Abstract
A large number of mutations in the human PLP1 gene lead to abnormal myelination and oligodendrocyte death in Pelizaeus–Merzbacher disease (PMD). Here we show that a major subgroup of PMD mutations that map into the extracellular loop region of PLP/DM20 leads to the failure of oligodendrocytes to form the correct intramolecular disulfide bridges. This leads to abnormal protein cross-links and endoplasmic reticulum retention and activates the unfolded protein response. Importantly, surface expression of mutant PLP/DM20 can be restored and the unfolded protein response can be reverted by the removal of two cysteines. Thus, covalent protein cross-links emerge as a cause, rather than as a consequence, of endoplasmic reticulum retention.
Keywords: cysteine bridge, membrane protein misfolding, proteolipid protein, quality control, dysmyelination
In the nervous system, integral membrane proteins, such as receptors, channels, or adhesion proteins, have been associated with a wide spectrum of neurodegenerative disorders. Some multimeric proteins, including ion channels and connexins, can only exit the endoplasmic reticulum (ER) when properly assembled (1). However, genotype–phenotype correlations remain difficult. Specifically, substitutions in extracellular loop regions rarely show obvious relationships to the presumed function of the protein. A better understanding of the essential features of misfolded proteins that are retained in the ER (2, 3) is required to develop therapeutic strategies.
Misfolded proteins in the ER can induce the unfolded protein response (UPR), which includes ER growth and transcriptional activation of genes encoding chaperones (4, 5). In mammalian cells, the UPR can also trigger apoptosis (reviewed in refs. 6 and 7). Not surprisingly, in many diseases abnormal cell death has been associated with mutant membrane proteins.
We have studied the molecular consequences of missense mutations in the PLP1 gene, encoding the major integral membrane protein of CNS myelin. Numerous PLP1 missense mutations cause ER retention and oligodendrocyte death in Pelizaeus–Merzbacher disease (PMD), whereas null mutations of the same gene are well tolerated and allow myelination (8, 9). (For a comprehensive list of PLP1 mutations, see www.med.wayne.edu/neurology/clinicalprograms/pelizaeus-merzbacher/plp.html.)
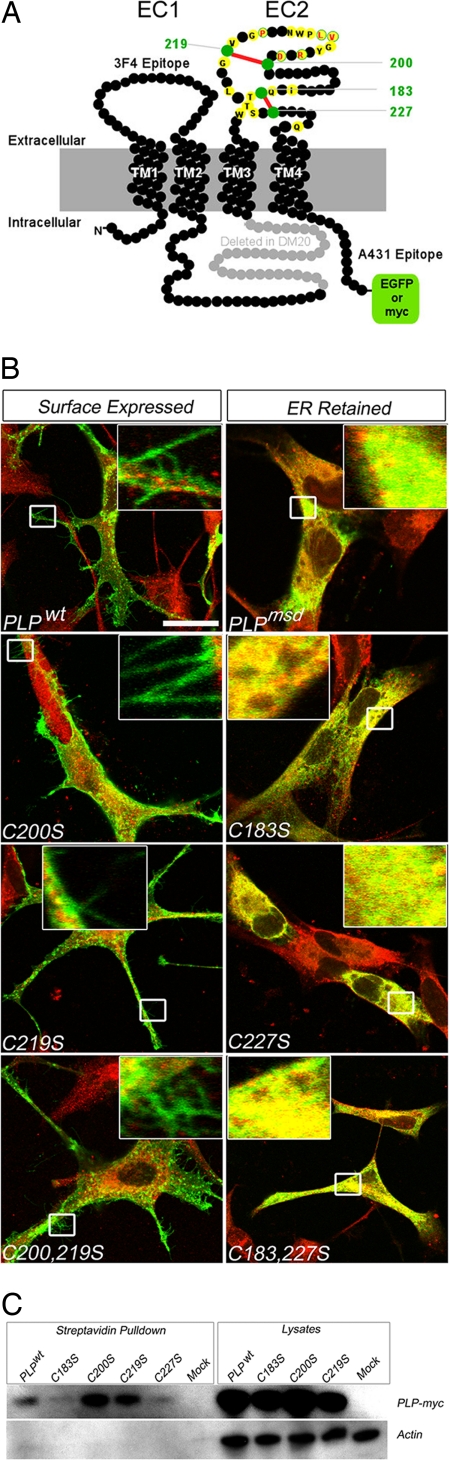
PLP and its splice isoform, DM20, are tetraspanins with two extracellular loop regions, EC1 and EC2, that interact in vivo with the opposing membrane in myelin (10, 11). Both the N and C termini of PLP protrude into the cytosol (Fig. 1A). The location of two disulfide bridges within EC2 is known (C183–C227 and C200–C219) (12).
Fig. 1.
Topology of proteolipid protein PLP/DM20 and the role of cysteine residues in subcellular trafficking. (A) Two-dimensional model of PLP (276 residues shown as black beads) and its splice isoform, DM20, lacking 35 residues (marked in gray) from an intracellular loop. The orientation of four transmembrane domains (TM1–TM4) positions both N and C termini in the cytoplasm. Within the second extracellular domain (EC2), the position of four cysteine residues (marked in green) forming two disulfide bridges (marked in red) is indicated. Also indicated are C-terminal epitope tags used in this study (EGFP or myc) and the approximate positions of extracellular (3F4) and intracellular (A431) antibody binding sites common to PLP and DM20. Positions of amino acids in EC2 that are substituted in patients with PMD are marked in yellow. Those that have been studied in detail here carry the single-letter code of the wild-type sequence, labeled in red. (B) In transfected, fixed, and permeabilized oli-neu cells, mutant PLP–myc (shown in green) colocalized with the endogenous chaperone and thiol-disulfide oxidoreductase PDI (shown in red). Only merged images are shown. (Left) Wild-type protein, PLPWT, reached the cell surface, as demonstrated by green fluorescent microspikes at the tip of processes (magnified in Insets). (Right) In contrast, PLPMSD (derived from jimpy-msd mice) failed to reach the cell surface. There are no labeled microspikes (magnified in Insets), but there is substantial overlap between PLPMSD and PDI (shown in yellow). Note also the paucity of cellular processes. From two disulfide bridges in EC2, the “outer” one (Cys200–Cys219) is dispensable for folding and cell-surface expression. To test the function of each disulfide bridge (see A) for PLP folding and colocalization with PDI (shown in red), single and double cysteine-to-serine substitutions were engineered for each disulfide bridge. Replacing one or both cysteines of the outer bridge did not interfere with cell-surface labeling of PLP (shown in green), as indicated by fluorescent microspikes (Left and magnified in Insets). In contrast, replacing one or both cysteines of the membrane-proximal bridge (Cys183–Cys227) led to severe misfolding, as visualized by ER retention and colocalization of PLP with PDI (shown as yellow overlay in Insets), similar to PLPMSD (topmost Right). Note also the paucity of cellular processes. (Scale bar: 20 μm.) (C) To obtain independent biochemical evidence that PLPC200S and PLPC219S (lacking the outer bridge) are cell-surface expressed in COS7 cells, all membrane proteins were biotinylated with membrane-impermeable sulfo-NHS biotin before cell lysis, and proteins were pulled down with streptavidin-conjugated agarose beads. Only myc-epitope labeled PLPWT, PLPC200S, and PLPC219S could be detected on Western blots (the first, third, and fourth lanes). (Left) The absence of actin in these six lanes confirms that only live cells were biotinylated. Total lysates served as positive controls for transfection and loading. Mock, transfected with plasmids lacking a cDNA insert.
Because many PLP1 missense mutations lead to oligodendrocyte death (13), it is difficult to dissect the subcellular pathomechanism in vivo. However, overexpression in nonglial cells has demonstrated ER retention of mutant PLP/DM20 and its interaction with chaperones (14–17), providing indirect evidence of misfolding also in the absence of structural data. For a specific substitution in transmembrane domain 4 of PLPA242V, it has been demonstrated that premature oligomerization causes ER retention (18). For the majority of PLP1 mutations, which involve the extracellular loop region (Fig. 1A), the disease mechanism is unclear.
Results and Discussion
In the search for an essential feature of membrane protein misfolding, we used the oligodendroglial cell line oli-neu (19), in which endogeneous proteolipids are practically undetectable by immunostaining (data not shown). We studied PLP with a myc epitope or fused to enhanced green fluorescent protein (EGFP) separated by a flexible linker (Fig. 1A). In all these experiments, the wild-type protein PLPWT, including tagged PLPWT isoforms, passed the “quality control” of the ER, exhibited surface expression within 8 h of transfection [see supporting information (SI) Movie 1], and accumulated in LAMP1-positive late endosomes within 24 h (Fig. 1B, SI Fig. 5A, and SI Movie 2). In contrast, PLP mutants were retained in the ER of oligodendrocytes (Fig. 1B and SI Movie 3). Surface expression of wild-type PLP was confirmed by live staining (SI Fig. 6A) with conformation-sensitive monoclonal antibody O10 (15).
Cysteine Residues Are Critical for PLP Folding and Surface Expression.
We hypothesized that in PMD cysteine residues in EC2 fail to form normal disulfide bridges as an essential feature of proteolipid misfolding and ER retention. First, we analyzed corresponding cysteine-to-serine and cysteine-to-alanine mutants (Fig. 1B and SI Fig. 5). PLP lacking the “inner” bridge C183–C227 was strictly retained in the ER, as indicated by a reticular immunostaining of cells that also lacked visible processes (Fig. 1B Right and SI Fig. 5B). Thus, the membrane-proximal bridge is essential to passing the quality control system of the ER. Unexpectedly, PLP lacking the “outer” bridge, C200–C219, was readily detectable on the cell surface (Fig. 1B Left), very similar to wild-type PLP. These oli-neu cells also exhibited a “mature” multipolar morphology with numerous filopodial processes (magnified in Fig. 1B Insets). Surface expression was confirmed by live staining with the monoclonal antibody 3F4 (20) against an extracellular PLP epitope (Fig. 1A and data not shown). As expected, a quadruple mutant (PLP lacking all four cysteines) was strictly retained in the ER (data not shown).
To biochemically confirm the presence (or absence) of PLP at the cell surface, we transfected COS7 cells and biotinylated all surface proteins before harvesting and precipitated the marked proteins with streptavidin-conjugated agarose beads. Subsequent Western blot analysis demonstrated that PLPWT, PLPC200S, and PLPC219S were biotinylated cell membrane proteins. In contrast, PLPC183S and PLPC227S were almost undetectable (Fig. 1C), confirming their intracellular retention. Interestingly, although PLP lacking the disulfide bridge C200–C219 reached the surface of transfected cells and accumulated in a LAMP1-positive endosomal compartment (similarly to PLPWT) (SI Fig. 5 A and C–E), it remained a monoclonal antibody O10-negative misfolded mutant (SI Fig. 6C). The absence of the conformation-sensitive O10 epitope (compare SI Fig. 6 A and C) in surface-expressed PLP cysteine mutants demonstrates that PLP misfolding and ER retention can, in principle, be uncoupled.
Although the outer disulfide bridge in EC2 (C200–C219) is dispensable for surface expression (Fig. 1B), we found a natural PMD mutation (21) of one of these cysteines (PLPC219Y) with obvious ER retention (Fig. 2 A and B). Because PLPC219S did not cause ER retention (Fig. 1B), we reasoned that the unpaired C200 would be sterically more exposed when a residue larger than serine (Y219) caused local misfolding and that the exposed C200 was the real cause of ER retention. Indeed, substituting C200 in a double-mutant protein (PLPC200S,C219Y) completely rescued the mutant phenotype (Fig. 2 C and D). Western blot analysis revealed that PLPC219Y (but not PLPWT or the rescued double mutant) formed dimers that were sensitive to reducing agents (Fig. 2E). This strongly suggests that the retention of PLPC219Y is caused by cysteine (C200) oxidation and abnormal cross-links (which we think prevent subsequent oligomerization). In these experiments, true dimers on gels could be distinguished from other adducts by coexpression of PLP–myc with PLP–EGFP and the emergence of novel hybrid bands (SI Fig. 7, lane 1). Apparently, the inability to form normal cysteine bridges leads to alternative intermolecular cysteine oxidation products, as was recently hypothesized for mutant TNFR1 and collagen X chains (22, 23).
Fig. 2.
Critical cysteine residue in PLP/DM20 for ER retention and protein dimerization. (A–D) oli-neu cells were transfected to express a myc-epitope-labeled natural PMD mutant, PLPC219Y (A and B), or a double mutant, PLPC200S,C219Y (C and D). Only merged images are shown. The PMD mutant was retained as visualized 24 h after transfection by lack of processes and colocalization of PLP (shown in green) with the ER marker PDI (shown in red in A). Mutant PLP (shown in green) also showed segregation from the late endosomal/lysosomal marker LAMP1 (shown in red in B and magnified in Inset). In contrast, the double mutant PLP behaved like a wild-type protein (see Fig. 1B and SI Fig. 5A), with reduced colocalization with PDI and the branched morphology of oli-neu cells (shown in C) and the emergence of green fluorescent microspikes on processes (shown in D and magnified in Inset). PLPC200S,C219Y also overlapped with the endosomal marker (shown as the yellow area in D). Thus, it is not Y219 but an unpaired C200 that emerges as the cause of PMD. (Scale bar: 10 μm.) (E) When oli-neu cell extracts were analyzed by semiquantitative Western blotting, mutant PLPC219Y revealed predominantly a dimer band, whereas PLPWT and the “rescued” PLPC200S,C219Y were monomeric. In the presence of ME, all forms were monomeric, demonstrating that dimerization of PLPC219Y can be attributed to cysteine oxidation. (F) SDS/PAGE of cellular extracts from primary oligodendrocytes (OL) and oli-neu cells with immunodetection of PLP/DM20. When analyzed under nonreducing conditions, endogenous PLP/DM20 expression in cultured oligodendrocytes (lane 1) led to a small percentage of dimerized PLP and DM20, detected by antibody 3F4. Absence of dimers in the presence of ME suggests that these are cysteine-mediated cross-links. Transfected oli-neu cells, expressing the slightly larger epitope-tagged PLP–myc (lane 2), also exhibited a small percentage of ME-sensitive PLP dimers, detected with antibody 3F4. Note that antibody 3F4 detects endogenous and induced PLP/DM20 expression. (G) Detection of mutant PLP as dimers in oli-neu glial cells, transfected to express the natural PMD mutant PLPD202N. By performing Western blot analysis of nonreducing gels (using antibody 3F4), >50% of total PLP was detectable in the 52-kDa putative dimeric form (lane 1). When PLP was further modified by the substitution of C200 and C219 for serine (see also Fig. 3), such dimer formation was largely prevented (lane 2) with most PLP being monomeric, similar to wild-type PLP (compare with F). All dimers were completely absent in the presence of 150 mM ME in the gels (lanes 4 and 5), demonstrating the involvement of cysteine cross-links. The same results were found for PLPR204G (data not shown). Note that endogenous DM20 (in mock-transfected cells) migrated exclusively as a monomer, even under nonreducing conditions (lanes 3 and 6).
Rescue of ER Retention and the UPR.
PLPWT derived from primary oligodendrocytes or transfected oli-neu cells migrated on gels largely as monomers (26 kDa), under both reducing and nonreducing conditions (Fig. 2F). In contrast, PMD-causing isoforms (PLPD202N, PLPR204G, and PLPC219Y) formed dimers that, upon the addition of mercaptoethanol (ME), were reduced to monomers (Fig. 2G). Again, when two cysteines were replaced (Cys200,219Ser), these PMD-causing mutations yielded predominantly monomeric PLP, also under nonreducing conditions (Fig. 2G and data not shown). Thus, it is a feature of diverse PMD mutations in EC2 to cause alternative oxidation products and abnormal PLP dimers.
To prove the critical role of cysteines in PMD mutations, independent of the position of the primary substitution in EC2, we generated EGFP-tagged PLP isoforms with the following PMD-causing mutations: PLPD202N, PLPR204G, PLPV208D, PLPL209H, and PLPP215S. As expected, all mutant PLP isoforms were strictly retained in the ER of oli-neu cells (Fig. 3A Left). However, when combined with the Cys200,219Ser substitution, these triple-mutant PLP isoforms showed a wild-type-like surface expression, as demonstrated by live staining with the monoclonal antibody 3F4 (Fig. 3 A Right and B). Similarly, in COS7 cells and in primary oligodendrocytes, EGFP-tagged PLP isoforms with a PMD mutation accumulated in the endosomal compartment when rescued with the substitutions Cys200,219Ser (Fig. 4A and data not shown).
Fig. 3.
PMD-causing PLP mutations can be rescued by the replacement of cysteines. (A) To distinguish PLP at the cell surface from PLP in intracellular compartments, oli-neu cells that express various EGFP-tagged mutant PLP isoforms (shown in green) were additionally live-stained for PLP by using monoclonal antibody 3F4 (shown in red; see Fig. 1A). Only merged images are shown. The same results were obtained with COS7 cells (data not shown). We analyzed various natural PMD-causing mutations that map into EC2 (PLPD202N, PLPR204G, PLPV208D, PLPL209H, and PLPP215S) for cell-surface expression of PLP in the absence (Left) or the presence (Right) of additional point mutations that substituted C200 and C219 for serine. Remarkably, in the absence of C200 and C219, the PMD-causing mutants were fully rescued from ER retention, and PLP was localized at the cell surface (labeled in red) and in the late endosomal/lysosomal compartment (shown in green). In the presence of EC2 cysteines, all PMD mutants were negative for 3F4 immunoreactivity and confined to the ER. Note also the lack of glial processes. This reveals that the ER retention of many natural PMD-causing PLP mutants, which do not alter cysteine residues, instead require cysteines for ER retention. Grayscale images, shown on the right, better reveal the distribution of double-labeled PLP at the cell surface (3F4) and in intracellular compartments (GFP). (Scale bar: 10 μm.) (B) PLPD202N trafficking to the cell surface in the presence or absence of C200 and C219. The percentage of cells live-stained by antibody 3F4 for surface-expressed PLP (in a population of cells expressing EGFP-tagged mutant PLP) were calculated from transfection experiments (n = 3). The PMD mutation PLPD202N was fully retained in the ER. Note that, in the absence of C200 and C219, PLPD202N was rescued from ER retention, because 95% of GFP-positive cells were stained by antibody 3F4.
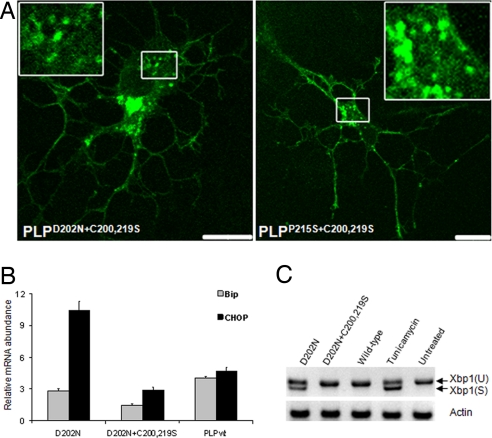
Fig. 4.
Rescue of PLP trafficking in primary oligodendrocytes and the attenuation of the UPR. (A) Confocal analysis of primary oligodendrocytes expressing either EGFP-tagged PLPD202N+C200,219S (Left) or EGFP-tagged PLPP215S+C200,219S (Right). Note that both proteins traffic to the cell surface and accumulate in an endosomal compartment (shown at higher magnification in Insets), similar to EGFP-tagged wild-type PLP (data not shown) and similar to their behavior in oli-neu cells (see Fig. 3). (B) Relative mRNA levels of the UPR markers BIP and CHOP in PLP-expressing oli-neu cells. For quantitative real-time PCR, cDNA was obtained 24 h after transfection. Expression of PLPD202N (an ER-retained mutant) resulted in the up-regulation of BIP and CHOP mRNA when compared with cells expressing wild-type PLP. Note that triple mutant PLP is comparable with wild-type PLP. BIP and CHOP mRNA levels were normalized to PLP expression. (C) Xbp1 mRNA was spliced in response to ER stress. oli-neu cells expressing ER-retained PLPD202N induced splicing of a Xbp1 mRNA, whereas cells expressing triple mutant PLP only exhibited unspliced Xbp1. Xbp1(U) and Xbp1(S) denote unspliced and spliced mRNA, respectively. Treatment of cells with tunicamycin served as a positive control to trigger the UPR.
The expression of misfolded PLP in mutant mice induces BIP and CHOP mRNA (24). In agreement with these observations, we found elevated BIP and CHOP mRNA in transfected oli-neu cells expressing PLPD202N, which was reduced to control levels by removal of the two luminal cysteines (Fig. 4B). ER resident Ire1 tranduces ER stress to the nucleus by splicing Xbp1 mRNA (25). Spliced Xbp1 mRNA encodes for an activated transcription factor that enhances the transcription of ER chaperone genes. We exclusively detected spliced Xbp1 mRNA in transfected oli-neu cells expressing PLPD202N. However, this splice product disappeared upon removal of the luminal cysteines (Fig. 4C).
Taken together, our data reveal that a subset of disease-associated point mutations in PLP1 converge mechanistically by perturbing the formation of an intramolecular disulfide bridge in PLP/DM20 in the lumen of the ER. This disulfide bridge itself is dispensable for normal PLP/DM20 folding and trafficking. Importantly, it is not the substituted amino acid itself that causes ER retention. However, when the unpaired cysteine becomes sterically exposed, it engages in intermolecular cross-links (with PLP itself or other proteins). Abnormal PLP adducts fail to oligomerize (i.e., are monoclonal antibody O10-negative) and become the primary cause of ER retention and, thus, oligodendrocyte dysfunction and death in vivo. ER retention of PLP may cause further abnormal protein cross-links.
Many studies have demonstrated a critical role of cysteines in protein retention that seems similar to our initial finding of PLPC219Y retention (Fig. 2A), which provides a plausible cause of PMD. Indeed, unpaired cysteines in secretory monomeric proteins, such as immunoglobulins, were proposed as physiological retention signals (26, 27). Here, we went an important step further by showing for PMD that several noncysteine mutations, which were mechanistically not understood (such as PLPD202N, PLPR204G, and others), do not alter the number of paired cysteines but can still be explained by the cysteine pathway.
By performing a successful rescue experiment with compensatory mutations, we could also solve an inherent problem of earlier studies, namely, to distinguish between cause and effect of protein retention. Theoretically, chaperone-mediated PLP retention could be the primary cause of the cysteine-mediated cross-links, if we assume that once proteins are retained in the ER such cysteine-mediated cross-links occur nonspecifically (14). Alternatively, specific mutations lead to PLP misfolding and the exposure of unpaired cysteines, which then become the primary cause of ER retention. Our study is in strong support of the second model, because we could release several of the known mutant PLP isoforms from ER retention by the removal of cysteines in triple-mutant proteins.
Our model is likely to apply to the subset of PMD cases associated with single substitutions in EC2, although not all of them can be rescued by removal of the dispensable cysteines [such as in L223P (data not shown)]. The mechanism is unlikely to explain the mutations that map into transmembrane domains and act by premature oligomerization (18). However, given that many other membrane proteins, not only tetraspanins, harbor intramolecular disulfide bridges in extracellular loop regions and are sensitive to point mutations in these domains (for example, see ref. 28), we suggest that the mechanism identified here might be relevant to a broader spectrum of diseases.
Materials and Methods
Molecular Cloning.
Plasmid pPLP-EGFP was generated by fusing the EGFP to the C terminus of PLP. The product was cloned into vector pEGFP-N1 by using EcoRI/NotI sites. All site-directed mutants were generated by circular amplification of this plasmid with PFU Turbo DNA polymerase (Stratagene, La Jolla, CA), followed by DpnI digestion (New England Biolabs, Ipswich, MA) and transformation into Escherichia coli. Individual clones were analyzed by analytic restriction digestion and DNA sequence analysis. Detailed cloning protocols and all primer sequences are available on request.
DNA Transfection.
COS7 cells were washed with PBS, trypsinized, pelleted, and resuspended in DMEM. Plasmids were used at 10 μg per 300 μl of cell suspension [4 × 106 cells per milliliter of DMEM and 10% (vol/vol) FBS] for electroporation with the Bio-Rad (Hercules, CA) Gene Pulser (350 V and 450 μF). oli-neu cells and primary oligodendrocytes were either transfected with FuGENE 6 (Roche Applied Science, Indianapolis, IN) or by electroporation. For electroporation, cells were detached mechanically by pipetting, pelleted, and resuspended in Sato medium (DMEM supplemented with transferrin, insulin, putrescine, sodium selenite, progesterone, l-thyroxine, and triiodo-l-thyronine). Plasmids were used at 10 μg per 400 μl of cell suspension (5 × 106 cells per milliliter, in Sato medium) for electroporation (250 V and 960 μF). (Scale bars: 20 μm.)
Protein Biotinylation.
For biotinylation of cell membrane proteins, the following steps were performed on water/ice slurry (unless otherwise stated), and reagents were precooled to 4°C. Twenty-four hours after transfection, cells grown in poly(l-lysine)-coated six-well Falcon plates (Becton Dickinson, Franklin Lakes, NJ) were washed twice with Dulbecco's PBS (DPBS) (0.7 mM CaCl2/2.6 mM KCl/1.5 mM KH2PO4/0.5 mM MgCl2/136 mM NaCl/8.1 mM Na2HPO4). Cells were then incubated for 30 seconds with 0.5 mg/ml N-hydroxysulfosuccinimide biotin (sulfo-NHS biotin) (Pierce, Rockford, IL) in DPBS (prepared freshly). Before lysing the cells in 400 μl of lysis buffer 1 (50 mM Tris·Cl, pH 7.5/140 mM NaCl/1 mM EDTA/1 mM PMSF/1% Triton X-100/0.1% SDS) at room temperature (RT), unbound sulfo-NHS biotin was quenched by washing once with DPBS containing 25 mM lysine monohydrochloride. Lysates were cleared by centrifugation at 13,000 × g for 20 min at RT and incubated with streptavidin-conjugated agarose beads for 2 h at RT. Agarose beads were washed five times with lysis buffer and once with PBS at RT. Beads were finally boiled with 4× lithium dodecyl sulfate (LDS) loading buffer, separated on NuPAGE 4–12% Bis-Tris precasted gels (Invitrogen, Carlsbad, CA), and immunoblotted for PLP and actin by following standard procedures.
SDS/PAGE and Western Blot Analysis.
Before lysing cells in 1× SDS loading dye [25 mM Tris, pH 6.7/1% SDS, 5% (vol/vol) glycerol/0.005% bromophenol blue] or in lysis buffer 2 (25 mM Tris, pH 7.5/150 mM NaCl/1 mM EDTA/1% Triton X-100), free cysteines were blocked by incubation in 13.3 mM iodoacetamide in DPBS. Samples were separated on 12% (wt/vol) SDS gels by using Bio-Rad Mini-PROTEAN 3 chambers and transferred to PVDF membranes by using Invitrogen blotting chambers. Membranes were blocked for at least 1 h at RT with 5% (wt/vol) nonfat dry milk in Tris-buffered saline (TBS) (50 mM Tris base/150 mM NaCl, pH 7.4). Primary antibody (blocking buffer) was applied for at least 1 h at RT (or overnight at 4°C). After four washes in buffer (0.05% Tween 20 in TBS), HRP-conjugated secondary antibodies were applied for 1 h, followed by four washes with wash buffer. Membranes were exposed by using the Enhanced Chemiluminescence Detection kit (PerkinElmer, Wellesley, MA).
RNA Isolation and Quantitative RT-PCR.
RNA was isolated from transfected cells by using the RNeasy Mini kit (Qiagen, Valencia, CA). Reverse transcription was performed on 1 μg of total RNA by using random nanomers and SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was performed according to the manufacturer's instructions by using a rapid thermal cycler system (LightCycler, Applied Biosystems, Foster City, CA). A master mixture containing SYBR green, Taq polymerase, nucleotides, and buffer was mixed with primers designed by using the Roche Applied Science universal probe library (http://qpcr2.probefinder.com/input.jsp?organism = mouse) and 20 ng of cDNA. Quantitative RT-PCR was performed in triplicates from pooled cDNA obtained from two independent transfectants. Actin and 18S mRNA were used as internal standards, and expression levels were normalized to PLP mRNA. As positive control, the UPR was induced by tunicamycin (2 μg·ml−1).
Antibodies.
Mouse monoclonal antibodies were anti-c-myc (clone 9E10 from Sigma, St. Louis, MO), anti-PLP (3F4, kindly provided by Marjorie B. Lees, Eunice Kennedy Shriver Center, Waltham, MA), and anti-actin (Chemicon, Temecula, CA). Rat monoclonal antibodies were anti-LAMP1 (clone cd107a from PharMingen, San Diego, CA) and anti-PLP [O10 (15)]. Polyclonal rabbit sera were against PDI (Stressgen Assay Designs, Ann Arbor, MI) and PLP (A431, against the C-terminal peptide GRGTKF). For immunofluorescence and Western blot analysis, fluorochrome- and HRP-conjugated secondary antibodies were purchased from Dianova (Hamburg, Germany). Antibody dilutions for immunocytochemistry were as follow: anti-c-myc, 1:2,000; 3F4 and O10, 1:50; anti-LAMP1, 1:200; anti-PDI, 1:200; and A431, 1:1,000. Cy2-conjugated secondary antibodies were diluted 1:1,000 and Cy3-conjugated antibodies were diluted 1:2,500. For Western analysis, dilutions were as follow: anti-c-myc, 1:1,000; 3F4, 1:50; anti-actin, 1:2,000; and anti-mouse HRP, 1:20,000.
Cell Culture.
COS7 cells were maintained on untreated tissue culture dishes (Falcon, Becton Dickinson) in DMEM and 10% (vol/vol) FBS. Cells were grown at 37°C in a 5% CO2 atmosphere, and medium was changed every third day. For passaging cells, confluent plates were washed once with PBS, followed by a short trypsination with 0.05% trypsin EDTA (Sigma). oli-neu cells (kindly provided by J. Trotter, Johannes Gutenberg University, Mainz, Germany) were maintained in Sato medium containing 1–5% (vol/vol) horse serum on poly(l-lysine)-coated tissue culture dishes (Falcon, Becton Dickinson). Cells were grown at 37°C in a 5% CO2 atmosphere. For passaging, confluent plates were washed once with medium, followed by a short trypsination with 0.005% trypsin EDTA.
Immunocytochemistry.
Immunostainings were carried out 18–20 h after transfection. All steps were performed at RT, unless stated otherwise. Cells grown on poly(l-lysine)-coated coverslips were washed once with TBS [25 mM Tris/136 mM NaCl/2.6 mM KCl, pH 7.5] and fixed for 5 min in 2% (wt/vol) paraformaldehyde/TBS. Cells were then washed twice for 10 min in TBS, permeabilized with 0.01% saponin in TBS for 10 min, and blocked in blocking buffer [TBS containing 2% (vol/vol) goat serum, 2% (wt/vol) BSA, 0.02% biotin, and 0.1% porcine skin gelatin] for at least 30 min. Primary antibodies diluted in blocking buffer were applied for at least 1 h at RT or overnight at 4°C. After three washes in TBS (10 min each), fluorochrome-conjugated secondary antibodies were applied for at least 45 min. After three washes with TBS (10 min each), cells were rinsed in distilled water and mounted in Aqua-Poly/Mount (Polysciences, Warrington, PA) on glass slides.
Live staining of cells with antibody 3F4 was performed at 4°C on water/ice slurry (unless stated otherwise). Cells grown on poly(l-lysine)-coated coverslips (24-well plates) were washed once with ice-cold DMEM, and primary antibodies diluted in ice-cold DMEM were applied directly to cells for 10 min. Cells were washed twice with DPBS and fixed with 2% (wt/vol) paraformaldehyde for 10 min. Cells were shifted to RT during fixation. After two additional washes in DPBS, Cy3-conjugated secondary antibodies diluted in DMEM were applied for at least 30 min. Cells were washed twice in DPBS, rinsed in doubly distilled H2O (ddH2O), and mounted. Live staining of cells with antibody O10 was performed as previously described (15).
Confocal Analysis.
Fluorescent images were captured on an LSM 510 confocal microscope (Zeiss Microimaging, Jena, Germany) with a 63× oil Plan Apochromat objective with an N.A. of 1.4 (Zeiss MicroImaging). For time-lapse live-cell imaging, coverslips with cells were mounted into a live-cell imaging chamber and observed in a low autofluorescence imaging medium [10 mM Hepes-buffered, phenol-red-free DMEM (Gibco, Carlsbad, CA) and 1% horse serum] at 37°C. Temperature was controlled by means of a Tempcontrol 37-2 digital system (PeCon, Erbach, Germany) or a custom-built perfusion system. Images were acquired at 10-sec intervals for the indicated time periods.
For final analysis, captured laser scanning microscopy (LSM) images were exported as tagged image file (TIF) images. Documentation and processing of TIF images were done with Photoshop 7.0.1 (Adobe Systems, San Jose, CA). For quantification of Western blots, only nonsaturated developed Western blots were used and scanned as 8-bit, 256-level grayscale images [at 1,200 dots per inch (1 inch = 2.54 cm) on an Epson (Long Beach, CA) F-3200 scanner]. Scanned images were first transformed to 512 × 512 pixels by using Photoshop 7.0.1 and exported as TIFs. Intensities of individual bands were quantified by using Bio-Rad Quantity One software.
Supplementary Material
Acknowledgments
We thank A. Helenius, M. Simons, E. Krämer, H. Werner, and members of the K.-A.N. Laboratory for helpful discussions. We also thank J. Trotter for oli-neu cells and M. Lees for providing antibody 3F4. Our work is supported by grants from the Deutsche Forschungsgemeinschaft Centre for Molecular Physiology of the Brain (SFB523), the National MS Society, the Hertie Institute of Multiple Sclerosis Research, the Myelin Project, and the Del Marmol Foundation.
Abbreviations
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- PMD
Pelizaeus–Merzbacher disease
- ME
mercaptoethanol
- DPBS
Dulbecco's PBS
- RT
room temperature
- TBS
Tris-buffered saline.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704975104/DC1.
References
- 1.Hurtley SM, Helenius A. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- 2.Ellgaard L, Helenius A. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 3.Ellgaard L, Molinari M, Helenius A. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 4.Rutkowski DT, Kaufman RJ. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Patil C, Walter P. Curr Opin Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 6.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 7.Federovitch CM, Ron D, Hampton RY. Curr Opin Cell Biol. 2005;17:409–414. doi: 10.1016/j.ceb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Klugmann M, Schwab MH, Puhlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave KA. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- 9.Boison D, Stoffel W. Proc Natl Acad Sci USA. 1994;91:11709–11713. doi: 10.1073/pnas.91.24.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoffel W, Subkowski T, Jander S. Biol Chem Hoppe-Seyler. 1989;370:165–176. doi: 10.1515/bchm3.1989.370.1.165. [DOI] [PubMed] [Google Scholar]
- 11.Popot JL, Pham Dinh D, Dautigny A. J Membr Biol. 1991;120:233–246. doi: 10.1007/BF01868534. [DOI] [PubMed] [Google Scholar]
- 12.Weimbs T, Stoffel W. Biochemistry. 1992;31:12289–12296. doi: 10.1021/bi00164a002. [DOI] [PubMed] [Google Scholar]
- 13.Gow A, Southwood CM, Lazzarini RA. J Cell Biol. 1998;140:925–934. doi: 10.1083/jcb.140.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanton E, High S, Woodman P. EMBO J. 2003;22:2948–2958. doi: 10.1093/emboj/cdg300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung M, Sommer I, Schachner M, Nave KA. J Neurosci. 1996;16:7920–7929. doi: 10.1523/JNEUROSCI.16-24-07920.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gow A, Lazzarini RA. Nat Genet. 1996;13:422–428. doi: 10.1038/ng0896-422. [DOI] [PubMed] [Google Scholar]
- 17.Southwood C, Olson K, Wu CY, Gow A. J Neurosci Res. 2007;85:471–478. doi: 10.1002/jnr.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanton E, Holland A, High S, Woodman P. Proc Natl Acad Sci USA. 2005;102:4342–4347. doi: 10.1073/pnas.0407287102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung M, Kramer E, Grzenkowski M, Tang K, Blakemore W, Aguzzi A, Khazaie K, Chlichlia K, von Blankenfeld G, Kettenmann H, et al. Eur J Neurosci. 1995;7:1245–1265. doi: 10.1111/j.1460-9568.1995.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 20.Greer JM, Dyer CA, Pakaski M, Symonowicz C, Lees MB. Neurochem Res. 1996;21:431–440. doi: 10.1007/BF02527707. [DOI] [PubMed] [Google Scholar]
- 21.Osaka H, Kawanishi C, Inoue K, Onishi H, Kobayashi T, Sugiyama N, Kosaka K, Nezu A, Fujii K, Sugita K, et al. Ann Neurol. 1999;45:59–64. doi: 10.1002/1531-8249(199901)45:1<59::aid-art11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Lobito AA, Kimberley FC, Muppidi JR, Komarow H, Jackson AJ, Hull KM, Kastner DL, Screaton GR, Siegel RM. Blood. 2006;108:1320–1327. doi: 10.1182/blood-2005-11-006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson R, Freddi S, Chan D, Cheah KS, Bateman JF. J Biol Chem. 2005;280:15544–15552. doi: 10.1074/jbc.M410758200. [DOI] [PubMed] [Google Scholar]
- 24.Southwood CM, Garbern J, Jiang W, Gow A. Neuron. 2002;36:585–596. doi: 10.1016/s0896-6273(02)01045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 26.Fra AM, Fagioli C, Finazzi D, Sitia R, Alberini CM. EMBO J. 1993;12:4755–4761. doi: 10.1002/j.1460-2075.1993.tb06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C. Cell. 1990;60:781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- 28.Kleopa KA, Scherer SS. Neuromol Med. 2006;8:107–122. doi: 10.1385/nmm:8:1-2:107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.