Abstract
Male and female germ cells can transmit genetic defects that lead to pregnancy loss, infant mortality, birth defects, and genetic diseases in offspring; however, the parental origins of transmitted defects are not random, with de novo mutations and chromosomal structural aberrations transmitted predominantly by sperm. We tested the hypotheses that paternal mutagenic exposure during late spermatogenesis can induce damage that persists in the fertilizing sperm and that the risk of embryos with paternally transmitted chromosomal aberrations depends on the efficiency of maternal DNA repair during the first cycle after fertilization. We show that female mice with defective DNA double-strand break repair had significantly increased frequencies of zygotes with sperm-derived chromosomal aberrations after matings with wild-type males irradiated 7 days earlier with 4 Gy of ionizing radiation. These findings demonstrate that mutagenic exposures during late spermatogenesis can induce damage that persists for at least 7 days in the fertilizing sperm and that maternal genotype plays a major role in determining the risks for pregnancy loss and frequencies of offspring with chromosomal defects of paternal origin.
Keywords: ionizing radiation, zygote, nonhomologous end joining, homologous recombination
Chromosomal defects transmitted through male and female germ lines are associated with pregnancy loss, developmental defects, infant mortality, infertility, and genetic diseases in the offspring (1). The parental origins of de novo genetic and chromosomal defects are not random: e.g., autosomal aneuploidy has a preferential maternal origin (2), whereas point mutations and structural chromosomal rearrangements have a preferential paternal origin (3). Male germ cells are susceptible to the accumulation of DNA lesions in fertilizing sperm because their DNA repair capacity declines during the latter part of spermatogenesis (4). In contrast, the mammalian oocyte is capable of repairing DNA damage throughout oogenesis and provides gene products that are responsible for repairing DNA damage in both parental genomes after fertilization (5, 6). These maternal gene products persist to sustain the zygote until its genome is fully activated, which occurs at the two-cell stage in the mouse (7) and even later in the human embryo (8). Early suggestion that the maternal genotype can affect the risk of transmission of genetic defects from the father came from mouse strains that were shown to vary in their frequencies of chromosomally defective embryos after paternal exposure to various mutagens (9). However, the molecular mechanisms responsible for these differences were not understood.
Chromosome-type aberrations, which affect both sister chromatids, were the main type of aberration found in zygotes after paternal exposure to ionizing radiation and chemical mutagens (10). Thus, we hypothesized that double-strand breaks (DSBs) are obligatory intermediates between DNA lesions in sperm and chromosomal aberrations in zygotes and that DSB repair occurs during the G1 phase of the zygotic cell cycle. DSB repair can occur along two mechanistically distinct pathways: nonhomologous end joining (NHEJ), i.e., direct rejoining of broken ends with minimal requirement for homology (11, 12), and homologous recombination (HR), in which a sister duplex provides information to repair the damaged duplex (13, 14). The NHEJ pathway involves the DNA–PK (DNA–protein kinase) complex, which is composed of the Ku70/Ku86 DNA end-binding heterodimer and the catalytic subunit DNA–PKcs (15); the latter is defective in the immunodeficient scid mouse strain (16). The HR pathway involves the RAD52 epistasis group including RAD54 (12), which interacts with RAD51 and is required for DSB repair and sister chromatid exchange (17, 18). Disruption of either DSB repair pathway in somatic cells confers increased radiation sensitivity (19).
We tested the hypotheses that paternal mutagenic exposure during late spermatogenesis, a time of diminished DNA repair capacity, can induce persistent damage in the fertilizing sperm and that the risk of embryos with paternally transmitted chromosomal aberrations depends on the efficiency of maternal DNA repair during the first cycle after fertilization. We used NHEJ-defective scid and HR-defective Rad54-null female mice to investigate how disruption of the NHEJ and HR pathways in the fertilized egg altered the repair of radiation-induced sperm DNA lesions by analyzing chromosomal aberrations at the first metaphase after fertilization. The results showed that genetic disruption of maternal DNA DSB repair pathways in mice significantly increased the frequency of zygotes with chromosomal structural aberrations after paternal exposure to ionizing radiation.
Results and Discussion
We used NHEJ-defective scid and HR-defective Rad54-null female mice to investigate how disruption of the maternal NHEJ and HR pathways in the fertilized egg altered the repair of radiation-induced sperm DNA lesions. B6C3F1 males were treated with 4-Gy γ-rays 7 days before mating with normal (C57BL/6J), scid, or Rad54-null females. First-cleavage zygote metaphases were collected for each maternal genotype and analyzed by the PAINT/DAPI chromosome painting procedure (Fig. 1) to determine the frequencies of zygotes with chromosomal structural aberrations and the types of aberrations involved, i.e., chromosome type vs. chromatid type, and rejoined vs. unrejoined.
Fig. 1.
Photomicrographs of first-cleavage (1-Cl) zygote metaphases using the PAINT/DAPI procedure. (Scale bars: 10 μm.) (A) Normal 1-Cl zygote metaphase from a C57BL/6J female with the Y-bearing sperm-derived chromosomes on the left. (B) Paternal chromosomes in a zygote from a scid female mated to an irradiated male showing a reciprocal translocation involving one chromosome painted in red and one unpainted chromosome (arrows). (C) Partial paternal chromosome complement showing an acentric fragment in a zygote from a scid female (arrowhead). (D and E) Examples of chromatid-type aberrations in paternal chromosomes in 1-Cl zygotes from Rad54-null females mated with irradiated males: triradial (D) and chromatid break (E). Arrows indicate rejoined aberrations; arrowheads indicate unrejoined aberrations.
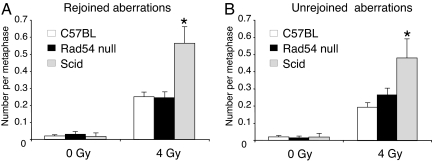
Disruption of the NHEJ pathway in female mice resulted in a 2.2-fold increase in the frequencies of zygotes with chromosomal aberrations with respect to C57BL/6J females after matings with irradiated males (P < 0.001; Table 1). Analysis of the types of chromosomal aberrations showed that there was a 2.4-fold increase (P < 0.001) in the number of chromosome-type aberrations in NHEJ-defective females, whereas chromatid-type aberrations were unchanged [supporting information (SI) Fig. 5]. Both unrejoined (i.e., acentric fragments and breaks) and rejoined (i.e., dicentrics, translocations, etc.) chromosomal aberrations were significantly higher in NHEJ-defective females with respect to C57BL/6J females (P < 0.001; Fig. 2 A and B). These results indicate that radiation-induced sperm DNA lesions persisted in the maturing sperm for at least 7 days before fertilization before they were repaired in the fertilized egg by the NHEJ pathway and that this repair occurred during the G1 phase of the first cell cycle of development (Fig. 3). Consistent with observations in somatic scid cells (20, 21), disruption of the NHEJ pathway in the fertilized egg through abrogation of DNA–PKcs function resulted in both a reduction in repair, as indicated by the increase in unrejoined aberrations, and an increase in misrepair of DSBs, as indicated by the increase in rejoined aberrations. This finding supports the existence of a NHEJ repair mechanism that is DNA–PK-independent and prone to misrejoining (22, 23).
Table 1.
Chromosomal aberrations in zygotes of three maternal genotypes mated to unirradiated (0 Gy) or irradiated (4 Gy) males
| Maternal genotype | Paternal exposure, Gy* | Zygotes analyzed | Zygotes with aberrations |
Zygotes with chromosome-type aberrations |
Zygotes with chromatid-type aberrations |
|||
|---|---|---|---|---|---|---|---|---|
| No.† | % ± SE‡ | No. | % ± SE ‡ | No. | % ± SE‡ | |||
| C57BL/6J | 0 | 117 | 2 | 1.7 ± 0.7 | 2 | 1.7 ± 0.7 | 0 | 0 |
| C57BL/6J | 4 | 261 | 56 (3) | 21.5 ± 1.6 | 55 | 21.1 ± 1.7 | 4 | 1.5 ± 0.9 |
| scid | 0 | 61 | 1 | 1.6 ± 1.1 | 1 | 1.6 ± 1.1 | 0 | 0 |
| scid | 4 | 71 | 34 (1) | 47.9 ± 6.6§¶ | 34 | 47.9 ± 6.6§¶ | 1 | 1.4 ± 1.9 |
| Rad54-null | 0 | 175 | 4‖ (1) | 2.3 ± 0.9 | 2 | 1.1 ± 1.0 | 3‖ | 1.7 ± 1.1 |
| Rad54-null | 4 | 155 | 45 (4) | 29.0 ± 2.4** | 38 | 24.5 ± 2.2 | 11 | 7.1 ± 1.8†† |
*Seven days prior to mating; males were B6C3F1 for all the experiments.
†Zygotes with both chromosome-type and chromatid-type aberrations are reported in parentheses.
‡Standard error across the minimum of three pools of zygotes.
§P < 0.01 vs. C57BJ/6J; 4 Gy.
¶P < 0.05 vs. Rad54-null; 4 Gy.
‖One zygote with a chromatid exchange in the maternal chromosomes.
**P = 0.08 vs. C57BL/6J; 4Gy.
††P < 0.05 vs. C57BL/6J; 4 Gy and Rad54-null; 0 Gy.
Fig. 2.
Relative induction of rejoined (A) and unrejoined (B) aberrations in C57BL/6J, Rad54-null, and scid females mated to unirradiated (0 Gy) or irradiated (4 Gy) male mice. The number of aberrations per metaphase analyzed is reported on the y axis, and the bars represent the SE. *, P < 0.001 vs. C57BL/6 females.
Fig. 3.
Model for the repair of radiation-induced sperm DNA lesions by maternal DSB repair pathways after fertilization. Our results with NHEJ-defective females show that DSBs induced by irradiating sperm before fertilization are repaired by the NHEJ pathway during the G1 phase of the first cell cycle. In the absence of a functional NHEJ pathway, unrepaired DSBs produce chromosome-type aberrations and increased unrejoined chromosomal breaks. Our results with HR-defective females show that some radiation-induced lesions are repaired during the S/G2 phase of the cell cycle. Thus, in the absence of a functional HR pathway, we observed an increase in chromatid-type aberrations. They may be a class of DSBs that escape repair by the functional NHEJ pathway during G1 or represent DSBs generated by misrepair of other sperm DNA lesions by other DNA repair pathways during G1.
Disruption of the HR pathway in female mice did not significantly change the frequencies of zygotes with chromosomal aberrations with respect to C57BL/6J females after matings with irradiated males (P = 0.08; Table 1). However, the frequency of zygotes with chromatid-type aberrations was increased 4.2-fold (P < 0.05; Table 1). Analysis of the types of chromosomal aberrations showed that chromosome-type aberrations were unaffected, whereas HR-defective females had a 5.5-fold increase in chromatid-type aberrations (P < 0.001; SI Fig. 5). Thus, HR deficiency and NHEJ deficiency showed an opposite effect on the production of chromosome- and chromatid-type aberrations. Disruption of the HR pathway did not affect the number of rejoined and unrejoined aberrations (Fig. 2 A and B). These findings demonstrate that the HR pathway also participates in the repair of radiation-induced sperm DNA lesions and postulate the existence of a class of DSBs that persisted unrepaired beyond the G1 phase and, in the absence of a functional HR pathway, produced chromatid-type aberrations during the S/G2 phase. This class of DSBs may represent lesions that (i) escaped repair by the functional NHEJ pathway during G1 or (ii) were generated by misrepair of other types of DNA lesions by other DNA repair pathways during the G1 phase of the cell cycle.
We next examined how disruption of the maternal NHEJ and HR pathways affected the progression through the first cell cycle of development (Table 2). Eggs were classified as unfertilized or fertilized (24), and fertilized eggs were further categorized as (i) arrested before S-phase (based on the absence of pronuclei or mitotic chromosomes); (ii) in S phase (based on the presence of the two parental pronuclei); and (iii) beyond S phase (based on the presence of mitotic chromosomes). Paternal exposure to radiation slightly reduced the number of eggs that were fertilized, across all three maternal genotypes (P = 0.09), but did not significantly affect the progression through the first cell cycle in NHEJ-defective females. However, when HR-defective females were mated with irradiated males, there was a significant reduction in the number of fertilized eggs that reached the zygotic metaphase stage compared with control mice mated to irradiated males (P < 0.001) and with HR-defective females mated to unirradiated males (P < 0.05). The data in Table 2 indicate that this reduction was caused by a failure of some fertilized eggs to form pronuclei rather than by a general delay in cell cycle progression. Formation of the male pronucleus is known to require dramatic chromatin remodeling of the fertilizing sperm DNA as protamines are removed and oocyte-supplied histones are assembled onto the sperm DNA (25). Our results suggest that, independently from its function in HR, Rad54 is involved in the remodeling of the sperm nucleus into the male pronucleus. This finding is consistent with the observation that Rad54 can catalyze nucleosome redistribution along the DNA independently of its association with Rad51 (26, 27).
Table 2.
Frequencies and types of eggs recovered from females with three maternal genotypes mated to unirradiated (0 Gy) or irradiated (4 Gy) males
| Maternal genotype | Paternal exposure, Gy* | Total eggs | Unfertilized eggs |
Fertilized eggs |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arrested before S phase |
In S phase |
Beyond S phase |
||||||||
| Total | % ± SE | Total | %† ± SE | Total | %† ± SE | Total | %† ± SE | |||
| C57BL/6J | 0 | 246 | 59 | 24.0 ± 2.7 | 29 | 15.5 ± 1.2 | 5 | 2.7 ± 1.2 | 153 | 81.8 ± 2.5 |
| C57BL/6J | 4 | 660 | 191 | 28.9 ± 2.3 | 87 | 18.6 ± 1.9 | 16 | 3.4 ± 1.6 | 366 | 78.0 ± 3.0 |
| scid | 0 | 160 | 43 | 26.9 ± 1.9 | 19 | 16.2 ± 4.7 | 3 | 2.6 ± 1.5 | 95 | 81.2 ± 6.2 |
| scid | 4 | 283 | 98 | 34.6 ± 2.8 | 47 | 25.4 ± 3.3 | 5 | 2.7 ± 1.2 | 133 | 71.9 ± 2.6 |
| Rad54-null | 0 | 466 | 135 | 29.0 ± 3.9 | 84 | 25.4 ± 6.2 | 7 | 2.1 ± 0.9 | 240 | 72.5 ± 6.7 |
| Rad54-null | 4 | 607 | 219 | 36.1 ± 3.1 | 155 | 39.9 ± 2.4‡§ | 18 | 4.6 ± 1.7 | 215 | 55.4 ± 1.4‡§ |
*Seven days prior to mating; males were B6C3F1 for all the experiments.
†Frequencies among fertilized eggs.
‡P < 0.05 vs. Rad 54 null; 0 Gy.
§P < 0.001 vs. C57BL/6J; 4 Gy.
Our findings show that deficiencies in maternal DSB repair increased both chromosome- and chromatid-type aberrations. Among chromosome-type aberrations, both unstable (dicentrics, acentric fragments, breaks, etc.) and stable (reciprocal translocations) aberrations were increased. These types of abnormalities have different consequences for the developing embryo. We showed previously that zygotes with unstable chromosomal aberrations after paternal exposure to 12 different germ cell mutagens are highly correlated (Fig. 4A; β = 0.73; R2 = 0.81; P < 0.001) with the frequencies of embryonic lethality in standard dominant lethal tests (10, 28). For this analysis, only aberrations detected by DAPI were considered. In this work, we found that that 19.5% of the zygotes in C57BL/6J females had unstable chromosomal aberrations (Table 3), which is not different (P = 0.2) from the 24.5% of embryonic lethality reported in the dominant lethal study after paternal irradiation with 4 Gy (29) and that a deficiency in the NHEJ pathway will more than double the risk of embryonic lethality after paternal exposure to ionizing radiation. Paradoxically, the inability of NHEJ-defective females to properly repair DSB carried by the sperm and the resulting elimination of embryos with unstable aberrations during pregnancy may confer protection toward transgenerational genomic instability (30).
Fig. 4.
Linear regression analyses of the relationship between the frequencies of zygotes with chromosomal aberrations and reproductive outcomes. ■, data from the present work with C57BL/6J females. ▵, literature data after paternal exposure to 13 mutagens, including ionizing radiation. (A) Correlation between unstable aberrations in zygotes and embryonic lethality as measured in standard dominant lethal tests. [Adapted from Marchetti and Wyrobek (10).] (B) Correlation between reciprocal translocations in zygotes and translocation carriers after birth as measured in standard heritable translocation tests. [Adapted from Marchetti et al. (28).]
Table 3.
Predicted frequencies of embryonic lethality (DL) and offspring with reciprocal translocations (HT) in mouse zygotes (1-Cl) zygotes of three maternal genotypes mated to unirradiated (0 Gy) or irradiated (4 Gy) male
| Maternal genotype | Paternal exposure, Gy* | DAPI analysis |
PAINT analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of 1-Cl analyzed | 1-Cl with unstable aberrations | Predicted DL† | No. of 1-Cl analyzed‡ | 1-Cl with unstable aberrations | 1-Cl with stable aberrations | Normal zygotes | Predicted HT§ | ||
| C57BL/6J | 0 | 117 | 2 | 1.3 ± 0.7 | 69 | 1 | 1 | 67 | 0 |
| C57BL/6J | 4 | 261 | 51 | 19.5 ± 1.4 | 149 | 22 | 11 | 116 | 8.7 ± 2.9 |
| scid | 0 | 61 | 1 | 1.6 ± 1.1 | 36 | 1 | 0 | 35 | 0 |
| scid | 4 | 71 | 31 | 43.7 ± 8.1 | 42 | 9 | 2 | 31 | 6.1 ± 3.2 |
| Rad54-null | 0 | 175 | 4 | 2.3 ± 0.9 | 103 | 3 | 0 | 100 | 0 |
| Rad54-null | 4 | 155 | 42 | 27.1 ± 2.4 | 76 | 11 | 3 | 62 | 4.6 ± 1.9 |
*Seven days prior to mating; males were B6C3F1 for all the experiments.
†Percentage ± SE observed frequency of dominant lethality: 24.5% (see ref. 29).
‡Cell equivalents. See Materials and Methods.
§1-Cl with reciprocal translocations over the number of viable zygotes (normal plus reciprocal translocations). Percentage ± SE. Observed frequency of translocation carriers: 14.5% (see ref. 31).
PAINT analysis of zygotic metaphases has shown that the frequencies of reciprocal translocations in zygotes are predictive of the frequencies of translocation carriers at birth (28). Analysis of zygotes from C57BL/6J females mated to irradiated males estimated a frequency of translocation carriers at birth of 8.7% (Table 3), which was not different (P = 0.2) from the 14.5% reported in offspring (31). In null females, the estimates of translocation carriers were 6.1% and 4.6% for NHEJ-null and HR-null females, respectively. Although the numbers in null females are too small to conclude whether deficiencies in DSB repair affect the frequencies of reciprocal translocations with respect to controls, these results confirm that reciprocal translocations in zygotes are predictive of translocations carriers at birth (Fig. 4B).
Less certain is the fate of zygotes with chromatid-type aberrations. As shown in Table 1, there were 10 zygotes with only chromatid-type aberrations (7 in the HR-null females). It is possible that these zygotes will lead to mosaic embryos. For example, the zygote with the aberration shown in Fig. 1E would be expected to produce a two-cell embryo with one blastomere containing a normal karyotype and one blastomere with an abnormal one. In the absence of selection, further development of the embryo will be expected to produce a blastocyst with 50% of normal cells. Because the fetus is derived from only few of the cells that make up the blastocyst (32), such a mosaic embryo has the chance of resulting in a healthy offspring.
Our findings demonstrate that exposure of males to ionizing radiation induces DNA lesions in germ cells that persist in sperm for at least 7 days and that these lesions are then repaired in the fertilized egg by the maternal DSB repair pathways. Both NHEJ and HR pathways are involved in the repair of radiation-induced sperm DNA lesions during the first cell cycle after fertilization, with the NHEJ pathway playing a greater role than the HR pathway. Because both chromosome- and chromatid-type aberrations were affected, our results suggest that maternal DSB repair mechanisms are operating in G1 as well as S/G2 phases of the zygotic cell cycle. Our findings also suggest that maternal Rad54 may be involved in male pronuclear formation.
The results of this work highlight the importance of maternal DNA repair during the early phases of mammalian development in assuring the genomic integrity of the conceptus. It remains to be determined whether more subtle mutations, such as heterozygosity for these genes, will produce similar effects. Nevertheless, because chromosomal aberrations in zygotes are quantitatively associated with various types of abnormal reproductive outcomes (28), our current findings suggest that quantitative and qualitative differences in the efficiency of maternal DNA repair genes can directly alter the risk of early pregnancy losses and offspring with chromosomal aberrations of paternal origin.
Materials and Methods
Animals.
Studies were conducted in accordance with the principles and procedures outlined by the National Institutes of Health (33) and were approved by the Lawrence Livermore National Laboratory (LLNL) Institutional Animal Care and Use Committee. B6C3F1 males were purchased from Harlan Sprague–Dawley, Inc. (Indianapolis, IN); C57BL/6J and scid females were purchased from the Jackson Laboratory (Bar Harbor, ME). A colony of Rad54-deficient mice was established and maintained at LLNL from two Rad54-null breeding pairs (18). Both DSB repair mutations were on a C57BL/6J background.
Animal Treatments.
Mice were 6–8 weeks of age at the beginning of the experiments and were maintained under a 12-h light/dark photoperiod at a room temperature of 21–23°C and relative humidity of 50 ± 5%. Pelleted food and sterilized tap water were provided ad libitum. Male mice received a whole-body irradiation dose of 4 Gy with a delivery rate of 0.61 Gy/min from a 137Cesium Mark 1 irradiator (J. L. Shepherd and Associates, Glendale, CA) and were mated with unirradiated females 7 days later. Control males were sham irradiated. Female mice were superovulated by an i.p. injection of 7.5 units of pregnant mare's serum (Sigma, St. Louis, MO) followed 48 h later by an i.p. injection of 5.0 units of human chorionic gonadotrophin (Sigma). scid females did not respond as well as the other maternal genotypes to superovulation. The numbers of ovulated eggs were 20.3 ± 4.6, 21.1 ± 3.9, and 13.2 ± 2.5 in C57BL/6J, Rad54-null, and scid females, respectively.
Zygote Collection and Statistical Analysis.
For each data point, a minimum of three repetitions, each using 12 mating pairs, were conducted. In each repetition, zygotes from all mated females were pooled and processed simultaneously. Many factors affect the number of metaphases that can be analyzed in each repetition, including the number of mated females, the number of ovulated eggs, the number of eggs that lysed during the fixation process, and the quality of the cytogenetic preparations. For example, the number of metaphases analyzed in scid females is lower than that in the other two female genotypes because of the significantly reduced number of eggs that were ovulated by these females.
Metaphases were hybridized with chromosome-specific painting probes for chromosomes 1, 3, 5, X, and Y labeled with FITC and chromosomes 2, 4, 6, X and Y labeled with biotin and signaled with Texas red (CAMBIO). With this probe combination, chromosomes 1, 3, and 5 appear green, chromosomes 2, 4, and 6 appear red, and the sex chromosomes appear yellow. Paternal chromosomes can be identified because they contain the Y chromosome or they are less condensed than maternal chromosomes. The total frequencies of zygotes with chromosomal aberrations, the frequencies of zygotes with unstable aberrations for correlation with the risk of embryonic lethality, and the frequencies of zygotes with stable aberrations for correlation with the risk for translocation carriers at birth were obtained as described previously (28). Additionally, to obtain a more precise assessment of how disruption of the NHEJ and HR pathways affected the repair of DSBs, chromosomal aberrations were classified into (i) chromatid- or chromosome-type, depending on whether one or both sister chromatids were affected, and (ii) rejoined or unrejoined, depending on whether there was or was not resealing of a DSB, as in the case of dicentrics and reciprocal translocations or as in the case of breaks and excess acentric fragments, respectively.
The ANOVA test was used for comparing the progression during the first cell cycle, and the χ2 test was used for comparing the frequencies of zygotes with chromosomal aberrations and types of chromosomal aberrations among the three maternal genotypes.
Supplementary Material
Acknowledgments
This work was performed under the auspices of the U.S. Department of Energy by the University of California, Lawrence Livermore National Laboratory, under Contract W-7405-ENG-48 with funding support from National Institutes of Health Grant ES 09117-03 and Lawrence Berkeley National Laboratory under Contract DE-AC02-05CH11231.
Abbreviations
- DSB
double-strand break
- HR
homologous recombination
- NHEJ
nonhomologous end joining.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705257104/DC1.
References
- 1.McFadden D, Friedman J. Mutat Res. 1997;396:129–140. doi: 10.1016/s0027-5107(97)00179-6. [DOI] [PubMed] [Google Scholar]
- 2.Hassold T, Hunt P. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 3.Crow JF. Nat Rev Genet. 2001;1:40–47. doi: 10.1038/35049558. [DOI] [PubMed] [Google Scholar]
- 4.Olsen AK, Lindeman B, Wiger R, Duale N, Brunborg G. Toxicol Appl Pharmacol. 2005;207:521–531. doi: 10.1016/j.taap.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 5.Brandriff B, Pedersen RA. Science. 1981;211:1431–1433. doi: 10.1126/science.7466400. [DOI] [PubMed] [Google Scholar]
- 6.Ashwood MJ, Edwards R. Mol Hum Reprod. 1996;2:46–51. doi: 10.1093/molehr/2.1.46. [DOI] [PubMed] [Google Scholar]
- 7.Hamatani T, Carter MG, Sharov AA, Ko MS. Dev Cell. 2004;6:117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- 8.Braude P, Bolton V, Moore S. Nature. 1988;332:459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 9.Generoso WM, Cain KT, Krishna M, Huff SW. Proc Natl Acad Sci USA. 1979;76:435–437. doi: 10.1073/pnas.76.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti F, Wyrobek AJ. Birth Defects Res C Embryo Today. 2005;75:112–129. doi: 10.1002/bdrc.20040. [DOI] [PubMed] [Google Scholar]
- 11.Lieber MR, Ma Y, Pannicke U, Schwarz K. Nat Rev Mol Cell Biol. 2003;4:712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 12.Valerie K, Povirk LF. Oncogene. 2003;22:5792–5812. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- 13.West SC. Nat Rev Mol Cell Biol. 2003;4:435–445. doi: 10.1038/nrm1127. [DOI] [PubMed] [Google Scholar]
- 14.Wyman C, Kanaar R. Annu Rev Genet. 2006;40:363–383. doi: 10.1146/annurev.genet.40.110405.090451. [DOI] [PubMed] [Google Scholar]
- 15.Smith GC, Jackson SP. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 16.Blunt T, Gell D, Fox M, Taccioli GE, Lehmann AR, Jackson SP, Jeggo PA. Proc Natl Acad Sci USA. 1996;93:10285–10290. doi: 10.1073/pnas.93.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dronkert MLG, Beverloo HB, Johnson RD, Hoeijmakers JHJ, Jasin M, Kanaar R. Mol Cell Biol. 2000;20:3147–3156. doi: 10.1128/mcb.20.9.3147-3156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essers J, Hendriks RW, Swagemakers SM, Troelstra C, de Wit J, Bootsma D, Hoeijmakers JH, Kanaar R. Cell. 1997;89:195–204. doi: 10.1016/s0092-8674(00)80199-3. [DOI] [PubMed] [Google Scholar]
- 19.van Gent DC, Hoeijmakers JH, Kanaar R. Nat Rev Genet. 2001;2:196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 20.Evans JW, Liu XF, Kirchgessner CU, Brown JM. Radiat Res. 1996;145:39–46. [PubMed] [Google Scholar]
- 21.Rothkamm K, Kuhne M, Jeggo PA, Lobrich M. Cancer Res. 2001;61:3886–3893. [PubMed] [Google Scholar]
- 22.DiBiase SJ, Zeng ZC, Chen R, Hyslop T, Curran WJ, Jr, Iliakis G. Cancer Res. 2000;60:1245–1253. [PubMed] [Google Scholar]
- 23.Perrault R, Wang H, Wang M, Rosidi B, Iliakis G. J Cell Biochem. 2004;92:781–794. doi: 10.1002/jcb.20104. [DOI] [PubMed] [Google Scholar]
- 24.Zudova D, Wyrobek AJ, Bishop J, Marchetti F. Reproduction. 2004;128:573–581. doi: 10.1530/rep.1.00333. [DOI] [PubMed] [Google Scholar]
- 25.McLay DW, Clarke HJ. Reproduction. 2003;125:625–633. doi: 10.1530/rep.0.1250625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexeev A, Mazin A, Kowalczykowski SC. Nat Struct Biol. 2003;10:182–186. doi: 10.1038/nsb901. [DOI] [PubMed] [Google Scholar]
- 27.Jaskelioff M, Van Komen S, Krebs JE, Sung P, Peterson CL. J Biol Chem. 2003;278:9212–9218. doi: 10.1074/jbc.M211545200. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti F, Bishop JB, Cosentino L, Moore IID, Wyrobek AJ. Biol Reprod. 2004;70:616–624. doi: 10.1095/biolreprod.103.023044. [DOI] [PubMed] [Google Scholar]
- 29.Ehling UH. Mutat Res. 1971;11:35–44. doi: 10.1016/0027-5107(71)90030-3. [DOI] [PubMed] [Google Scholar]
- 30.Hatch T, Derijck AA, Black PD, van der Heijden GW, de Boer P, Dubrova YE. Oncogene. 2007;26:4720–4724. doi: 10.1038/sj.onc.1210253. [DOI] [PubMed] [Google Scholar]
- 31.Searle AG, Beechey CV. Mutat Res. 1974;22:63–72. doi: 10.1016/0027-5107(74)90009-8. [DOI] [PubMed] [Google Scholar]
- 32.Markert CL, Petters RM. Science. 1978;202:56–58. doi: 10.1126/science.694518. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: Natl Res Council; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.