Abstract
Mismatches between the composition of a time-averaged death assemblage (dead remains sieved from the upper mixed-zone of the sedimentary column) and the local living community are typically attributed to natural postmortem processes. However, statistical analysis of 73 molluscan data sets from estuaries and lagoons reveals significantly poorer average “live-dead agreement” in settings of documented anthropogenic eutrophication (AE) than in areas where AE and other human impacts are negligible. Taxonomic similarity of paired live and dead species lists declines steadily among areas as a function of AE severity, and, for data sets comprising only adults, rank-order agreement in species abundance drops where AE is suspected. The observed live-dead differences in composition are consistent with eutrophication (anomalous abundance of seagrass-dwellers and/or scarcity of organic-loving species in the death assemblage), suggesting compositional inertia of death assemblages to recent environmental change. Molluscan data sets from open shelf settings (n = 34) also show higher average live-dead discordance in areas of AE. These results indicate that (i) live-dead discordance in surficial grab samples provides valuable evidence for strong anthropogenic modification of benthic communities, (ii) actualistic estimates of the ecological fidelity of molluscan death assemblages tend to be erroneously pessimistic when conducted in nonpristine settings, and (iii) based on their high fidelity in pristine study areas, death assemblages are a promising means of reconstructing otherwise elusive preimpact ecological baselines from sedimentary records.
Keywords: ecological baseline, eutrophication, marine communities, paleoecology
Human activities affect living systems in many ways, directly by means of harvesting and indirectly by means of processes ranging from habitat conversion to climate change. Many of these activities have deep roots in human history, but virtually all have intensified and become increasingly global in effect over the last two centuries and especially the last several decades (1–6). Acquiring baseline information on ecosystems before the onset of human activities of a particular type or intensity is thus essential to evaluating anthropogenic impacts and to developing targets for remediation. However, such baselines have been unobtainable in many settings where human impacts preceded biomonitoring. Sedimentary records can be a powerful means of reconstructing ecological and physical environmental changes in such situations, by using a variety of proxies to extend chronologies beyond the reach of available scientific observations (7). Such records are becoming more widely used to determine the historical trajectories of ecological change and to assess the likely role of humans as drivers (7–10). However, in nonvarved, estuarine and open-shelf sedimentary settings, time-averaging of biotic assemblages (the mixing of durable dead remains from multiple generations within the upper part of the sedimentary column) has the potential to blur the ecological record to decadal, centennial, or coarser resolution (11–14), a seeming obstacle to extraction of meaningful baseline information.
Mismatches between the composition of a death assemblage (dead remains sieved from the upper mixed-zone of the sedimentary column) and the local living community are typically attributed to natural processes such as postmortem transportation, differential production and destruction rates of species, and the time-averaging of natural stochastic variation and change in community composition (15). However, such mismatches might also reflect recent, relatively rapid anthropogenic change(s) in community composition (16–18). The stronger the shift in composition, the lower the rate of production of dead shells of new cohorts relative to old cohorts, the greater the relative durability of dead shells from the original community, or the slower the rate of permanent burial (with the last two factors determining the window for time-averaging), then the more likely that the composition of the death assemblage will lag behind the changing composition of the living community. Although a memory of past populations is fundamental to the concept of time-averaging, taphonomic inertia (that is, the lag in response of death assemblage composition) to changing ecological conditions should be particularly likely under conditions of anthropogenic modification, given that the rapidity of many human-driven changes is rare if not unprecedented in natural systems. If so, then (i) many actualistic estimates of taphonomic bias will be overly pessimistic, and (ii) live-dead mismatch, which is relatively easy and inexpensive to determine, might be a valuable tool in environmental assessment. A phenomenon generally seen as an obstacle to paleoecological analysis thus might be used neontologically to detect anthropogenic shifts in community composition in the absence of direct historical observations, with anomalous dead occurrences of species providing valuable insights into past populations.
Here, I evaluate this approach using 73 molluscan data sets from bays and lagoons to test for an association between human modification of the ecosystem [specifically, anthropogenic eutrophication (AE; nutrient enrichment caused by human activities)] and “live-dead” agreement in the taxonomic composition and relative abundance of species. I find that, despite considerable (natural) variability in live-dead agreement even among apparently pristine settings, average death assemblage fidelity is significantly poorer in areas of known human impact. Statistical association is only the first step in developing a widely applicable method of environmental assessment built on live-dead discordance. However, the results so far are encouraging and identify productive directions for further research and application.
Results
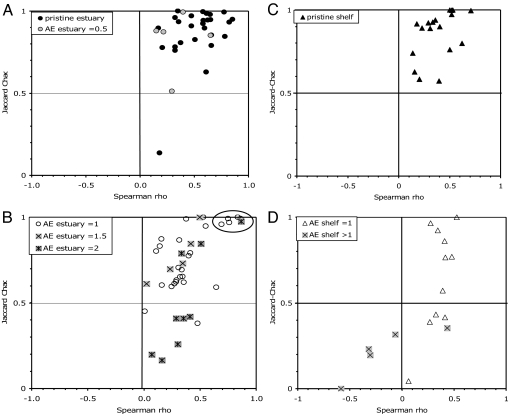
The intensity of AE at the time the living community was sampled can be assessed with confidence for 73 live-dead data sets ranging from (AE0) absent or negligible (“pristine”) to (AE1) mild or diffuse (significant human habitation or commercial activity in the watershed), to (AE2) severe [one or more nearby point sources of pollution, commonly in addition to diffuse sources; supporting information (SI) Table 1]. All but one of the 27 data sets from pristine settings show high taxonomic similarity of live and dead species lists (using the sample-size adjusted Jaccard–Chao (J–C) index; ref. 19), and all show positive correlations in species rank-order abundance (Spearman's rho), thus occupying the upper-right quarter of a cross-plot of these two metrics (Fig. 1A). Average taxonomic similarity is high (mean 0.86 ± 0.06, with 95% confidence intervals calculated on the standard error; median = 0.92; if the one outlier is excluded, mean = 0.89 ± 0.03, median unchanged; Fig. 2Upper). The range of rank-order agreement is broad, but all correlations are positive and most are significantly so (63% of rhos positive at P < 0.05, 51% at P < 0.01, after sequential Bonferroni correction; mean rho = 0.52 ± 0.07, median = 0.58; Fig. 2 Lower).
Fig. 1.
Cross-plots of live-dead taxonomic similarity (Jaccard-Chao Index) and rank-order correlation of species abundances (Spearman's rho). In each plot, data sets occupying the upper-right quarter are characterized by the highest live-dead agreement, and those in the lower-left quarter the poorest. In both coastal embayments (A and B; 73 data sets analyzed here) and in open shelf settings (C and D; 34 large data sets used in ref. 18), molluscan death assemblages from pristine areas show consistently high agreement to the local living community by both metrics (A and C). Death assemblages from areas of suspected (AE0.5) and documented anthropogenic eutrophication (AE ≥1) overlap the pristine range but extend down to significantly poorer live-dead agreement by one or both metrics, with live-dead agreement generally decreasing as the intensity of AE increases (B and D). Lagoonal AE data sets having unusually high live-dead agreement are circled in B. Only the lowermost right corner of the cross-plot cannot be occupied.
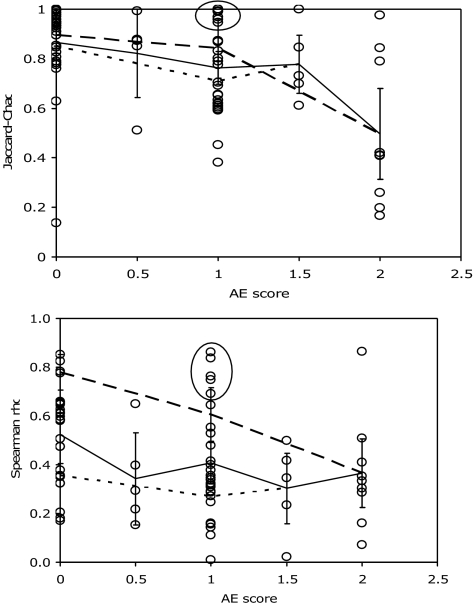
Fig. 2.
With increasing certainty and severity of AE, living and death assemblages from estuaries diverge in (Upper) taxonomic similarity and (Lower) among coarse-mesh data sets composed largely of adult specimens, rank-order agreement in species abundance. Solid line connects mean values for each AE score with 95% confidence intervals on the standard error; dotted line connects mean values of fine-mesh data sets (≤ 1 mm; no confidence intervals marked); dashed line connects mean values of 24 identically processed coarse-mesh data sets from the Gulf of Mexico (1.5 mm; AE scores 0, 1 and 2). See SI Text for counterpart plot of shelf data sets.
With increasing certainty and severity of AE, data sets tend toward lower live-dead agreement (Figs. 1 and 2). The five data sets from estuarine areas where mild AE is possible (AE0.5) tend to occupy the left edge of the pristine distribution in the cross-plots (lower rho; Fig. 1A). The 41 data sets from areas of definite AE (AE1 to AE2) overlap with the pristine distribution, but mostly show lower taxonomic similarity (J–C <≈0.7; mean = 0.71 ± 0.07, median = 0.70) and, like the suspected-AE data sets of Fig. 1A, relatively low rank-order agreement (rho ≈0 to ≈0.4; mean = 0.38 ± 0.07, median = 0.34) (Fig. 1B). Median taxonomic similarity declines significantly from AE0 (0.92) to AE1 (0.77) and AE2 (0.41), despite the overlap of values (Kruskal–Wallis test, H = 20.9, P = 0.0003; mean values decline but not significantly so until AE2; Fig. 2 Upper). Mean live-dead agreement in species rank-abundance drops to 0.34 ± 0.15 where AE0.5 (median = 0.29; 40% of rank-order correlations significantly positive at P < 0.05 and 20% at P < 0.01; n = 5), and this approximate level of agreement persists across the spectrum of higher AE intensity (median = 0.34; 20% positive at P < 0.05, 15% positive at P < 0.01; n = 41; Fig. 2 Lower). The decline is not significant when all data sets are used (Kruskal–Wallis test H = 8.58, P = 0.073; but see mesh-size effects below). A cluster of exceptionally high-agreement AE1 data sets is from a single lagoon complex (circled in Figs. 1B and 2; see discussion below).
These patterns are not artifacts of sampling effort or of the smaller numbers of live individuals and lower species richness typically retrieved in AE2 settings (SI Fig. 4 A–F). Although J–C values generally increase with sampling intensity (number of stations) and sampled community richness (number of live species, which is roughly correlated with the log number of live individuals in the data set), high taxonomic similarity is common among small data sets. Similarly, rho values funnel symmetrically as sampling increases from a wide range of values among smaller data sets. In addition, neither metric varies with the ratio of dead:live individuals, which ranges over almost four orders of magnitude (J–C corrects for differences in sample size, but rho does not), nor with bottom habitat type, which ranges from muds to gravels and grassbeds (SI Fig. 4 I and J).
The mesh size used to process samples (determining whether newly settled juveniles ≤1 mm were included in counts of individuals) has no effect on the taxonomic similarity of live and dead species lists (linear regression of both raw and normalized J–C values against mesh size in mm, P > 0.05; SI Fig. 4G, by using all data). Also, both fine-mesh (≤1 mm) and coarse-mesh data sets respond similarly to AE (compare dotted and dashed lines in Fig. 2A; note that no fine-mesh data sets are available for AE2 settings; SI Table 1). In pristine settings, the mean J–C value for fine-mesh data sets is high (0.85 ± 0.06) and indistinguishable from that for coarse-mesh (≥1.5 mm; 0.87 ± 0.08).
However, mesh size does influence live-dead agreement in species relative abundance (SI Fig. 4H). Fine-mesh data sets show no variation in rho with AE (dotted line connecting mean values in Fig. 2B). The coarsest mesh data sets (2–5 mm, composed only of adult individuals) are with one exception exclusively from pristine settings (SI Table 1), and have a significantly higher average rho than pristine fine-mesh data sets (rho = 0.52 ± 0.08, versus 0.35 ± 0.09; coarse-mesh mean rho = 0.57 ± 0.08 if 1.5 mm data sets are included). A powerful check of these results is provided by a subset of 24 coarse-mesh (1.5 mm) data sets from 11 lagoons in Veracruz and Tabasco, Mexico, spanning the full range of AE: variation in live-dead agreement as a function of methodology should be minimal, because all were collected by using identical gear and species identifications were by a single research group (SI Table 1). For these data sets, rho declines significantly as a function of AE, both by means of comparison of means (dashed line in Fig. 2B; AE0 mean = 0.78 ± 0.07, AE1 = 0.60 ± 0.11, AE2 = 0.36 ± 0.14) and medians (Kruskal–Wallis test, H = 8.14, P = 0.017; medians = 0.80, 0.64, and 0.33).
Discussion
Live-Dead Discordance as a Product of Taphonomic Inertia.
The strength of the association between live-dead disagreement and AE is remarkable, given the array of natural processes, other human impacts, and methodological issues that might undermine the match between a single census of the living community and a time-averaged death assemblage. The higher average discordance exhibited by AE settings implies that death assemblages lag behind the shift in ecological baseline caused by human perturbation – it takes time for the “new” community to dilute the dead residua of the “old” preimpact community. Live-dead discordance is thus in part a signal of human impacts in the recent past, that is within the time frame of time-averaging, which has been successfully calibrated elsewhere by using other methods. High-resolution dating of shells in the poorest condition indicates that most were produced in the last century (11, 14), and dating of randomly drawn shells indicates dominance by production in the last several decades (20–22).
The temporal changes implied by comparing localities from a spectrum of AE are also consistent with taphonomic inertia (Fig. 2). The community first shifts in the proportional abundances of species already present, eroding rank-order agreement, followed by the total loss of some species (which may become “dead-only”) and, in some instances, the entry of new “live-only” immigrants, progressively reducing taxonomic similarity. The composition of the death assemblage equilibrates to stochastic variation within the new living community only as the remains of the previous community are destroyed and/or buried below the mixing zone, and are diluted by the input of dead shells from the new community.
It makes sense that coarse-mesh data sets register community change more strongly than fine-mesh data sets, that is, exhibit greater inertia and thus greater live-dead discordance. The composition of the adult community should be less subject to stochastic variation within a given community state, so that a single census is a more accurate sample of the living community, and the larger shells should tend to have longer postmortem persistence and greater spatial fidelity. Taxonomic similarity, being fundamentally a presence-absence test, should be less sensitive to the swings in abundance among subadults and thus to mesh size. These results are also consistent with the mesh-size effect detected in metaanalyses across marsh to outer shelf assemblages (refs. 23–25; fortuitously, this earlier database was drawn largely from pristine study areas; and see ref. 26).
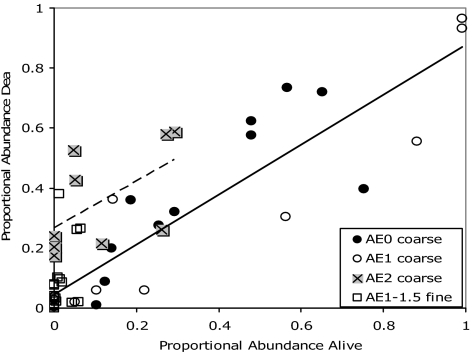
If the correlation of live-dead discordance and AE is causal, then live-dead differences in species identity should also be consistent with eutrophication. Analysis of data sets from the Gulf of Mexico, where all five AE2 lagoons are located, reveals that many species that occur “dead only” or at very low abundance alive prefer grassy substrata (Fig. 3; see SI Text for details). In the 38 coarse-mesh data sets from the 11 pristine to mildly AE lagoons (AE0 to AE1) in that same region, the proportional abundance of grass-dwellers in the death assemblage is positively correlated with that in the local living community, with a slope remarkably close to 1:1 and a y-intercept near zero (Fig. 3). In contrast, with two exceptions out of nine data sets, death assemblages in the five AE2 lagoons are enriched in grass-dwellers relative to living communities by 20% or more. Both exceptional AE2 data sets (high live-dead agreement in grass-dwellers) are from a single lagoon (Alvarado) where Ruppia seagrass has persisted despite pollution and where rootmats of invasive water hyacinths may seasonally provide a substitute habitat (SI Text). In AE2 lagoons, the most abundant living mollusks tend to be predatory and deposit-feeding gastropods or the opportunistic bivalve Mulinia lateralis, with grass-dwellers constituting at most 30% (in AE0 and AE1 lagoons their abundance ranges up to nearly 100%). Loss of seagrass is a common consequence of eutrophication: in addition to increasing epiphytic growth, nutrient enrichment increases phytoplankton production beyond zooplankton grazing capacity, leading to increased water turbidity, decreased light levels, and morbidity and mortality of subaquatic vegetation (6, 27, 28). The tendency for AE1 data sets to plot among pristine data sets makes sense in this regard, because fertilization initially stimulates grass growth. The relative abundance of the opportunist Mulinia is also consistent with the episodic hypoxia, stunted animal growth, and pulsed mortality that can accompany eutrophication (e.g., ref. 29). Mulinia is rare both living and dead in the pristine lagoons, but tends to be among the most abundant species both living and dead in AE1 and AE2 lagoons, where colonization and strong mortality would alternate with seasonal or other variation in water stratification (SI Fig. 5).
Fig. 3.
The proportional abundances of grass-dwelling mollusks in death assemblages are, in general, positively correlated with those in the local living communities, based on analysis of 47 large coarse-mesh data sets from 16 lagoons in the Gulf of Mexico (scatterplot of raw data). In the 11 lagoons with negligible (AE0, black circles) to mild AE (AE1, open circles), grass-dwellers constitute up to 100% of individuals in the living community, which is closely reflected by death assemblages (solid trendline for raw data; linear regression of arcsin-normalized proportions r2 = 0.81, P ≪ 0.001, slope = 0.83, y-intercept = 0.01 after back-transformation). In the five lagoons where anthropogenic eutrophication is severe, grass-dwellers are absent to sparse in the living assemblage and are enriched in the death assemblage, usually by ≥20% (AE2 asterisks with gray shadow, 9 data sets; dashed trendline is for raw AE2 data; for normalized data, r2 = 0.39, P = 0.07, slope = 0.83, y-intercept = 0.23 after back-transformation). Fine-mesh data sets from the Gulf of Mexico region are all from northern Texas lagoons, where grasses are commonly suppressed by freshwater runoff and where AE is long-standing (11 data sets from two AE1 lagoons, 5 data sets from two AE1.5 lagoons; open squares). Grass-dwellers occur in only trace abundance in both living and death assemblages there, with the exception of death assemblages from three marginal sands that are enriched in grass-dwellers and are reasonable past habitats for seagrass.
Fine-mesh data sets from the Gulf of Mexico (n = 17) yield virtually no grass-dwellers either alive or dead (Fig. 3), but all are from northern Texas where grass diversity tends to be naturally depressed from low salinity and all have long-standing AE conditions. Grassbed extent has been in strong decline in the Galveston complex, for example, since at least the 1950s and probably since ≈1900 (SI Text). Many of the northern Texas death assemblages also include moderately abundant “dead-only” relicts from past salinity regimes (low-salinity Rangia and/or marine-salinity Anadara and Ostrea s.s.). These live-dead matches suggest that (i) death assemblages retrieved by grab samples have a multidecadal ecological memory and (ii) where grass-dwellers are rare dead as well as alive, grass has not been a significant element over that period (reasonably assuming that salinity-sensitive species are no more prone to postmortem transport than grass-dwellers).
Molluscan live-dead data sets from open shelves segregate similarly with respect to AE (Fig. 1 C and D; SI Table 2 and SI Fig. 6 for data set sources and for a counterpart to Fig. 2). Death assemblages from pristine areas are limited to the high-agreement quarter of the cross-plot, especially the upper half of that quarter, even though these shelves encompass naturally nutrient-poor (Yucatan) to nutrient-rich extremes (Patagonia and Amazon shelves). AE shelves overlap with the lower half of the range of pristine shelves and range down to much lower taxonomic similarity and rank-order correlations, including negative rho values on AE2 shelves (data sets from Suruga Gulf of Japan and Rhodes Island, collected near pollution point-sources). Live-dead differences in species identity again suggest eutrophication rather than postmortem transportation (living assemblages are dominated by small-bodied chemosymbiontic and deposit-feeding bivalves, which are disproportionately rare in the death assemblage; ref. 18). Variation is not an artifact of sampling, mesh size, or bottom habitat type (SI Fig. 7), nor does live-dead agreement vary with bottom-trawling (but virtually all shelves are trawled). However, live-dead agreement is lowest on AE shelves that are also narrow, suggesting that multiple extrinsic environmental factors contribute, including perhaps a tendency for narrow shelves to be more susceptible to ecological damage from human impacts (18).
Toward a Diagnostic Tool.
The concordance of estuarine/lagoonal and open shelf results strongly supports an association between AE and live-dead discordance, but this remains a correlation rather than a demonstrated causal link. Given that other anthropogenic stresses typically accompany AE (SI Tables 1 and 2), any or all of these stresses might also contribute to, or even be the proximate driver of, the inferred biotic changes in a given habitat. For example, seagrass health might decline from the near-permanent turbidity created by frequent channel-dredging, from depletion of herbivores, or from large-scale removal of suspension-feeding shellfish, which reduces the rate at which water is filtered (6, 28, 30). The key point is that the community composition has changed, and that, although natural changes have probably also occurred in many cases, human impacts are strongly implicated.
Live-dead disagreement is clearly prone to false negatives, making it a conservative indicator of AE and co-occurring human impacts. Forty-three percent of data sets from estuarine areas of certain modification (score ≥ AE1) fall within a rectangle defined by two standard deviations (SD) of the pristine population mean (29% fall inside a one-SD rectangle; SD calculated excluding the very low AE0 outlier in Fig. 1A; for open shelves, 38% fall inside two-SD and one-SD rectangles; SI Fig. 8). For an estuarine habitat to be recognized as “AE” with confidence by using live-dead discordance, the operational target would be a J–C index <≈0.7 and rho <≈0.2 (Fig. 2, SI Fig. 8 A and B); for open shelves, the target would be a J–C <≈0.6 and rho <≈0.1 (SI Figs. 6 and 8 C and D). Understanding the origins of variability within the pristine fields of Fig. 1 is thus an important next phase of metaanalysis, especially the role of intrinsic factors such as inter-specific differences in shell production and durability, given that many methodological and environmental factors have been identified (analyses here, plus refs. 18 and 23–26).
Understanding the wide range of live-dead mismatch observed among areas of definite AE (particularly the occurrence of high live-dead agreement) will also be critical to refining this method. Local history is clearly important. For example, if the duration of anthropogenic impacts exceeds the window of time-averaging, the seafloor death assemblage is likely to have equilibrated to the “new” community state. Equilibration has probably occurred in brackish Lake Hamana, where anoxia intensified ≈100 years before live-dead sampling (AE1; the two data sets have J–C ≈0.7 and rho = 0.1 and 0.3; SI Text), and in the Galveston Bay complex, where petrochemical refineries have operated for 100 years (AE1.5; four of the five data sets yield J–C ≥0.7 and rho >0.2; SI Text). Equilibration of a death assemblage with an AE living community is more likely in estuaries and lagoons than on open shelves, both because such embayments are natural sediment traps and because human activities there are generally more deeply rooted. The occurrence of high live-dead agreement in AE ≥1 estuaries (that is, agreement as high as that observed in pristine settings) is thus scientifically reasonable, albeit counterintuitive when first encountered.
High live-dead agreement might also arise if other human activities or natural conditions counteract AE. For example, four of the five high-agreement AE1 outliers circled in Figs. 1B and 2 are from a single lagoonal complex (Carmen-Machona). This complex receives petrochemical wastes and agricultural runoff, but with the opening of a new artificial inlet became strongly flushed with clean Gulf waters (SI Text), thus perhaps minimizing change in the soft-bottom molluscan community. The lone high-agreement AE2 outlier is from the mouth of Laguna Alvarado, where seagrass has persisted despite major pollution outfalls, perhaps because of natural flushing with Gulf waters (SI Text).
Thus in AE settings, death assemblage compositions will be arrayed along a mixing curve between old and new states of the living community. A significant live-dead mismatch within a seafloor habitat requires that the ecological shift (i) was strong and “permanent” (i.e., reached a state outside the range of natural variability in the previous system, establishing a new mean and variance) and (ii) occurred within the window of time-averaging. If sedimentation rates are high, or if the new AE state is deeply rooted in time, then live-dead mismatch within the mixing zone will be minimal, and ecological shifts will only be detected by coring into historical layers. Where the two necessary conditions (above) are met, the accuracy with which live-dead agreement registers a strong ecological change will depend upon the relative preservation potentials of the two communities. (i) Where the new community has a higher preservation potential than the old community (higher average per-species production and/or greater shell durability), the signal of the old community will rapidly fade or be diluted, and thus live-dead mismatch will be suppressed and the magnitude of ecological change underestimated. (ii) Where the new and old communities have comparable preservation potentials, the death assemblage is a modern, anthropogenic analog of the paleontologic concept of faunal condensation, and characterized by moderate live-dead mismatch owing to the mix of shells from pre- and postimpact communities. This is the likely situation where seagrass-dwellers are the guild most sensitive to AE, because they exhibit a range of body sizes, skeletal compositions, and production rates similar to those of fauna on nonvegetated substrata. (iii) Where the new community has a lower preservation potential than the old community, the mismatch will be accentuated and the death assemblage is effectively a time-capsule of preimpact conditions. This is a likely situation where AE results in an increase in organic-loving species such as prosobranch and solemyid bivalves, which employ intrinsically low-durability, high-organic aragonitic shell microstructures that are rare among the suspension-feeding infaunal bivalves that characterize noneutrophied seafloors. This asymmetry in preservation potential is also likely on seafloors subject to bottom-trawling or dredging for fin- and shellfish. Slower growing and less frequently recruiting macrobenthos (many infaunal mollusks with relatively large or thick shells) are disadvantaged in such conditions along with epifauna, which include the only molluscan groups producing calcitic shells (oysters, scallops, mussels, among others). Shells from the original community are thus likely to be more durable than those produced by the replacement community, whether degradation of the original community is due to direct harvesting or a trophic cascade (e.g., refs. 31–33).
Field studies where the cultural and ecological history is known in detail will be essential to calibrate the magnitude of taphonomic inertia against the magnitude of ecological change and differential preservation potential of pre- and postimpact communities. The ideal test would involve quantitative “before-and-after” data on both the composition of the living and the death assemblage. The limited studies available indicate a range of time-lags in response to ecological change. On the one hand, two live-dead molluscan surveys of back-reef habitats in Smuggler's Bay, Virgin Islands, separated by 20 years, indicate that both living and death assemblages changed significantly but in the same direction, thereby maintaining good live-dead agreement with no inertia (37). On the other hand, molluscan death assemblages collected in 2003 from the southern California shelf are positively correlated to all six live censuses conducted over the preceding 30 years of biomonitoring, but are most similar to the earliest, lucinoid-dominated community, implying decadal-scale taphonomic inertia to (declining) AE and climatic regime change.†
Conclusions
Molluscan death assemblages from regions subject to AE (and usually to other human impacts as well) tend to exhibit significantly poorer agreement with the local living community than death assemblages from areas of negligible human modification. As a method of environmental assessment, live-dead agreement is imperfect, with AE areas showing considerable overlap with pristine areas. However, given the sparseness of data on the preimpact composition of most communities and the urgent need for ecological baselines to support management decisions (e.g., ref. 34), this approach using live-dead discordance should be vigorously evaluated, particularly because it is clear how to weight positive versus negative results. In both estuarine/lagoonal (analysis here) and open shelf settings (18), high taxonomic similarity and positive rank-order correlation in species abundance are not definitive evidence for an undisturbed environment. However, low taxonomic similarity and/or low to negative rank-order correlation are almost always associated with anthropogenic modification (Fig. 1). Live-dead discordance should have general utility as an inherently conservative means of recognizing strong, recent change in ecological systems, given taphonomic inertia. It should not be limited to the detection of anthropogenic eutrophication, despite the focus of the present analysis, nor in principle to the molluscan component of skeletal death assemblages.
This analysis also indicates that the human footprint is a significant peril to actualism: modified systems yield artificially pessimistic estimates of the ecological fidelity of time-averaged death assemblages. Assuming no significant postburial biases (which is reasonable for Holocene records that have not been uplifted into meteoric zones), the relatively high live-dead agreement values obtained in pristine areas indicates the confidence with which preimpact baselines can be extracted from historical layers, with comparable pooling of samples into habitat-level data sets. These results thus strengthen the already compelling cases made by others for historical transformation of benthic communities by using time-averaged skeletal records, for example a 20-fold reduction in molluscan abundance in the estuarine Colorado River delta associated with damming of freshwater outflow since the 1930s (35), a 5-fold reduction in molluscan species diversity and 60% reduction in abundance during the 100-year history of scallop fishing in Tasmania (32), and AE-related decline of Florida coral reefs since the 1950s signified by a 3-fold increase in sedimentary coral debris (36), to mention but a few.
The paleontological phenomenon of faunal condensation, usually considered an obstacle to ecology and paleoecology, thus presents a valuable means not only to recognize recent anthropogenic modification, but to identify the most likely drivers of change, based on contrasts in the ecological preferences of live and dead species. The high fidelity of death assemblages formed under pristine conditions also indicates that the composition of a true preimpact community can be inferred from older sedimentary layers with considerable confidence. Live-dead discordance is a promising additional tool to bring to bear on the increasingly critical need to evaluate anthropogenic impacts on ecological communities.
Methods
Ninety-three molluscan live-dead data sets, each based on pooling counts of live and dead individuals sieved from sediment samples at two or more stations within a subtidal habitat (maximum = 74 stations; median = 9), are available from 28 mostly North American estuaries, lagoons, and other coastal embayments protected from the full force of ocean energy (SI Table 1). Habitats are defined on the basis of sedimentary grain size and other physical features that would be distinguishable in sedimentary cores (e.g., bay-head delta versus mid-reach mud versus bay margin or tidal inlet sands within a single lagoon). All data sets are based on a single survey of the habitat by the original author for live and dead individuals, which mimics a likely sampling protocol for environmental assessment. If an author surveyed a habitat repeatedly, the visit yielding the largest sample size of live individuals is used. If an area was resampled by a different author, that second study is entered as a separate, independent analysis, despite the geographic overlap.
Of the 93 data sets, 77 are based on at least 20 live and 20 dead individuals, which is a minimal acceptable sample size for establishing species rank-order and proportional abundances (see SI Fig. 4 for lack of sensitivity of results to sampling effort). Of these 77 large data sets, 73 could be scored confidently for AE (see SI Text for details). Twenty-seven are from eight coastal embayments judged to have negligible AE at the time of sampling; 5 additional data sets are from two areas less confidently included in this category, and thus scored as AE0.5; 41 data sets are from 16 areas of definite AE (AE1 to AE2; 14 data sets are from 7 areas where AE >1). AE scores are based on independent scientific reports and the expert knowledge of original authors of the molluscan surveys, which was deferred to in the few cases of disagreement (experts tended to recognize more human impact than reported in the literature). The AE designation indicates only that nutrient supply was elevated above natural levels, but not necessarily to fully eutrophic conditions. As AE scores increase, other human impacts also tend to increase, including sediment toxicity, solid sediment runoff, dredging and spoil-dumping, commercial shell-fishing, and salinity modification related to channelization (SI Text).
For each data set, live and dead species lists are compared by using two standard ecological metrics: (i) Similarity in taxonomic composition, by using Chao et al. 's (19) abundance-based Jaccard index to compensate for differences in (live and dead) sample sizes. The J–C index has a potential range from 0 (no shared species) to 1; and (ii) rank-order agreement in species relative abundance, by using the correlation coefficient rho of a nonparametric Spearman rank-order test, with a potential range from −1 (species in one list are ranked in opposite order to the other list) to +1.
The 34 large data sets from open shelf settings plotted in Fig. 1 C and D were handled the same way, and are drawn from the 38 data sets used in Kidwell (ref. 18; SI Table 2). Eighteen data sets are from 8 shelves judged to have negligible AE; 16 data sets are from 9 areas of definite AE, of which four areas are AE >1 (5 data sets). Commercial harvesting of bottom-dwelling fin- or shell-fish was long-standing, mechanized, or both in all but three areas (6 data sets).
Supplementary Material
Acknowledgments
For unpublished data on molluscan assemblages and insights into cultural and environmental history, I am grateful to D. Bosence, T. R. Calnan, A. A. Ekdale, A. Garcia-Cubas, S. W. Henderson, J. B. C. Jackson, A. I. Miller, M. Peharda, E. N. Powell, M. M. Reguero, Edmund Smith (associate of the late R. G. Johnson), Elizabeth Smith (now Fischer), G. M. Staff, J. E. Warme, and W. A. White. I am especially grateful to M. M. Reguero for her thoughtful exchanges on human impacts and guidance into the regional literature. Errors of analysis and interpretation are, however, my own. I also thank librarians of the U.S. National Museum of Natural History, The Natural History Museum (London), Texas Bureau of Geology, California Academy of Sciences, and Crerar Science Library of the University of Chicago for assistance with their holdings, and D. Jablonski, P. Harnik, A. Tomasovych, M. Kowalewski, J. W. Valentine, and an anonymous reviewer for helpful comments. This work was supported by National Science Foundation Earth Sciences (EAR) Grant 0345897.
Abbreviations
- AE
anthropogenic eutrophication
- J–C
Jaccard-Chao index of taxonomic similarity.
Footnotes
The author declares no conflict of interest.
See Commentary on page 17563.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707194104/DC1.
Tomasovych A, Kidwell SM, Rothfus TA (2007) Ecol Soc Am Ann Mtg Abstr, no 92.
References
- 1.Paerl HW. Limnol Oceanogr. 1997;42:1154–1165. [Google Scholar]
- 2.Jackson JB, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, et al. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 3.Schöne BR, Flessa KW, Dettman DL, Goodwin DH. Est Coastal Shelf Sci. 2003;54:715–726. [Google Scholar]
- 4.Roessig JM, Woodley MJ, Cech JJ, Jr, Hansen LJ. Rev Fish Biol Fisheries. 2004;14:251–275. [Google Scholar]
- 5.Parmesan C. Annu Rev Ecol Evol Syst. 2006;37:637–669. [Google Scholar]
- 6.Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, et al. BioScience. 2006;56:987–996. [Google Scholar]
- 7.National Research Council (USA) The Geological Record of Ecological Dynamics. Washington, DC: Natl Acad Press; 2005. [Google Scholar]
- 8.Cooper SR, Brush GS. Science. 1991;254:992–996. doi: 10.1126/science.254.5034.992. [DOI] [PubMed] [Google Scholar]
- 9.Finney BP, Gregory-Eaves I, Sweetman J, Douglas MSV, Smol JP. Science. 2000;290:795–799. doi: 10.1126/science.290.5492.795. [DOI] [PubMed] [Google Scholar]
- 10.Rabalais NN, Turner RE, Sen Gupta BK, Platon E, Parsons ML. Ecol Appl. 2007;17:S129–S143. [Google Scholar]
- 11.Meldahl KE, Flessa KW, Cutler AH. Paleobiology. 1997;23:207–229. [Google Scholar]
- 12.Anderson LC, Sen Gupta BK, McBride RA, Byrnes MR. Geology. 1997;25:127–130. [Google Scholar]
- 13.Carroll M, Kowalewski M, Simões MG, Goodfriend GA. Paleobiology. 2003;29:382–403. [Google Scholar]
- 14.Kidwell SM, Best MMR, Kaufman D. Geology. 2005;33:729–732. [Google Scholar]
- 15.Kidwell SM, Flessa KW. Annu Rev Ecol Syst. 1995;26:269–299. [Google Scholar]
- 16.Pandolfi JM, Greenstein BJ. Limnol Oceanogr. 1997;42:1505–1516. [Google Scholar]
- 17.Staff GM, Powell EN. Cont Shelf Res. 1999;19:717–756. [Google Scholar]
- 18.Kidwell SM. Lethaia. 2008;41 in press. [Google Scholar]
- 19.Chao A, Chazdon RL, Colwell RK, Shen T-J. Ecol Lett. 2005;8:148–159. [Google Scholar]
- 20.Goodwin DH, Flessa KW, Téllez-Duarte MA, Dettman DL, Schöne BR, Avila-Serrano GA. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;205:1–21. [Google Scholar]
- 21.Barbour Wood SL, Krause RA, Jr, Kowalewski M, Wehmiller J, Simões MG. Quat Res. 2006;66:323–331. [Google Scholar]
- 22.Kosnik M, Hua Q, Jacobsen G, Kaufman D, Wus R. Geology. 2007;35:811–814. [Google Scholar]
- 23.Kidwell SM. Science. 2001;294:1091–1094. doi: 10.1126/science.1064539. [DOI] [PubMed] [Google Scholar]
- 24.Kidwell SM. Geology. 2002;30:803–806. [Google Scholar]
- 25.Kidwell SM. Geobios Mém Spec. 2002;24:107–119. [Google Scholar]
- 26.Olszewski TA, Kidwell SM. Paleobiology. 2007;33:1–23. [Google Scholar]
- 27.Twilley RR, Kemp WM, Staver KW, Stevenson JC, Boynton WR. Mar Ecol Progr Ser. 1985;23:179–191. [Google Scholar]
- 28.Newell RIE, Koch EW. Estuaries. 2004;27:793–806. [Google Scholar]
- 29.Powers SP, Peterson CH, Christian RR, Sullivan E, Powers MJ, Bishop MJ, Buzzelli CP. Mar Ecol Progr Ser. 2005;302:233–243. [Google Scholar]
- 30.Grant J, Bugden G, Horne E, Archambault M-C, Carreau M. Can J Fish Aquat Sci. 2007;64:387–390. [Google Scholar]
- 31.Kaiser MJ, Ramsay K, Richardson CA, Spence FE, Brand AR. J Anim Ecol. 2000;69:494–503. [Google Scholar]
- 32.Edgar GJ, Samson CR. Conserv Biol. 2004;18:1579–1588. [Google Scholar]
- 33.Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH. Science. 2007;315:1846–1850. doi: 10.1126/science.1138657. [DOI] [PubMed] [Google Scholar]
- 34.Gray JS, Dayton P, Thrush S, Kaiser MJ. Mar Poll Bull. 2006;52:840–843. doi: 10.1016/j.marpolbul.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Kowalewski M, Avila Serrano GE, Flessa KW, Goodfriend GA. Geology. 2000;28:1059–1062. [Google Scholar]
- 36.Lidz BH, Hallock P. J Coastal Res. 2000;16:675–697. [Google Scholar]
- 37.Ferguson CA, Miller AI. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;254:418–429. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.