Abstract
Hormonal regulation of salt excretion and water balance by the kidneys is well documented. Before 1961, it was widely believed that the glomerular filtration rate and the steroid hormone aldosterone controlled sodium balance in the body. In 1961, deWardener et al. [de Wardener HE, Mills IH, Clapham WF, Hayter CJ (1961) Clin Sci 21:249–258] showed that when these two variables were controlled, the kidney was still able to increase sodium excretion in response to a salt load. Several lines of evidence argued for a small-molecule signal as a definitive modulator of sodium excretion by the kidney. However, the chemical nature of the suspected natriuretic agent remained unknown. Here we report the identification and natriuretic activity of two closely related small molecules isolated from human urine, xanthurenic acid 8-O-β-d-glucoside and xanthurenic acid 8-O-sulfate. The two compounds were partially purified by activity-guided fractionation and subsequently identified by using NMR spectroscopic analyses of enriched active fractions. Both compounds caused substantial and sustained (1- to 2-h) natriuresis in rats and no or minimal concomitant potassium excretion. We believe these compounds constitute a class of kidney hormones that also could influence sodium transport in nonkidney tissues given that these tryptophan metabolites presumably represent evolutionarily old structures.
Keywords: diuresis, kidney, nonpeptidic, sodium homeostasis, NMR spectroscopy
In 1985, de Wardener and Clarkson (1) reviewed the status of the search for the putative natriuretic hormone, a signaling molecule that could regulate sodium excretion by the kidney (2, 3). In numerous experimental models, several groups had consistently observed natriuretic activity from a small molecule (<1,000 Da) contained in mammalian urine that eluted as a postsalt peak in Sephadex G-25 gel chromatography. A natriuretic substance was shown to be present in urine and/or serum extracts of patients with chronic renal disease (CRD) (4, 5), normal man (6), salt-loaded man (7, 8), volume-loaded man (9), and volume-loaded rat (10). Although inferred in the experiments mentioned earlier, no molecule was ever isolated or identified. Numerous isolation and assay techniques have been applied to natriuretic urine fractions derived from Sephadex G-25 chromatography to further characterize the small-molecule components with varied results (1). Other laboratories, initially in pursuit of the low-molecular-weight natriuretic hormone, focused on a digitalis-like substance that specifically and with high affinity inhibited sodium/potassium ATPase (11). In contrast to the compounds described herein, the digitalis-like substance is kaliuretic and inconsistently natriuretic (11, 12).
Additional evidence supporting the existence of a natriuretic hormone includes experiments in which small-molecule-containing fractions from serum (13) or urine extracts of CRD patients were shown to inhibit short-circuit current (SCC) across the frog skin (13, 14) and toad bladder (15). These urine extracts also inhibited sodium transport in isolated perfused rabbit renal tubular collecting ducts (16). Furthermore, the urine of salt-loaded dogs was reported to contain a small molecule that inhibited sodium current in the toad bladder (17, 18).
Results
In the present study, we used the frog skin assay (Rana pipiens) (19) to screen for an inhibitor of sodium transport obtained from the urine of CRD patients (13). It was assumed that the CRD patients' urine would be a rich source of natriuretic hormone because it would be the signal to the remaining nephrons, in advancing CRD, to increase sodium excretion per nephron, per unit of sodium intake (20). Its low molecular weight also should make it ultrafiltrable and thus more likely to appear in the urine.
Urine from CRD patients was lyophilized and fractionated by Sephadex G-25 chromatography (5, 13) (see Materials and Methods). Fractions eluting immediately after the salt peak were collected, lyophilized, reconstituted in water, and then assayed for inhibition of SCC in the frog skin when applied to the serosal surface. The active fractions (equivalent to 6 h of original urine) decreased the SCC from 40–50 to 20–30 μamp/cm2. SCC values returned to or toward initial levels upon washout and replacement with fresh anuran Ringers. Urine fractions obtained by using a similar protocol were previously shown to induce natriuresis in the uremic rat (5). In the present study, these active fractions were purified further by two iterations of reversed-phase HPLC again by using the frog skin SCC inhibition assay for monitoring activity. In addition, HPLC fractions were monitored for fluorescence and UV absorbance as previously reported (5).
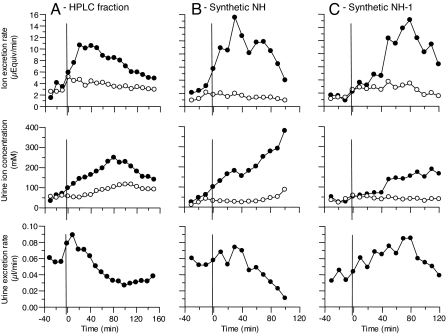
Active fractions from the second stage of HPLC were lyophilized, reconstituted in isotonic saline, and infused into a uremic rat that responded with strong and sustained natriuresis (see Fig. 1A). Natriuresis began within 10 min and continued for 100 min, during which time the mean Δ sodium excretion rate (ΔUNaV: experimental UNaV − control UNaV) was 4.21 (± 1.5 SEM) μEq/min. In contrast, the mean Δ potassium excretion rate remained virtually unchanged (ΔUKV = −1.05 (± 0.15 SEM) μEq/min. Concomitantly, the mean urinary sodium concentration increased from a mean control value of 55.0 (± 19.0 SEM) mEq/liter to 150 (± 49.8 SEM) mEq/liter, reaching a peak of 249 mEq/liter at 80 min. The mean urinary volume increased from a control rate of 57 (± 1.5 SEM) nl/min to 77 (± 4.5 SEM) nl/min during the first 40 min and gradually decreased to 26 nl/min 100 min after administration of the fraction. The natriuretic and nonkaliuretic responses of the isolated urine fraction, both in terms of magnitude and duration, are consistent with previous reports that followed a similar HPLC fractionation scheme (5).
Fig. 1.
Comparison of sodium (filled circles) and potassium (open circles) urinary excretion rates, urine sodium and potassium concentrations, and urinary volumes. Female Sprague–Dawley rats were intraarterially infused with partially purified NH (A), 6.3 nmol of synthetic NH (B), and 15 nmol of synthetic NH-1 (C). As described in Materials and Methods, pooled HPLC II fractions from ≈42 h of urine were reconstituted in 1.0 ml of saline and intraarterially infused (A). Then, ≈8 nmol of partially purified NH was infused based on the fluorescence of the sample and a retrospective standard curve of synthetic NH. At time 0 min (vertical bar), 1.0 ml of sample was infused over the course of 10 min. Data obtained before infusion of natriuretic material (left of vertical bars) served as control.
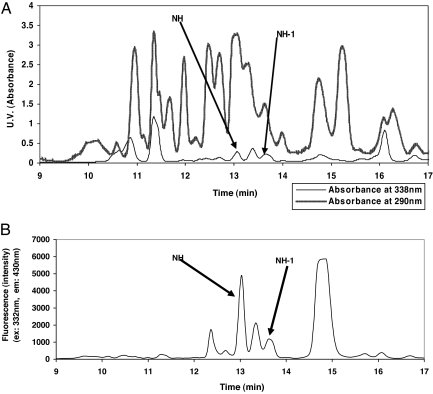
The natriuretic HPLC fractions consistently showed two peaks [xanthurenic acid 8-O-β-d-glucoside (NH) and xanthurenic acid 8-O-sulfate (NH-1)] with strong fluorescence (excitation, 332 nm; emission, 430 nm) and characteristic UV absorption (UVmax at 338 nm) (Fig. 2). These spectroscopic signatures were subsequently used as the main pooling criteria for further HPLC-based purification of NH (Fig. 3B) and NH-1 (Fig. 3C).
Fig. 2.
HPLC I fractionation of human urine Sephadex G-25 postsalt peak. As described in Materials and Methods, 6 h of urine was lyophilized, reconstituted in water, and then run through the Sephadex G-25 column. The postsalt peak was lyophilized, reconstituted in water, and then fractionated by using the HPLC I method. (A) HPLC chromatograms for UV absorption at 338 nm and 290 nm. (B) HPLC chromatogram for fluorescence (excitation, 332 nm; emission, 430 nm).
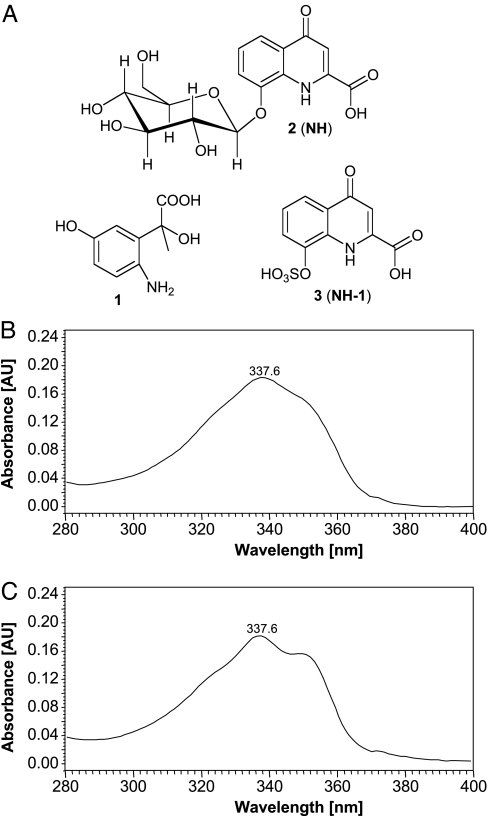
Fig. 3.
Chemical properties of identified compounds. (A) Structures of 1, 2 (NH), and 3 (NH-1). (B) The 280- to 400-nm region of the UV spectrum of NH. (C) UV spectrum of NH-1.
The fluorescence and characteristic UV spectra suggested an aromatic or heteroaromatic moiety as part of NH and NH-1 (Fig. 3 B and C). Furthermore, their chromatographic properties suggested that these compounds may have acidic and/or basic properties because chromatographic separation was generally poor unless carefully buffered solvents were used. In addition, prolonged exposure to acidic conditions (pH <5) resulted in precipitation and loss of fluorescence of NH, indicating decomposition of the active principles. The later-eluting NH-1 was noted to have similar fluorescent properties, but a slightly different UV spectrum at 338–350 nm (Fig. 3C). Samples containing either NH or NH-1 were purified further by a third HPLC step and, after removal of residual buffer, subjected to NMR-spectroscopic analyses.
1H-NMR and (1H,1H)-dqf-COSY NMR spectra immediately revealed that both fractions represent mixtures each containing two major aromatic compounds, as well as smaller amounts of other aromatic and aliphatic components. However, even the major components in these mixtures represented <50 μg of material. Therefore, to prevent additional losses of material, further fractionation aiming at the isolation of individual components was not pursued. Instead, building on recent examples for successful NMR-based characterization of individual compounds in complex small-molecule mixtures (21, 22), we continued with a comprehensive series of MS and NMR-spectroscopic experiments designed to characterize the individual components in the active fractions without further purification. Additional (1H,13C)-HMQC and (1H,13C)-HMBC NMR spectra of the fraction containing NH suggested the presence of a trisubstituted benzene derivative (compound 1) and a heteroaromatic bicyclic compound featuring a glucosylated phenol (compound 2) [supporting information (SI) Tables 1 and 2]. In combination with high-resolution positive-ion electrospray mass spectra, these data indicated that compound 1 corresponded to 2-(6-amino-3-hydroxyphenyl)-2-hydroxypropanoic acid, whereas compound 2 corresponded to NH (Fig. 3). Because the natriuretic activity of the active urine fractions did not appear to correlate with the amounts of compound 1 present, stereochemical characterization of compound 1 was not pursued. To confirm the identity of compound 2, an authentic sample of NH was chemically synthesized (see Materials and Methods). Because the NMR-spectroscopic data of compound 2 were highly pH- and concentration-dependent, we conducted an NMR-spectroscopic mixing experiment (NMR-spectroscopic coanalysis) (23), which provided final proof that compound 2 corresponded to NH (Fig. 4). Subsequent assays using synthetic NH clearly showed that this compound represents the suspected natriuretic factor NH (see Fig. 1B).
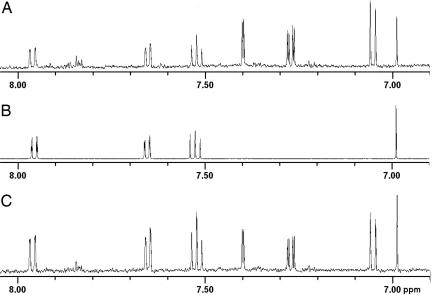
Fig. 4.
The 6.90- to 8.05-ppm region of 1H-NMR spectra. (A) Natriuretic fraction from HPLC III. (B) Synthetic NH (compound 2 in Fig. 3). (C) Natriuretic fraction from HPLC III plus 80 nmol of synthetic NH. Addition of synthetic NH to isolated fraction of NH increased the intensity of the signals derived from NH at 6.92, 7.53, 7.66, and 7.97 ppm relative to the intensity of the signals derived from other components of the HPLC III fraction, such as the signals at 7.06, 7.28, and 7.40 ppm representing the aminophenol 1. This finding confirms the identity of the natural product NH with synthetic NH.
Similarly, NMR-spectroscopic analysis of the other natriuretic HPLC fraction revealed a major component whose structure appeared to be closely related to that of NH. In conjunction with MS evidence, the NMR-spectroscopic data suggested NH-1 (compound 3) (Fig. 3) as the structure of NH-1 (SI Table 3). This finding was confirmed by the synthesis of a reference sample (see Materials and Methods) and NMR-spectroscopic coanalysis (23).
Similar to the isolated material, infusion of 6.3 nmol of synthetic NH (Fig. 1B) or 15 nmol of synthetic NH-1 (Fig. 1C) stimulated natriuresis within 10 min, which continued for ≈100 min. The time course of NH and NH-1 in representative rats is compared with the isolated HPLC fraction (Fig. 1A). In eight assays using NH, ΔUNaV averaged 3.68 (± 0.55 SEM) μEq/min, and ΔUKV values averaged 0.41 (± 0.27 SEM) μEq/min. Using NH-1 in five assays, ΔUNaV averaged 4.33 (± 0.71 SEM) μEq/min and ΔUKV values averaged 0.53 (± 0.32 SEM) μEq/min.
As a control, additional experiments were performed in which isotonic saline (without either NH or NH-1) was infused. Sodium excretion values before infusion showed a UNaV of 0.48 (± 0.07 SEM) μEq/min and a mean postinfusion value of 0.37 (± 0.18 SEM) μEq/min. These two values did not differ significantly. Furthermore, the infusion of xanthurenic acid, the parent compound of NH and NH-1, produced neither significant natriuresis (ΔUNaV = 0.56 μEq/min) nor kaliuresis (ΔUKV = −0.12 μEq/min). Synthetic NH also reversibly inhibited SCC in frog skin (data not shown).
Discussion
Our results show that the xanthurenic acid derivatives NH and NH-1 represent natriuretic hormones, with regard to both their biosynthetic origin and activity profile. Xanthurenic acid is believed to an play important role in many biological processes, and thus it is possible that NH and NH-1, in addition to their natriuretic properties, may have other biological functions as well (24, 25). However, a more detailed understanding of these compounds' physiological role will have to await further study.
NH was isolated based on its ability to reversibly inhibit SCC from the serosal side of the frog skin. The epithelial sodium channel, ENaC, is generally thought to be responsible for this SCC (26). ENaC also is expressed in the distal nephron of mammalian kidneys (27). The natriuresis induced by NH and NH-1 suggests a signaling pathway, in which these molecules act on the basolateral surface of the distal nephron and a transduced signal is sent to the luminal side of the tubule, where ENaC activity and sodium reabsorption are inhibited (16). This mode of action is consistent with the ability of NH and NH-1 to enhance acetazolamide-induced natriuresis and block acetazolamide-induced kaliuresis (data not shown). This lack of potassium excretion is consistent with decreased ENaC activity because potassium exchanges for sodium across the apical membrane during sodium reabsorption. The relatively quick natriuretic response and the hydrophilic properties of NH and NH-1 suggest a membrane receptor-dependent mode of action (possibly analogous to dopamine's well characterized binding to membrane receptors that trigger G protein-linked second messengers) (28). Given their activity in both amphibian skin and mammalian kidneys, it seems likely that NH and NH-1 bind to a generally expressed and evolutionarily old class of receptors.
Materials and Methods
Isolation.
First, 2- to 4-liter samples of human urine were lyophilized and then reconstituted with 100 ml of water (5). Aliquots of reconstituted urine were applied to a Sephadex G-25 medium column (Spectra/Chrom LC Column; 500 ml; i.d., 2.5 cm; 4.91 ml/cm). The mobile phase was 0.01 M ammonium acetate (pH 6.8) with a flow rate of 1.0 ml/min at 4°C. The UV absorbance was monitored at 285 nm, and conductivity was monitored online. After the elution of the salt peak (5), the osmolality dropped to <100 mOsm/kg. Following this step, ten 10-ml fractions were collected, lyophilized, resuspended (in 1.0 ml of water), and assayed for inhibition of SCC in the frog skin (10).
Active fractions, as already defined, from the Sephadex column were then injected into a semiprep C18 column (Synergi 4u Hydro-RP 80A 250 × 10 mm, 4 μm; Phenomenex, Torrance, CA). The HPLC system included a Waters 600E Multisolvent Delivery System (Milford, MA). The detectors included a Waters 474 Fluorescent Detector and a 996 Photodiode Array Detector. Chromatograms were extracted at 290 and 338 nm. The mobile phase was 0.1 M pyridium acetate (pH 5.5) (solvent A) and methanol (solvent B). In the HPLC I method, sample volumes were 0.8–1.0 ml. The flow rate was 4 ml/min with 100% solvent A for 3 min and then in a linear gradient from 0–40% solvent B for the next 11 min. Subsequently, the column was washed with solvent B and then equilibrated with 100% solvent A. The retention time for NH was 13 min, as determined by the combination of a fluorescent peak (excitation, 332 nm; emission, 430 nm) and the UV spectrum (maximum at 338 nm) (Fig. 2). Then 2-ml fractions from multiple runs were pooled, and the volume was reduced by centrifugation under vacuum with medium heat. In the pooling and volume reduction of HPLC I fractions, the recovery of the fluorescent peak was typically 80–90%. Samples were periodically spiked with pyridine to keep the pH >5. Typically, 10 HPLC I runs were pooled together for each HPLC II injection of 1.0 ml. The HPLC II method differed from the HPLC I method only in the use of an isocratic gradient of 8% methanol, which was run for 13 min. Retention time for NH ranged from 11.2–11.6 min. Typically, 10 HPLC II runs were pooled for each HPLC III injection. The HPLC III method was the same as the HPLC II method, except that an analytical column was used with a flow rate of 1.0 ml/min by using a 0.15-ml injection volume (Synergi 4μ Hydro-RP 80A 250 × 4.6 mm, 4 μm; Phenomenex). The retention time for NH was 10.6 min. After the HPLC III chromatography, NH was relatively pure based on the lack of any additional UV absorbance at 290 nm and its characteristic spectrum at 338 nm (Fig. 3B). The largely purified NH was then applied to the same analytical column by using only methanol (20%) and water (80%) as solvents to eliminate pyridium acetate.
During the HPLC I chromatography, a second fluorescent peak, subsequently determined to be NH-1 (retention time 13.7 min), was eluted after NH. This peak had a UV spectrum similar to NH as noted earlier (Fig. 3 B and C). From this point on, NH-1 was purified through all of the same steps as NH. The final HPLC III retention time was 11.6 min.
The amounts of NH in the urine of CRD patients ranged from 2–130 nmoles per 24-hr urine collection, with an average of 10 nmoles. This calculation is based on a standard curve of synthetic NH and the fluorescence peaks from HPLC I method chromatograms (Fig. 2B). Similarly, amounts of NH-1 ranged from 0–105 nmoles per 24-hr urine with an average of 8 nmoles.
Rat Assays.
The protocol to measure sodium and potassium excretion in rats has been used previously at multiple institutions and has been described in detail (5). In summary, following an equilibration period of 60 min (to allow for stabilization from the anesthesia and surgical procedures), urine was collected from rats for three 10-min control periods. At time 0, the test material was infused in 1.0 ml of saline by constant infusion over 10 min. Thereafter, urine was collected at 10-min intervals. UNaV is sodium excretion rate in μEq/min, and UKV is potassium excretion rate in μEq/min. Sodium and potassium concentrations were measured with ion specific probes.
NMR Spectroscopy.
All NMR-spectroscopic analyses were carried out by using a Varian Inova 600 MHz NMR spectrometer (Palo Alto, CA), which was equipped with a 5-mm inverse-detection HCN probe. Shigemi tubes were used as needed. NMR spectra were acquired at 25°C by using the standard pulse sequences provided by Varian. For each sample, a standard phase-cycled, phase-sensitive dqf-COSY spectrum was obtained by using the following parameters: 600-ms acquisition time, 512 increments in F1, and 16 or 64 scans per increment. For further characterization, nongradient HMQC (phase-sensitive), gradient HSQC (phase-sensitive), nongradient HMBC, and phase-cycled, phase-sensitive NOESY spectra (using a 600-ms mixing time) were acquired. HMQC spectra were acquired without decoupling.
MS.
Positive- and negative-ion electrospray ionization mass spectra were acquired by using a Micromass (Manchester, U.K.) Quattro I mass spectrometer. High-resolution mass spectra were recorded on a Micromass Autospec instrument operated in a positive- or negative-ion electrospray mode. High-resolution positive-ion electrospray mass spectra of synthesized NH revealed a strong ion at 390.0811 (C16H18NO9Na); the calculated molecular weight of this ion was 390.0801. After removal of the sodium, the measured molecular weight was 368.0995 (C16H19NO9), with a calculated molecular weight of 368.0982. Negative-ion electrospray MS of NH-1 showed a strong peak at 283.9887 (C10H6NO732S), with a calculated molecular weight of 283.9865 and a calculated molecular weight of 285.9803 (C10H6NO734S).
Synthesis of NH.
Step 1.
Synthesis of xanthurenic acid 2,3,4,6-tetra-O-acetyl 8-O-β-d-glucoside.
First, 4.53 mmol of xanthurenic acid was dissolved in 10 ml of aqueous 1 M NaOH and cooled to 10°C. Then 4.94 mmol of 2,3,4,6-tetra-O-acetyl-8-α-d-glucopyranosylbromide in 16 ml of oacetone was added dropwise over 10 min, and the resulting mixture was stirred at room temperature for 4 h. Subsequently, an additional 3 ml of aqueous 1 M NaOH was added slowly over 30 min. The reaction mixture was stirred for an additional 30 min and then diluted with 20 ml of water and extracted with 40 ml of diethyl ether. The aqueous portion was acidified to pH 3.5 and further extracted with 100 ml of a 1:1 tetrahydrofuran:ethylacetate mixture. The combined organic layers were washed with saturated aqueous NaCl and dried over magnesium sulfate. After filtration, the combined extracts were concentrated in vacuo to give crude xanthurenic acid 2,3,4,6-tetra-O-acetyl 8-O-β-d-glucoside, which was triturated with 28 ml of 4:1 dimethyl sulfoxide:water and filtered and dried to yield 215 mg of xanthurenic acid 2,3,4,6-tetra-O-acetyl 8-O-β-d-glucoside as an off-white solid intermediate.
Step 2.
Synthesis of xanthurenic acid 2,3,4,6-tetra-O-acetyl 8-O-β-d-glucoside.
First, 0.35 mmol of xanthurenic acid 2,3,4,6-tetra-O-acetyl 8-O-β-d-glucoside from step 1 was added to a 0.7-mmol solution of sodium methoxide in 5 ml of methanol and stirred for 1 h. The mixture was adjusted to pH 3.5 with aqueous 1 M HCl. The slurry was diluted with 20 ml of diethyl ether and filtered. The filter cake was washed with 1:1 methanol:diethyl ether and dried in vacuo to give 118 mg of pure NH.
Synthesis of NH-1.
First, 2.92 mmol of sulfurtrioxide complex and 5 ml of acetone were added to a 1.46-mmol solution of xanthurenic acid in 2.9 ml of 1 M NaOH and 2.1 ml of water at room temperature. Then the reactor tube was sealed under nitrogen and stirred at 70°C for 16 h. It was cooled to room temperature and concentrated to dryness under reduced pressure. The residue was washed with acetone, acetonitrile, dichloromethane, ethyl acetate, and diethyl ether consecutively and then dried under vacuum overnight. The brown solid was dissolved in 3 ml of water and loaded on Sephadex SP-C25 column (swollen in water) to remove the sodium salt. The yield was 92.8%.
Supplementary Material
Acknowledgments
We thank Boaz Cotton, Carrie Cottrell, Sam Gbadebo, Tony Mai, and Deborah Thomas for providing technical assistance with the isolation and bioassays.
Abbreviations
- CRD
chronic renal disease
- NH
xanthurenic acid 8-O-β-d-glucoside
- NH-1
xanthurenic acid 8-O-sulfate
- SCC
short-circuit current.
Footnotes
Conflict of interest statement: Five of the six authors hold stock in Naturon, which is incorporated in the State of Delaware. The sixth author (F.C.S.) participated as a paid consultant. All costs for the present studies were paid for by Naturon. No dividends have been issued. A patent application on the two molecules (NH and NH-1) has been submitted (patent application no. US 2006-0217322-A1).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705553104/DC1.
References
- 1.de Wardener HE, Clarkson EM. Physiol Rev. 1985;65:658–759. doi: 10.1152/physrev.1985.65.3.658. [DOI] [PubMed] [Google Scholar]
- 2.de Wardener HE, Mills IH, Clapham WF, Hayter CJ. Clin Sci. 1961;21:249–258. [PubMed] [Google Scholar]
- 3.Mills IH, de Wardener HE, Hayter CJ, Clapham WF. Clin Sci. 1961;21:259–264. [PubMed] [Google Scholar]
- 4.Bourgoignie J, Hwang KH, Ipakchi E, Bricker NS. J Clin Invest. 1974;53:1559–1567. doi: 10.1172/JCI107706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bricker NS, Zea L, Shapiro M, Sanclemente E, Shankel S. Kidney Int. 1993;44:937–947. doi: 10.1038/ki.1993.335. [DOI] [PubMed] [Google Scholar]
- 6.Clarkson EM, Young DR, Raw SM, de Wardener HE. In: Hormone Regulation of Sodium Excretion. Lichardus B, Schrier RW, Ponec J, editors. Amsterdam: Elsevier/North-Holland; 1980. pp. 333–340. [Google Scholar]
- 7.Viskoper RJ, Czaczkes JW, Schwartz N, Ullmann TD. Nephron. 1971;8:540–548. doi: 10.1159/000179959. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson EM, Raw SM, de Wardener HE. Kidney Int. 1976;10:381–394. doi: 10.1038/ki.1976.124. [DOI] [PubMed] [Google Scholar]
- 9.Bricker NS, Klahr S, Purkerson M, Schultze RG, Avioli LV, Birge SJ. Nature (London) 1968;219:1058–1059. doi: 10.1038/2191058a0. [DOI] [PubMed] [Google Scholar]
- 10.Gonick HC, Saldanha LF. J Clin Invest. 1975;56:247–255. doi: 10.1172/JCI108087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrandi M, Manuta P. Curr Opin Nephrol Hypertens. 2000;9:165–171. doi: 10.1097/00041552-200003000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Klingmuler D, Weiler E, Kramer HJ. Klin Wochenschr. 1982;60:1249–1253. doi: 10.1007/BF01716732. [DOI] [PubMed] [Google Scholar]
- 13.Bourgoignie J, Klahr S, Bricker NS. J Clin Invest. 1971;50:303–311. doi: 10.1172/JCI106495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzurik R, Lichardus B, Spustova J, Ponec J, Gerykova A, Bakos PA. Physio Bohemosdov. 1982;31:573–576. [PubMed] [Google Scholar]
- 15.Kaplan MA, Bourgoignie JJ, Rosecan J, Bricker NS. J Clin Invest. 1974;53:1568–1577. doi: 10.1172/JCI107707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine LG, Bourgoignie JJ, Hwang KH, Bricker NS. J Clin Invest. 1976;58:590–597. doi: 10.1172/JCI108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckalew VM, Jr, Martinez FJ, Green WE. J Clin Invest. 1970;49:926–935. doi: 10.1172/JCI106312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favre H, Hwang KH, Schmidt RW, Bricker NS. J Clin Invest. 1975;56:1302–1311. doi: 10.1172/JCI108206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ussing HH, Zerahn K. Acta Physiol Scand. 1951;23:110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 20.Fine LG, Bourgoigne JJ, Weber H, Bricker NS. Kidney Int. 1976;10:364–372. doi: 10.1038/ki.1976.122. [DOI] [PubMed] [Google Scholar]
- 21.Schröder FC, Farmer J, Attygalle AB, Smedley SR, Eisner T, Meinwald J. Science. 1998;281:428–431. doi: 10.1126/science.281.5375.428. [DOI] [PubMed] [Google Scholar]
- 22.Taggi AE, Meinwald J, Schroeder FC. J Am Chem Soc/I. 2004;126:10364–10369. doi: 10.1021/ja047416n. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder FC, Weibel DB, Meinwald J. Org Lett. 2004;6:3019–3022. doi: 10.1021/ol049233b. [DOI] [PubMed] [Google Scholar]
- 24.Stone TW, Darlington LG. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 25.Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y. Am J Physiol. 2005;289:C1075–C1084. doi: 10.1152/ajpcell.00619.2004. [DOI] [PubMed] [Google Scholar]
- 26.Anne Shane M, Nofziger C, Blazer-Yost B. Gen Compar Endocrin. 2006;147:85–92. doi: 10.1016/j.ygcen.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Loffing J, Kaissling B. Am J Physiol. 2003;284:F628–F643. doi: 10.1152/ajprenal.00217.2002. [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.