.11 ± 0.5 nmol of Ca2+ per mg·min−1; sln−/− = 27.47 ± 1.0 nmol of Ca2+ per mg·min−1; P < 0.01) but not in the sln−/− ventricles. Of note, in our experimental conditions, the rate of Ca2+ uptake in the atrial homogenate was similar to that of previously published results (18). However, the rate of Ca2+ uptake for ventricles was much higher than for atria. We do not have any satisfactory explanation for this difference, and future studies are needed to explore it.
Fig. 3.
Calcium uptake function in sln−/− atria and ventricles. Ca2+ uptake assays were performed by using total homogenates from atria (A) and ventricles (B) of 24-week-old mice. For each atrial experiment, atria from four mice were pooled. n = 4 for each group. The Vmax of Ca2+ uptake was obtained at pCa 6.0.
SLN Knockout Mice Showed an Increased Maximum Rate of Contraction in Isolated Work-Performing Heart Preparations.
We next examined the effects of SLN ablation on myocardial contractility using the anterograde-perfused work-performing heart preparation in parallel with WT control hearts under identical load conditions. The maximum rate of contraction (+dP/dt) was significantly increased in sln−/− hearts (Table 1). The maximum rate of relaxation (−dP/dt) and systolic and end diastolic pressures in the sln−/− hearts were not significantly different from WT control hearts. The other parameters of cardiac function, such as time to peak pressure and half-relaxation pressure derived from intraventricular pressure tracings, were not altered in the sln−/− hearts (Table 1).
Table 1.
Contractile parameters in sln−/− hearts in the isolated work-performing heart preparations at 6 Hz
| Mice | Maximal rate pressure development, mmHg/s | Maximal rate pressure decline, mmHg/s | Left ventricular systolic pressure, mmHg | Left ventricular diastolic pressure, mmHg | Left ventricular end diastolic pressure, mmHg | Time to peak pressure, ms/mmHg | Half relaxation pressure, ms/mmHg |
|---|---|---|---|---|---|---|---|
| WT (n = 5) | 3,295 ± 98 | 3,186 ± 207 | 107.7 ± 5.6 | −8.4 ± 1.6 | 8.3 ± 1.6 | 0.43 ± 0.039 | 0.52 ± 0.024 |
| sln−/− (n = 8) | 3,840 ± 108* | 3,770 ± 170 | 122.0 ± 6.1 | −8.2 ± 2.1 | 11.6 ± 3.7 | 0.42 ± 0.021 | 0.49 ± 0.051 |
*Statistically significant from WT.
To determine the sln−/− heart's ability to respond to β-adrenergic stimulation, hearts from sln−/− and WT littermates were subjected to perfusion with increasing concentrations of ISO under similar load conditions and the maximal rates of pressure development (dP/dt) were measured. At low concentrations of ISO, the rate of contraction (+dP/dt) was significantly higher in the sln−/− hearts than in the WT control hearts. However, at high doses of ISO infusion, the maximal rate of contraction in sln−/− hearts was not different from that of control hearts [supporting information (SI) Fig. 6].
Ablation of SLN Increases Contractility of the Atria.
SLN is abundant in atria (2–4), and therefore we next studied how loss of SLN affects atrial muscle contractility. The left atria contracted spontaneously, and the frequency of contraction was not different between WT and sln−/− atria. Under basal conditions with 2 mmol/liter [Ca]o, isometric force generation was ≈1.6 times higher in the sln−/− atria (WT = 3.74 ± 0.40 mN; sln−/− = 6.00 ± 0.47 mN; P < 0.01) compared with the age- and sex-matched WT controls (Fig. 4A). When the extracellular Ca2+ was increased from 2 mM to 4 and 6 mM, the force generation gradually increased in the WT atria. However, this increase did not reach the basal (in 2 mM Ca2+) contractility of sln−/− atria (Fig. 4A). Increase in [Ca]o did not have any significant effect on the isometric force generation of sln−/− atria, indicating that the basal contraction is already maximal in these tissues. The time taken for 50% relaxation (RT50) and 90% relaxation (RT90) were significantly decreased in the sln−/− atria (Fig. 4 B and C) compared with the WT controls, indicating faster relaxation of sln−/− atria. Time to peak tension was similar between WT and sln−/− atria (WT = 83 ± 6 ms; sln−/− = 84 ± 4 ms).
Fig. 4.
Mechanical properties of sln−/− atria. (A) Effect of extracellular Ca2+ and ISO on the isometric force generation in isolated left atria from WT and sln−/− mice. #, isometric force generation in sln−/− atria (at 2 mM Ca2+) was significantly different from WT atria at all Ca2+ concentrations; *, isometric force generation in WT atria at 6 mM Ca2+ and ISO stimulation was significantly different from the basal contraction at 2 mM Ca2+ (P < 0.05). NS, not significant. Time taken for 50% relaxation (RT50) (B) and 90% relaxation (RT90) (C) was significantly faster in the sln−/− atria. *, P < 0.05 (significant difference between WT and sln−/− groups). n = 5.
To determine how loss of SLN affects β-adrenergic-mediated atrial contraction, the atrial muscles were challenged with 1 μM ISO and the contractile parameters were studied. ISO significantly increased the frequency of contraction in WT and sln−/− atria, as expected, but no difference was observed between the groups. In response to ISO, the force generation in the WT atria was increased (WT without ISO = 3.74 ± 0.40 mN; WT with ISO = 7.35 ± 0.52 mN; P < 0.01) and reached levels similar to those of sln−/− atria (Fig. 4A). On the other hand, ISO stimulation of sln−/− atria did not have an effect on isometric force generation, indicating a blunted response of the sln−/− atria to β-adrenergic response.
Ca2+ Transient Amplitude Was Significantly Increased in sln−/− Atrial Myocytes.
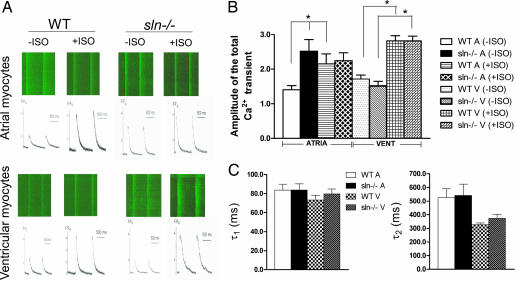
Increased Ca2+ uptake, as observed in the sln−/− atria, could affect overall Ca2+ homeostasis. Therefore, we determined how loss of SLN affects Ca2+ transient amplitudes in sln−/− atrial compared with ventricular myocytes using confocal line scan imaging. Ca2+ transients were fitted by using two exponential functions. Confocal line scan images of Ca2+ transients for WT and sln−/− cardiac myocytes (Fig. 5A) show a significant increase in the Ca2+ transients in atrial myocytes of sln−/− compared with WT. In contrast, there were no significant changes in the Ca2+ transient in the ventricular myocytes. Further analyses shown in Fig. 5B indicate that the amplitude of the total Ca2+ transient (Fig. 5A) was significantly increased in the atrial myocytes from sln−/− compared with WT control. In addition, there were no significant differences in the amplitude of the total Ca2+ transient in ventricular myocytes isolated from WT and sln−/− mice. These data implicate SLN as an important modulator of SERCA pump activity in atria. Fig. 5C shows the summary data for the fast and slow time constants (τ1 and τ2) of the Ca2+ transient decay at baseline using two exponential functions. There were no significant differences in the fast or slow time constants between atrial or ventricular myocytes isolated from WT and sln−/− animals.
Fig. 5.
Ca2+ transients in atrial and ventricular myocytes isolated from WT and sln−/− mice. (A) Representative examples of line-scan images in response to ISO stimulation showing kinetics of Ca2+i transients recorded from atrial and ventricular myocytes of sln−/− mice compared with respective WT littermates. Sample records show a line-scan fluorescence images (Upper) and Ca2+i, measured as F/Fo (Lower). (B) Summary data for the amplitude of the total Ca2+ transient (A) obtained at baseline and after ISO stimulation for atrial and ventricular myocytes. n = 28 [WT A (−ISO)], 17 [sln−/− A (−ISO)], 20 [WT V (−ISO)], 19 [sln−/− V (−ISO)], 9 [WT A (+ISO)], 13 [sln−/− A (+ISO)], 17 [WT V (+ISO)], and 32 [sln−/− V (+ISO)] isolated from five to seven animals per group. (C) Summary data for the fast and slow time constants (τ1 and τ2) of the Ca2+ transient decay at baseline using two exponential functions.
Effects of ISO.
We additionally evaluated the effect of β-adrenergic stimulation on Ca2+ transients in atrial and ventricular myocytes using ISO. As shown in Fig. 5, in WT atrial myocytes the total Ca2+ transient amplitude was significantly increased upon ISO treatment. In contrast, in sln−/− atrial myocytes the total Ca2+ transient amplitude did not further increase after ISO treatment. These results indicate that the SERCA pump is maximally activated in the sln−/− atrial myocytes and that ISO stimulation did not further increase pump activity. In sln−/− ventricular myocytes ISO stimulation resulted in a significant increase in the amplitude of the total Ca2+ transient (Fig. 5A), and these increases were similar to that of ISO-stimulated WT myocytes.
Discussion
SLN was discovered nearly 20 years ago; however, the role of SLN has only been investigated in the past few years. Although recent studies have demonstrated that SLN is an inhibitor of SERCA2a (reviewed in refs. 8, 9, 19, and 20), its precise role in cardiac contractility is not fully understood. In this study we generated a SLN knockout mouse model and demonstrate that SLN is a key regulator of SERCA2a in atrial myocytes.
A major finding of the present study is that ablation of SLN increases the affinity of the SERCA pump for Ca2+, resulting in the enhanced rates of SR Ca2+ uptake. Our data also lend support to the previous findings, which showed that SLN overexpression can decrease the rates of SR Ca2+ uptake and cardiac contractility (3, 15–17). In addition, loss of SLN in the ventricles resulted in an enhanced rate of Ca2+ uptake, suggesting that low levels of SLN in the ventricles (4) may have functional significance. This was further supported by the increased basal contractility observed in ex vivo heart preparations. On the other hand, SLN is predominantly expressed in atrial myocytes, and the effects of SLN ablation are more pronounced in atria than in the ventricles. Although SLN has been shown to affect the affinity of the SERCA pump for Ca2+, its effect on the maximum velocity (Vmax) of Ca2+ uptake is less well defined. We report that ablation of SLN is associated with an increase in the Vmax of Ca2+ uptake rates in atria, suggesting that SLN could regulate the kinetics of the ATPase activity at one or more steps. Our data are also supported by the previous findings by Tupling et al. (21), which showed that transient expression of SLN in rat soleus muscle can decrease the maximal Ca2+ transport activity. In contrast, the absence of SLN did not affect the Vmax of Ca2+ uptake rates in the ventricles. This could be explained by the differential expression of SLN and PLB in the ventricles (2–4). PLB is the predominant regulator of SERCA2a in the ventricles, whereas SLN is a minor component. Therefore, ablation of SLN function is not expected to have a significant effect on the Vmax of Ca2+ uptake rates in the ventricles. The finding that SLN regulates Vmax of Ca2+ uptake suggests that SLN functionally differs from PLB. Taken together, our data suggest that SLN could play unique roles in atrial Ca2+ uptake and may contribute to the differences in Ca2+ handling between atria and ventricles (22).
Another important finding of this study is increased Ca2+ transient amplitude in the sln−/− atrial myocytes, and this is most likely because of the increase in the rate of Ca2+ uptake. Consistent with the enhanced SR Ca2+ uptake, the rate of atrial muscle relaxation is also faster in the sln−/− mice. On the other hand, ablation of SLN in the ventricles did not affect the rates of Ca2+ transient amplitude and muscle relaxation. Nonetheless, cautions need to be taken in our interpretation of the Fluo-4 measurement of the Ca2+ transients. Fluo-4, like fluo-3, has a relatively high affinity for Ca2+ with the dissociation constant (kd) of 1.1 μM (in accordance with the value for fluo-3 in cells) (23, 24). Accordingly, the dynamic range achievable for these studies may be limited. Therefore, the lack of differences observed between the groups after ISO may result from the inherent limitations. On the other hand, fluo-4 has been widely used for intracellular Ca2+ measurement in cardiac myocytes (25). We also found that loss of SLN function resulted in an increase in rate of contraction in isolated atria. This is in contrast to PLB, which has been shown to be only a repressor of basal relaxation in the atria (26). Thus, our data strongly suggest that SLN is the major regulator of the SERCA pump in the atria and muscle contraction.
In this study we further investigated whether SLN is a key mediator of β-adrenergic responses in atria. Earlier studies have shown that the β-adrenergic activation of SR Ca2+ uptake and contractile indices are higher in atria despite low levels of PLB (27). Studies from PLB transgenic and knockout mouse models also suggest that PLB contributes, only in part, to the β-adrenergic-mediated atrial contractile response (26), and there may be proteins other than PLB involved in mediating the β-adrenergic response in atria. In this study we demonstrate that ablation of SLN in the atria resulted in a blunted response to ISO whereas the ventricular myocytes from sln−/− preserve the β-adrenergic response. These results suggest that SLN is a key player in mediating the β-adrenergic responses in atria. This finding is further supported by the data from a transgenic mouse model overexpressing SLN in a PLB-null background, which demonstrate that the inhibitory effect of SLN can be relieved upon ISO treatment (17). Taken together, our results point to the important distinction between atrial and ventricular myocytes under β-adrenergic control, namely, that the increase in SERCA pump function under β-adrenergic stimulation in the atria is largely mediated by SLN, whereas in the ventricles it is mediated by PLB, as suggested by previous literature (reviewed in refs. 28 and 29).
The mechanism by which SLN responds to β-adrenergic activation is yet to be determined. SLN has a highly conserved threonine residue (T5) at the amino terminus (1), and it was recently shown that mutation of T5A abolished the inhibitory effect of SLN on SERCA function (7, 17). These studies further identified T5 as a potential phosphorylation site and showed that serine–threonine protein kinase 16 (STK16) could phosphorylate SLN (17). However, the physiological relevance of STK16 and its role during β-adrenergic stimulation are yet to be investigated. Preliminary in vitro phosphorylation data from our laboratory (our unpublished results) showed that SLN can be phosphorylated by CaMKII at the T5. Therefore, it is fitting to suggest that SLN phosphorylation might play an important role in mediating β-adrenergic responses of atria. Future studies examining the T5 amino acid are needed to shed light on the regulatory mechanisms of SLN.
In summary, using a SLN-deficient mouse model, we have demonstrated that SLN is a key modulator of SERCA function and plays a critical role in atrial contractility. Increased Ca2+ uptake, Ca2+ transient amplitudes, and muscle contractility observed in sln−/− atria provide strong evidence for the regulatory role of SLN in SR Ca2+ uptake function and the functional significance of SLN expression in the atria. Furthermore, our study provides evidence that SLN could play a major role in mediating β-adrenergic responses in atria.
Materials and Methods
All experiments were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at Ohio State University, the University of Cincinnati, and the University of California, Davis.
Generation of SLN Knockout Mice.
A 20-kb genomic DNA fragment, containing the entire coding and 5′ and 3′ regions of the SLN gene, was isolated from a λ/129 mouse genomic library. To ablate the SLN gene, a targeting vector consisted of 7.9 kb of mouse genomic sequence in which the internal 2.1-kb HindIII fragment, containing the coding exon of SLN gene and some 5′ and 3′ untranslated regions, was replaced with a neomycin gene under the phosphoglycerate kinase promoter. A copy of the thymidine kinase gene was attached to the 3′ end of the targeting construct (Fig. 1A). Electroporation and blastocyst injection of targeted ES cells were performed by the University of Cincinnati Gene Knockout Mouse service facility. Chimeric male mice were mated to B6 females, and germ-line transmission of the targeted allele was detected by PCR as described above. Heterozygous male and female mice were intercrossed to generate SLN-null mice. Five chimeric male mice were generated by blastocyst injection of targeted ES cell lines. To generate heterozygous mice for SLN (sln+/−), male chimeras were mated to C56 Black Swiss female mice. Heterozygous mice were intercrossed to generate SLN homozygous knockout (sln−/−) mice.
Determination of SLN Expression by RT-PCR.
Total RNA was isolated from atria and ventricles by using the ULTRASPEC-II RNA Isolation System (Biotecx Laboratories, Houston, TX). RT-PCR analysis was performed by using 1 μg of total RNA as described earlier (3). After oligo(dT) primed first-strand cDNA synthesis, a 1-μl portion of the first-strand cDNA mixture was subjected to PCR using primers specific for mouse SLN (forward, 5′-GCACTAGGTCCTTGGCATGT-3′; reverse, 5′-ACTCAAGGGACTGGCAGAGA-3′) and mouse GAPDH (forward, 5′-CCCATCACCATCTTCCAGGA-3′; reverse, 5′-TTGTCATACCAGGAAATGAGC-3′). The PCR protocols were as follows: 94°C for 30 sec, 55°C for 30 sec, and 75°C for 60 sec (35 cycles) with a 72°C extension for 7 min.
Western Blot Analysis.
Total tissue homogenate and microsomal fraction enriched with SR membrane proteins were prepared from WT and sln−/− atria and ventricles as described earlier (4, 16). For Western blot analysis, equal amounts of protein from SLN WT and sln−/− tissues were separated by using SDS/PAGE (5% for RyR, 8% for SERCA and CSQ, 10% for DHPRα2 and triadin, and 14% for PLB) or 16% Tricine gel for SLN and transferred to nitrocellulose membrane. Membranes were immunoprobed with the following primary antibodies: anti-rabbit SLN (4), anti-rabbit SERCA2a, anti-rabbit PLB, anti-rabbit CSQ (Affinity BioReagents), anti-mouse DHPRα2, anti-rabbit triadin, anti-rabbit S16, or T17 PLB antibody (Cyclacel, Dundee, U.K.). Protein loading was normalized to α-actin levels by using Coomassie staining. Signals were detected by Super Signal WestDura substrate (Pierce) and quantitated by densitometry.
Ca2+ Uptake Assay.
Atria and ventricles from WT and sln−/− mice were used for SR Ca2+ uptake assays as described earlier (16, 30). Briefly, atrial or ventricular tissue was homogenized in 8 volumes of protein extraction buffer (50 mmol/liter KPi, 10 mmol/liter NaF, 1 mmol/liter EDTA, 300 mmol/liter sucrose, 0.5 mmol/liter DTT, and 0.3 mmol/liter PMSF), and Ca2+ uptake was measured by the Millipore filtration technique. The rate of SR Ca2+ uptake and the Ca2+ concentration required for half-maximal velocity of Ca2+ uptake (EC50) were determined by nonlinear curve fitting analysis using Prism 4.0 software (GraphPad).
Isolated Work-Performing Heart Preparations.
Work-performing hearts were studied as described previously (16).
Mechanical Studies on Atria.
Left atria from WT and sln−/− mice were used for this study, and the right atria were excluded because of automatic pacemaker activity. The left atrium from each mouse (14–16 weeks old) was attached to an isometric force transducer (sensitivity 2 V/g; model 400A; Aurora Scientific) and a fixed post by using quick-setting glue and suspended in a 20-ml organ bath containing constantly oxygenated (95% O2 and 5% CO2) Ringer's bicarbonate buffer containing 137 mM NaCl, 5 mM KCl, 13 mM NaHCO3, 1.8 mM KH2PO4, 2 mM CaCl2, 11 mM glucose, and 1 mM MgSO4 (pH 7.4) at 23°C. The tip of the atrial appendage was glued to a fine wire that was attached to the force transducer, and the base of the atrium (just below the atrioventricular septum) was glued to the plastic rim of a syringe needle that was clamped to the fixed post in the chamber. The chamber was held in the frame of an in vitro muscle test apparatus (model 800A; Aurora Scientific) with an integrated electrode assembly consisting of two parallel platinum electrodes. Most of the isolated atria that were studied spontaneously contracted at a frequency of ≈1.5 Hz. The atria were stretched to determine the maximum contraction amplitude. We measured the amplitude of force generation, the time to peak isometric force, and the times to 50% and 90% relaxation. These parameters were measured in Ringer's solution containing 2, 4, and 6 mM Ca2+ and in Ringer's solution containing 2 mM Ca2+ plus 1 μM ISO. The force transducer output was digitized by using a DaqBoard/2000 and Daqview software (IOtech, Cleveland, OH). Force records were viewed and analyzed by using DASYLab (version 5.5; DASYTEC, Amherst, NH).
Confocal Ca2+ Imaging of Atrial Myocytes.
Single-mouse free-wall left ventricular and atrial myocytes were isolated from 10- to 12-week-old WT and sln−/− mice as previously described (31, 32). Mice were anesthetized with pentobarbital (i.p. 40 mg·kg−1), and hearts were rapidly excised. Because of the known electrophysiological heterogeneity in various regions of the heart, we used only left ventricular free-wall cells for our recordings. Enzymatically isolated free-wall left ventricular and atrial myocytes were loaded with the Ca2+ indicator fluo 4-AM (21). Cells were field-stimulated at a frequency of 1 Hz (IonOptix, Milton, MA). Confocal line-scan imaging was performed by using a Zeiss Pascal confocal microscope equipped with an argon laser (488 nm) and a ×40, 1.3 N.A. oil immersion objective. Line-scan images were acquired at sampling rates of 0.7 ms per line and 0.07 μm per pixel, with radial and axial resolutions of 0.4 and 1.0 μm, respectively. Ca2+ transients were expressed as the normalized local fluorescence (F/Fo), where Fo refers to the fluorescence level before depolarization, as described (24). Where appropriate, pooled data are presented as means ± SEM. Significant differences between groups were tested by using ANOVA. The null hypothesis was rejected when the two-tailed P value was <0.05.
Statistics.
Results are expressed as mean ± SEM. Statistical significance was estimated by an unpaired Student t test. A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by funds from the Heart and Lung Research Institute and Ohio State University (to G.J.B.) and from National Institutes of Health Grant HL-64140 (to M.P.).
Abbreviations
- SR
sarcoplasmic reticulum
- SLN
sarcolipin
- SERCA
SR Ca2+ ATPase
- PLB
phospholamban
- ISO
isoproterenol
- RyR
ryanodine receptor
- CSQ
calsequestrin
- DHPR
dihydropyridine receptor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707722104/DC1.
References
- 1.Odermatt A, Taschner PE, Scherer SW, Beatty B, Khanna VK, Cornblath DR, Chaudhry V, Yee WC, Schrank B, Karpati G, et al. Genomics. 1997;45:541–553. doi: 10.1006/geno.1997.4967. [DOI] [PubMed] [Google Scholar]
- 2.Minamisawa S, Wang Y, Chen J, Ishikawa Y, Chien KR, Matsuoka R. J Biol Chem. 2003;278:9570–9575. doi: 10.1074/jbc.m213132200. [DOI] [PubMed] [Google Scholar]
- 3.Babu GJ, Zheng Z, Natarajan P, Wheeler D, Janssen PM, Periasamy M. Cardiovasc Res. 2005;65:177–186. doi: 10.1016/j.cardiores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. J Mol Cell Cardiol. 2007;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahi M, Sugita Y, Kurzydlowski K, De Leon S, Tada M, Toyoshima C, MacLennan DH. Proc Natl Acad Sci USA. 2003;100:5040–5045. doi: 10.1073/pnas.0330962100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odermatt A, Becker S, Khanna VK, Kurzydlowski K, Leisner E, Pette D, MacLennan DH. J Biol Chem. 1998;273:12360–12369. doi: 10.1074/jbc.273.20.12360. [DOI] [PubMed] [Google Scholar]
- 8.MacLennan DH, Kranias EG. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 9.Bhupathy P, Babu GJ, Periasamy M. J Mol Cell Cardiol. 2007;42:903–911. doi: 10.1016/j.yjmcc.2007.03.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asahi M, Kurzydlowski K, Tada M, MacLennan DH. J Biol Chem. 2002;277:26725–26728. doi: 10.1074/jbc.C200269200. [DOI] [PubMed] [Google Scholar]
- 11.Minamisawa S, Uemura N, Sato Y, Yokoyama U, Yamaguchi T, Inoue K, Nakagome M, Bai Y, Hori H, Shimizu M, et al. FEBS Lett. 2006;580:2247–2252. doi: 10.1016/j.febslet.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Shimura M, Minamisawa S, Yokoyama U, Umemura S, Ishikawa Y. Biochem Biophys Res Commun. 2005;334:861–866. doi: 10.1016/j.bbrc.2005.06.186. [DOI] [PubMed] [Google Scholar]
- 13.Trivieri MG, Oudit GY, Sah R, Kerfant BG, Sun H, Gramolini AO, Pan Y, Wickenden AD, Croteau W, Morreale de Escobar G, et al. Proc Natl Acad Sci USA. 2006;103:6043–6048. doi: 10.1073/pnas.0601072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura N, Ohkusa T, Hamano K, Nakagome M, Hori H, Shimizu M, Matsuzaki M, Mochizuki S, Minamisawa S, Ishikawa Y. Eur J Clin Invest. 2004;34:723–730. doi: 10.1111/j.1365-2362.2004.01422.x. [DOI] [PubMed] [Google Scholar]
- 15.Asahi M, Otsu K, Nakayama H, Hikoso S, Takeda T, Gramolini AO, Trivieri MG, Oudit GY, Morita T, Kusakari Y, et al. Proc Natl Acad Sci USA. 2004;101:9199–9204. doi: 10.1073/pnas.0402596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, et al. J Biol Chem. 2006;281:3972–3979. doi: 10.1074/jbc.M508998200. [DOI] [PubMed] [Google Scholar]
- 17.Gramolini AO, Trivieri MG, Oudit GY, Kislinger T, Li W, Patel MM, Emili A, Kranias EG, Backx PH, Maclennan DH. Proc Natl Acad Sci USA. 2006;103:2446–2451. doi: 10.1073/pnas.0510883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann J, Boknik P, DePaoli-Roach AA, Field LJ, Rockman HA, Kobayashi YM, Kelley JS, Jones LR. J Mol Cell Cardiol. 1998;30:1991–2002. doi: 10.1006/jmcc.1998.0760. [DOI] [PubMed] [Google Scholar]
- 19.Asahi M, Nakayama H, Tada M, Otsu K. Trends Cardiovasc Med. 2003;13:152–157. doi: 10.1016/s1050-1738(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 20.Vangheluwe P, Sipido KR, Raeymaekers L, Wuytack F. Biochim Biophys Acta. 2006;1763:1216–1228. doi: 10.1016/j.bbamcr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Tupling AR, Asahi M, MacLennan DH. J Biol Chem. 2002;277:44740–44746. doi: 10.1074/jbc.M206171200. [DOI] [PubMed] [Google Scholar]
- 22.Maier LS, Bers DM, Pieske B. J Mol Cell Cardiol. 2000;32:2249–2258. doi: 10.1006/jmcc.2000.1252. [DOI] [PubMed] [Google Scholar]
- 23.Wang SQ, Song LS, Lakatta EG, Cheng H. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 24.Harkins AB, Kurebayashi N, Baylor SM. Biophys J. 1993;65:865–881. doi: 10.1016/S0006-3495(93)81112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Proc Natl Acad Sci USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadambi VJ, Koss KL, Grupp IL, Kranias EG. J Mol Cell Cardiol. 1998;30:1275–1284. doi: 10.1006/jmcc.1998.0688. [DOI] [PubMed] [Google Scholar]
- 27.Kaasik A, Paju K, Vetter R, Seppet EK. Cardiovasc Res. 1997;35:106–112. doi: 10.1016/s0008-6363(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 28.Chu G, Kranias EG. Basic Res Cardiol. 2002;97(Suppl 1):I43–I48. doi: 10.1007/s003950200028. [DOI] [PubMed] [Google Scholar]
- 29.Mattiazzi A, Mundina-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Cardiovasc Res. 2005;68:366–375. doi: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Ji Y, Loukianov E, Periasamy M. Anal Biochem. 1999;269:236–244. doi: 10.1006/abio.1999.4059. [DOI] [PubMed] [Google Scholar]
- 31.Ahmmed GU, Xu Y, Hong Dong P, Zhang Z, Eiserich J, Chiamvimonvat N. Circ Res. 2001;89:1005–1013. doi: 10.1161/hh2301.100801. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Zhang Z, Timofeyev V, Sharma D, Xu D, Tuteja D, Dong PH, Ahmmed GU, Ji Y, Shull GE, et al. J Physiol. 2005;562:745–758. doi: 10.1113/jphysiol.2004.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.