Abstract
Protein quality control is accomplished by inducing chaperones and proteases in response to an altered cellular folding state. In Escherichia coli, expression of chaperones and proteases is positively regulated by σ32. Chaperone-mediated negative feedback control of σ32 activity allows this transcription factor to sense the cellular folding state. We identified point mutations in σ32 altered in feedback control. Surprisingly, such mutants are resistant to inhibition by both the DnaK/J and GroEL/S chaperones in vivo and also show dramatically increased stability. Further characterization of the most defective mutant revealed that it has almost normal binding to chaperones and RNA polymerase and is competent for chaperone-mediated inactivation in vitro. We suggest that the mutants identify a regulatory step downstream of chaperone binding that is required for both inactivation and degradation of σ32.
Keywords: heat shock transcription factor, proteolysis, GroEL, DnaK, stress response
The heat shock response is a major homeostatic mechanism for controlling the state of protein folding and degradation in all cells (1–3). Upon heat stress, a set of highly conserved heat shock proteins (hsps), including chaperones and proteases, is rapidly and transiently induced. Hsps maintain optimal states of protein folding and turnover during normal growth and also minimize cellular damage from stress-induced protein misfolding and aggregation (4, 5). The level of hsps is controlled primarily by heat shock transcription factors that sense the cellular folding environment through negative feedback control mediated by chaperones (6–9). Understanding this mode of regulation is central to our understanding of protein quality control as well as cellular stress responses. Here, we report the determinants required for chaperone regulation of σ32, the heat shock transcription factor in Escherichia coli (10–12).
σ32 regulon members control both the activity and stability of σ32. The DnaK/J/GrpE and GroEL/S chaperone machines each constitute a negative feedback loop that couples σ32 activity to cellular protein folding state: overexpression of either chaperone machine decreases σ32 activity; conversely, chaperone depletion or overexpression of chaperone substrates increases σ32 activity (13–16). The chaperones are likely to act directly on σ32 because they bind to σ32 and inhibit its activity in a purified in vitro transcription system (17–19). Regulated degradation of σ32 is mediated by the FtsH protease and facilitated by DnaK/J/GrpE and GroEL/S in vivo, but this process has not been completely recapitulated in vitro, where degradation of σ32 by FtsH is slow and not facilitated by chaperones (20, 21).
We selected and characterized feedback-resistant mutants of σ32. The residues altered by the mutations map to a small patch in σ32. Surprisingly, most mutants simultaneously diminished all three negative feedback loops operative in vivo; the most severe mutant essentially eliminated chaperone-mediated activity control and degradation by FtsH protease. In striking contrast to the in vivo phenotype, this strong mutant exhibited few defects when tested either for binding or chaperone-mediated inactivation in vitro. The implications of these findings for the mechanism of chaperone-mediated negative feedback control will be discussed.
Results
Isolation of σ32 Mutants That Are Not Fully Responsive to Chaperone-Mediated Inactivation.
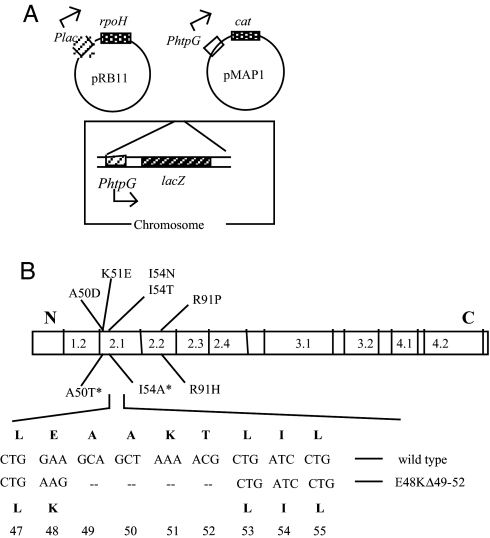
When σ32 is overexpressed, the resultant accumulation of chaperones triggers a negative regulatory loop that inactivates σ32 (13). The features in σ32 that permit chaperone-mediated inactivation are unknown. To identify σ32 mutants defective in this process, we overexpressed σ32 and selected mutants with higher σ32 activity than expected if the inactivation loop were operative. A high-copy plasmid carrying mutagenized rpoH driven from an isopropyl β-d-thiogalactoside (IPTG)-inducible promoter was transformed into cells with two σ32-dependent reporters, one driving expression of cat (chloramphenicol resistance) and a second driving expression of lacZ (Fig. 1A). Cells were selected for higher than normal σ32-dependent transcription of cat by plating on LB/chloramphenicol/IPTG plates having a concentration of chloramphenicol that inhibits growth of cells with wild-type (WT) σ32 activity. To eliminate mutations in the cat promoter, all candidates were then screened for increased σ32-dependent transcription of lacZ on IPTG-containing lactose indicator plates (red color). Sequencing the entire rpoH gene of each mutant revealed five independent single-base substitutions, four of which were located in conserved region 2.1 (Fig. 1B, above bar). Three other mutants obtained from a slightly different screen (22) that mapped in the same region were included for analysis (Fig. 1B, below bar).
Fig. 1.
Selection for σ32 mutations on a high-copy rpoH plasmid. (A) Plasmid and chromosomal constructs of the parental strain used for selection. (B) Location of σ32 mutations. Region 2 mutants obtained in this selection are shown above the bar, which illustrates nine conserved regions of the bacterial σ factor; the three mutants indicated below the bar were identified from a slightly different selection described in ref. 19. *, mutants reported previously (19). The E48KΔ49–52 deletion mutant, shown at the bottom, was obtained during transfer of the plasmid-encoded A50D mutation onto the chromosome (see Results).
We compared the activity of the plasmid-encoded σ32 mutants by quantifying the differential rate of β-galactosidase synthesis in strains carrying the σ32-dependent PhtpG-lacZ reporter at 30°C. All mutants exhibited somewhat higher σ32 activity than their WT counterparts when rpoH expression was not induced (1.5- to 2.5-fold; data not shown) and severalfold higher activity when induced with IPTG (3- to 6-fold; Table 1, first column). These mutants also had increased σ32 levels (data not shown) and stability (Table 1, fourth column). Thus, these mutants were altered both in the activity and stability of σ32.
Table 1.
The σ32 mutants are altered in activity, stability, and response to chaperone overexpression
| σ32 | Relative σ32 activity* |
Relative σ32 stability† | ||
|---|---|---|---|---|
| Control | GroEL/S | DnaK/J | ||
| WT | 1.0 | 0.33 | 0.27 | 1.0 |
| A50D | 6.1 | 4.9 | 5.0 | 5.4 |
| A50T | 3.2 | 2.6 | 2.0 | |
| K51E | 4.2 | 3.3 | 2.6 | 5.2 |
| I54N | 4.9 | 4.9 | 3.8 | 47.0 |
| I54T | 3.0 | 1.8 | 1.7 | 3.7 |
| I54A | 5.4 | 3.5 | 3.4 | |
| R91P | 3.2 | 2.2 | 2.1 | 2.2 |
| R91H | 3.6 | 1.2 | 1.5 | |
*σ32 activity is taken as the differential rate of β -galactosidase synthesis from PhtpG-lacZ reporter determined starting 30 min after induction of σ32 only (first column), σ32 and GroEL/S (second column), or σ32 and DnaK/J (third column) (see Materials and Methods). All activities are normalized to that of the WT control shown as 1.0. Results of a representative experiment are shown; variation among two to four experiments was <20%.
†The amount of σ32 remaining after the addition of 200 μg/ml chloramphenicol was determined by immunoblotting (see Materials and Methods). Stability relative to WT from a typical experiment is shown. The variation among two to four experiments was <20%.
Effect of Chaperone Overexpression on σ32-Dependent Transcription.
The activity of WT σ32 decreases after overexpression of GroEL/S or DnaK/J chaperones because excess chaperones inactivate σ32. We investigated whether the σ32 mutants had decreased susceptibility to chaperone overexpression as expected if they were defective in this feedback loop. These experiments were performed in a strain with the chromosomal GroEL/S or DnaK/J operons under control of an inducible σ70 promoter so that chaperone expression could be initially adjusted to that of the WT strain despite differences in the activity of σ32 mutants. Overexpression of σ32 (Table 1, first column) and GroEL/S (Table 1, second column) were from plasmids encoding, respectively, rpoH or groEL/S under control of an inducible σ70 promoter; DnaK/J overexpression (Table 1, third column) was from the chromosomal locus by increasing inducer concentration. GroEL/S or DnaK/J overexpression markedly inhibited the activity of WT σ32 (≈3-fold), as expected from previous studies (15, 16). In contrast, all region 2.1 mutants and one of the region 2.2 mutants exhibited varying levels of resistance to excess GroEL/S and DnaK/J. In particular, I54N was essentially resistant to inhibition. Thus, these mutants are less sensitive to negative feedback inhibition by both GroEL/S and DnaK/J chaperones.
Activity Control of Chromosomally Located I54N and E48KΔ49–52 Mutants.
We performed detailed characterization of two mutants in the physiologically relevant genomic context. Our goal was to compare the phenotypes of a strong mutant (I54N) with those of a somewhat weaker mutant (A50D). We were unsuccessful in transferring A50D to the chromosome, but fortuitously we isolated a chromosomal mutant consisting of both a small deletion (Ala49 to Thr52) and an E48K substitution, hereafter called E48KΔ49–52 (Fig. 1B) while trying to transfer A50D (see Materials and Methods). Chromosomal I54N-σ32 showed 7-fold higher activity and E48KΔ49–52 σ32 ≈4-fold higher activity than WT σ32 both in LB and M9 medium (Table 2, first two columns), but they had almost normal growth at 30°C, 37°C, or 42°C (data not shown).
Table 2.
Relative σ32 activity, level, and stability in chromosomal σ32 mutants
| Strain | σ32 Activity |
σ32 Level |
Specific activity (activity/level) |
Stability |
|||
|---|---|---|---|---|---|---|---|
| LB | M9 | LB | M9 | LB | M9 | LB | |
| WT | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| I54N | 6.9 ± 0.6 | 6.8 ± 0.8 | 10 | 15 | 0.7 | 0.5 | 42.0 |
| E48KΔ 49–52 | 3.8 ± 0.4 | 3.9 ± 0.5 | 4 | 6 | 1.0 | 0.6 | 2.7 |
| SfhC | 1.5 | 1.2 | 1.3 | 1.3 | |||
| sfhC Δ ftsH | 3.0 | 2.3 | 25 | 35 | 0.12 | 0.07 | 100 |
Isogenic WT (CAG48238) and σ32 mutant strains, and sfhC (suppressor of Δ ftsH lethality) and its isogenic Δ ftsH strain were grown at 30°C. Activity was determined by measuring the differential rate of β -galactosidase activity (LB; first column) or by pulse-labeling experiments measuring HtpG synthesis (M9; second column); the σ32 level (third and fourth columns) was determined by immunoblotting (level in M9 was about one-third of that in LB); stability data (seventh column) were taken from SI Fig. 6.
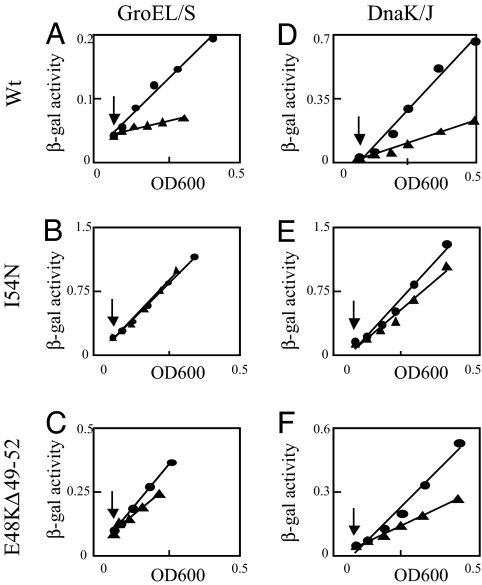
We used two different assays to characterize the effect of chaperone overexpression on σ32 activity. Measuring the differential rate of β-galactosidase synthesis from the σ32-dependent PhtpG-lacZ reporter (Fig. 2) indicated that I54N cells were completely resistant and E48KΔ49–52 cells partially resistant (30% inhibited) to inhibition by excess GroEL/S (Fig. 2 A–C); both mutants were also more resistant to inhibition by excess DnaK/J than cells with WT σ32 (Fig. 2 D–F). Because this assay measures accumulation of β-galactosidase, it is inherently insensitive to small changes in rates of synthesis. Therefore, to characterize the instantaneous extent of inhibition, we determined the rate of HtpG synthesis at different times after overexpression of either GroEL/S (Fig. 3A) or DnaK/J (Fig. 3B) with a pulse-chase–immunoprecipitation protocol. After 10 min of GroEL/S overexpression, WT σ32 activity was severely inhibited (≈10-fold), the E48KΔ49–52 mutant was inhibited somewhat less (≈5-fold), and the I54N mutant was essentially resistant to inhibition (<30% inhibition). Indeed, the level of inhibition exhibited by I54N is equivalent in magnitude to the nonspecific effects of GroEL/S overexpression manifest also at σ70 and σE promoters (16). After 30 min of DnaK/J overexpression, WT σ32 activity was inhibited 80%, the E48KΔ49–52 mutant was inhibited ≈60%, and I54N was almost completely resistant (20% inhibition). Taken together, these results indicate that σ32 activity of the “strong” I54N mutant is more resistant to both GroEL/S and DnaK/J overexpression than the “moderate” E48KΔ49–52 mutant.
Fig. 2.
Effect of chaperone overexpression on σ32-dependent transcription in the chromosomal σ32 mutants. WT and σ32 mutants carrying the PhtpG-lacZ reporter and either Para-groESL (Left) or PA1/lacO-1-dnaKJ (Right) on the chromosome were grown at 30°C in LB medium containing, respectively, 0.2% arabinose or 5 μM IPTG to maintain normal chaperone levels. (A–C) GroEL/S was overexpressed from pGro11 by addition of 25 ng/ml anhydrotetracycline. (D–F) DnaK/J was overexpressed by addition of 1 mM IPTG. ●, control cultures; ▲, chaperone overexpression cultures. Arrows, time of induction. Western blots taken at the last time point indicated an ≈5- to 8-fold increase in GroEL and ≈4- to 5-fold increase in DnaK.
Fig. 3.
Effect of chaperone overexpression on HtpG synthesis in the chromosomal σ32 mutants. WT and mutant strains with a chaperone expression plasmid (pGro11 or pKJE8) were grown in M9 medium containing all amino acids except methionine and cysteine at 30°C. At time 0, GroEL/S was induced by the addition of 40 ng/ml anhydrotetracycline (A), or DnaK/J/GrpE was induced by addition of 0.2% l-arabinose (B). At the times indicated, samples were pulse-labeled for 1 min with [35S]Met/Cys, chased with unlabeled methionine for 1 min, and immunoprecipitated to determine HtpG synthesis.
We used these same strains to deplete GroEL/S or DnaK/J, to determine whether I54N and E48KΔ49–52 σ32 increase their activity under these conditions. Whereas the activity of WT σ32 increased 3- to 10-fold after depletion of GroEL/S or DnaK/J over the time course of 2–3 h, the mutants showed little (E48KΔ49–52) or no (I54N) response to depletion during most of this period [supporting information (SI) Table 5). Thus, when expressed in single copy from the chromosome, these two σ32 mutants are less sensitive than WT σ32 to sudden change (both increase and decrease) in cellular chaperone levels.
Level and Stability of Mutant σ32.
Quantification of the level of σ32 revealed that I54N is much more abundant (10- to 15-fold) and E48KΔ49–52 is somewhat more abundant (4- to 6-fold) than WT σ32 (Table 2, third and fourth columns). Assessment of σ32 stability with immunoblotting after the addition of chloramphenicol in LB medium revealed that an increased σ32 level resulted from increased stability of the mutants, with E48KΔ49–52 showing an ≈3-fold increase and I54N exhibiting a ≈40-fold increase in stability compared with WT (Table 2, seventh column, and SI Fig. 6). The increase in stability of the mutants is sufficient to explain their increase in level. σ32 synthesis was unaffected by the I54N mutation, as expected (data not shown).
As an independent assessment of feedback inhibition of the mutant σ32s, we compared the specific activity of the mutant proteins with that of WT σ32 (σ32 activity/σ32 level; Table 2, fifth and sixth columns). Setting the activity of WT σ32 as 1.0 (first line), the specific activity of the mutant proteins is very close to that of WT (between 0.5 and 1.0), depending on the mutant and the medium used (second and third lines) even though the levels of the mutant proteins are sufficiently high that they should be feedback-inhibited. For comparison, when the level of WT σ32 is increased by the absence of the FtsH protease, its specific activity decreases to ≈0.1 (fifth line), consistent with previous reports (23).
Behavior of the Mutants upon Temperature Upshift.
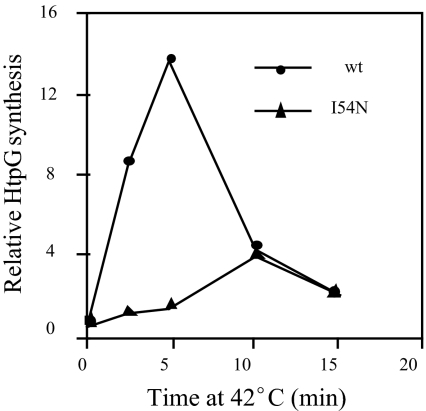
After shift from 30 to 42°C, synthesis of hsps transiently increases ≈10-fold as a consequence of increased translation of σ32 and its transient stabilization (24). In contrast, neither I54N nor E48KΔ49–52 strains exhibited a heat shock response as demonstrated for both strains by using the reporter assay for σ32-dependent transcription (data not shown) and for I54N by examining HtpG synthesis (Fig. 4). Lack of a heat shock response was somewhat unexpected because the translational response of the mutant remains intact (data not shown). We therefore considered the possibility that the mutant proteins were destabilized at 42°C. Indeed, both mutant σ32s were destabilized compared with 30°C, exhibiting t½ of 2.5 min (I54N) and 0.5 min (E48KΔ49–52). Thus, a lack of a classical heat shock response is explained by the fact that the σ32 level in the mutant strains decreased upon heat shock rather than transiently increasing (data not shown). Enhanced degradation at 42°C is not a reflection of complete protein unfolding because the specific activity of the mutant proteins at 42°C (1.0 for I54N and 1.4 for E48KΔ49–52) was similar to that at 30°C (0.5–1.0) (data not shown).
Fig. 4.
Heat shock response of WT and I54N mutant. Cells were grown to log phase in M9 medium at 30°C and shifted to 42°C. Samples were taken at the times indicated to determine HtpG synthesis by immunoprecipitation as described in Fig. 3.
Analysis of I54N σ32 in vitro.
Altered binding of I54N σ32 to either chaperones or to RNA polymerase could explain why I54N is resistant to chaperone-mediated feedback inhibition in vivo. We quantified binding to GroEL and DnaK by using fluorescence anisotropy and to DnaJ by using surface plasmon resonance (see Materials and Methods). Surprisingly, I54N σ32 bound as well as WT σ32 to DnaK and GroEL and bound only 2-fold less tightly to DnaJ than WT σ32 (Table 3). The measurements for WT σ32 reported here are consistent with previously reported values (16, 18). Thus, decreased binding to chaperones cannot explain the observed feedback resistance. Because chaperones are believed to compete with RNA polymerase for binding to σ32, another explanation for feedback resistance is that I54N σ32 binds more tightly to RNA polymerase than WT. We used fluorescence anisotropy to measure binding of mutant and WT σ32 to RNA polymerase. Our determined value for binding of WT σ32 is consistent with the previously reported value (25); I54N exhibited a slightly lower affinity than WT for RNA polymerase (Table 3). Thus, resistance to chaperone-mediated inhibition cannot be explained by increased affinity for RNA polymerase.
Table 3.
Binding of σ32 to its relevant partners in vitro
| σ32 | Dissociation constants, Kd |
|||
|---|---|---|---|---|
| GroEL, μM | DnaK, μM | DnaJ, nM | RNA polymerase, nM | |
| WT | 2.72 ± 0.22 | 5.70 ± 2.39 | 39.6 ± 4.10 | 5.38 ± 1.41 |
| I54N | 3.83 ± 0.66 | 4.38 ± 1.51 | 86.6 ± 21.7 | 13.64 ± 4.00 |
The affinity of σ32 for its binding partners was determined by using fluorescence anisotropy (GroEL, DnaK, and RNA polymerase) or surface plasmon resonance (DnaJ) using purified proteins. Averages from four measurements ± SD are presented.
We examined the transcriptional capacity of I54N σ32 in vitro by using a multiround transcription assay conducted at 30°C. Titration of both WT and mutant σ32 (His-tagged and untagged) with a constant level of RNA polymerase core revealed that both proteins have similar transcriptional activity in vitro (data not shown). We then tested chaperone-mediated inhibition of σ32-dependent transcription. Surprisingly, addition of either DnaK/J/GrpE or GroEL/S to the transcription reaction inhibited both WT and mutant σ32 to a similar extent (Table 4). Addition of either DnaK/J or GroEL alone to the reaction also inhibited mutant and WT proteins similarly (data not shown). Finally, a similar extent of inhibition of WT and I54N σ32 was observed with a single-round transcription protocol or when the reaction was carried out at 37°C or 42°C rather than 30°C (data not shown).
Table 4.
Chaperone-mediated inhibition of σ32-dependent transcription in vitro
| σ32 | Relative activity |
||
|---|---|---|---|
| Control | +GroEL/S | +DnaK/J/GrpE | |
| WT | 1.0 | 0.3 ± 0.02 | 0.53 ± 0.06 |
| I54N | 1.0 | 0.28 ± 0.13 | 0.55 ± 0.07 |
σ32-Dependent transcription of htpG was determined essentially as described previously (16) using purified His-tagged σ32, core RNA polymerase, template DNA, and chaperone proteins as indicated. Multiround transcription reactions were run at 30°C, and 32P-labeled htpG transcript was analyzed by a PhosphorImager scanning system. Averages from four measurements with standard deviation are presented.
Discussion
In the present work, we report the characterization of σ32 mutants selected to be resistant to chaperone-mediated feedback inhibition. Our work casts doubt on several accepted features of σ32 regulation. First, chaperone-mediated sequestration of σ32 from RNA polymerase was believed to underlie inactivation. However, the present results indicate that the only mutants thus far defective in this process affect a step not consistent with this simple model. Second, chaperone-mediated inactivation and regulated degradation of σ32 were believed to be linked only by a requirement for binding chaperones. However, the present results suggest that the two pathways are functionally interconnected in an additional manner. Finally, these mutants do not recapitulate their inactivation defective phenotype in vitro.
The prevalent model for chaperone-mediated inactivation is that chaperone binding to σ32 sequesters it from RNA polymerase (6, 7). Therefore, we expected our inactivation-defective mutants to be altered either in interaction with chaperones or RNA polymerase. Our demonstration that I54N σ32 is almost completely defective in inactivation without appreciably altering binding to chaperones or RNA polymerase argues against the sequestration model in its simplest form. Instead, these results argue that there is an unanticipated step required for chaperone-mediated inactivation. Because I54N is defective in feedback regulation mediated by both GroEL/S and DnaK/J without significantly affecting chaperone binding, it most probably affects a step downstream of chaperone binding.
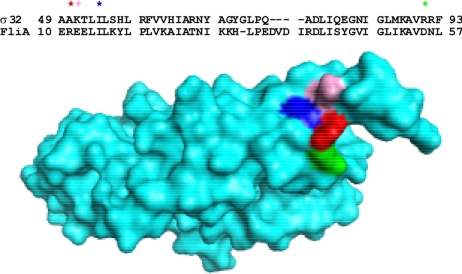
Surprisingly, all of our σ32 mutants exhibited a dual phenotype: although they were selected for defects in inactivation, they also exhibited a defect in degradation control. Importantly, a screen that selected σ32 mutants based solely on their increased stability identified mutants in the same or nearby residues as our selection (26). These residues could be essential for both inactivation and degradation because (i) they have two different functions, one necessary for activity control and the other necessary for degradation control; or (ii) they have a single function, necessary for both processes. Although we cannot eliminate the first possibility, our data are most consistent with the proposition that these residues define a single function. First, mutant defects are of similar severity for activity and degradation control. Thus, E48KΔ49–52 is moderately defective, and I54N is severely defective in both control processes. Such a correlation is consistent with the expectations of a model in which the mutations disrupt a single function. Second, homology modeling with FliA (27) (like σ32, FliA is a group 3 σ) reveals that the mutants define a cluster of surface-exposed residues centered in σ region 2.1 (Ala50, Lys51, Ile54, and Arg91), suggestive of a patch with a single function (Fig. 5). We note that alterations in adjacent residues not on the same surface (Glu48, Ala49, Thr52 and Leu53) have no phenotype (22), reinforcing the idea that these mutations define a small patch with a crucial function in both degradation and inactivation. Chaperone binding is the only currently known commonality between degradation and inactivation, and these mutations have little effect on that process (Table 3). We suggest that the mutants identify a regulatory step that operates downstream of chaperone binding and is required for both inactivation and degradation of σ32.
Fig. 5.
Sequence alignment of σ32 and FliA illustrates that the mutations define a cluster of surface-exposed residues. Regions 2.1 and 2.2 of Ec σ32 and Aa FliA were aligned (ClustalW; DNASTAR, Madison, WI). Residues corresponding to σ32 mutations were identified on the FliA structure (27) and mutated to the naturally occurring residues of σ32 (asterisks) in PyMOL. Shown are Arg11 mutated to Ala (Ala50 in σ32; red), Glu12 mutated to Lys (Lys51 in σ32; pink), Ile15 (Ile54 in σ32; blue), and Asp55 mutated to Arg (Arg91 in σ32; green). These residues form a surface-exposed patch.
It was surprising to find that the in vivo and in vitro phenotypes of I54N σ32 were quite discrepant: I54N is almost completely resistant to chaperone-mediated inactivation in vivo (Figs. 2 and 3) but indistinguishable from WT σ32 in both GroEL/S and DnaK/J chaperone-mediated inactivation in vitro (Table 4). It is conceivable but unlikely that the in vivo phenotype is an indirect consequence of the mutational alteration in the protein. Both the strength of the in vivo phenotypes and the fact that selections for both activity and degradation control identify this tight cluster of residues argue against this possibility. Alternatively, the in vitro system may only partly recapitulate inactivation in vivo. For example, the mutant σ may lack a conformational change that is required for inactivation in vivo but is not necessary for inactivation under in vitro conditions. Alternatively, the mutant may be defective in interacting with an additional factor that is bypassed in the current in vitro system. In this regard, it is interesting that degradation of σ32 by FtsH protease in vitro does not mimic degradation in vivo, possibly because of a missing factor (21). It is unclear whether the missing factor is the same as the one postulated to be missing from our in vitro inactivation system or yet an additional factor. Resolving this discrepancy is crucial for understanding how the chaperone-mediated control adjusts the activity of σ32 to a level appropriate to maintain protein-folding homeostasis in the cell.
Finally, it is interesting to note that σ region 2.1 is commonly used for controlling σ activity. In addition to the region 2.1 cluster identified here, E. coli σE uses a residue comparable with Ile54 to contact its anti-σ factor RseA (28). Likewise, Rhodobacter sphaeroides σE interacts with its zinc-containing anti-σ (ChrR) by using residues in region 2.1 (29). Indeed, structural homology modeling suggests that many σ–anti-σ pairs may interact in a similar manner (30). Region 2.1 of σ has many surface-exposed residues and does not itself contact either core RNA polymerase or DNA. This region may be free to evolve regulatory capacities so that the activity of σ can be controlled appropriately.
Materials and Methods
Strains.
All strains used were derivatives of E. coli K-12 strain MG1655. σ32 mutants originally isolated on pRB11 or slightly different rpoH plasmids were placed under the control of an IPTG-inducible promoter in strain CAG48238 carrying ΔlacX74 and prophage λJW2 (PhtpG-lacZ) (31) and its ΔrpoH derivative.
Chaperone Overexpression Studies.
For chaperone overexpression experiments, chromosomal Para-groEL/S (32) or PA1/lacO-1-dnaK/J-lacIq (15) was transduced into CAG48238 to obtain CAG48239 or CAG48275, respectively. For Table 1, derivatives of CAG48239 carrying a plasmid-encoded rpoH mutations and a pACYC184-based plasmid pGro11 (Ptet-groESL) (33) were grown in LB medium containing l-arabinose (0.2%) at 30°C to obtain the WT level of GroEL/S; rpoH was induced by the addition of 1 mM IPTG, and GroEL/S was overexpressed by the addition of 25 ng/ml anhydrotetracycline. For DnaK/J overexpression, derivatives of CAG48275 carrying plasmid-encoded rpoH mutations were grown in 5 μM IPTG at 30°C to achieve the WT level of DnaK/J from the chromosomal PA1/lacO-1-dnaK/J-lacIq locus. DnaK/J and σ32 were simultaneously overexpressed by the addition of 1 mM IPTG; alternatively, DnaK/J was overexpressed from another compatible plasmid pKJE8 (Para-dnaK/J/grpE) by the addition of l-arabinose (33). The differential rate of β-galactosidase synthesis was determined over a 2-h time course immediately after chaperone induction.
Media and Antibiotics.
LB medium and M9 medium were prepared as described (34) except that M9 medium was supplemented with 0.2% glucose/1 mM MgSO4/all amino acids (40 μg/ml) except methionine and cysteine/vitamin mixture. When required, antibiotics were added to the medium as follows: 100 μg/ml ampicillin, 30 μg/ml kanamycin, and 20 μg/ml chloramphenicol.
λred-Mediated Recombination.
Synthetic deoxyoligonucleotides (30–70 bp) containing a specific rpoH mutation were electroporated into cells carrying pKD46 (λred) essentially as described (35, 36), and dark blue colonies were screened on LB agar medium containing X-Gal. The rpoH mutant candidates were confirmed by β-galactosidase assay, linkage to a nearby tetracycline resistance (Tn10) marker, and by sequencing. The chromosomal rpoH mutations thus obtained were transduced into CAG48238 by selecting for the nearby tet marker.
β-Galactosidase Assay.
Overnight cultures (LB medium) were diluted 200- to 500-fold and grown to exponential phase (OD600 = 0.05–0.3). Samples were taken at intervals starting at OD600 = 0.05, and σ32 activity was monitored by measuring β-galactosidase activity expressed from the σ32-dependent htpG promoter by the standard procedure (37).
Pulse Labeling and Immunoprecipitation.
Cells were grown in supplemented M9 medium and pulse-labeled with [35S]methionine for σ32 synthesis, or with EasyTag EXPRESS35S protein labeling mix (PerkinElmer, Waltham, MA) for HtpG synthesis and immunoprecipitated as described previously (16).
Immunoblotting.
Cells were treated with cold 5% tricholoroacetic acid, kept on ice for 30 min, precipitated by centrifugation, washed in acetone, and resuspended in Laemmli buffer. Serial dilutions of WT and mutant samples were loaded onto a polyacrylamide gel, and the proteins were transferred to nitrocellulose membranes. The blots were first probed with rabbit primary antibodies and then with anti-rabbit horseradish peroxidase-conjugated secondary antibody. Immunoblots were developed with chemiluminescence and exposed to film. Fold increase (level experiments) was estimated by comparison with a dilution series of samples from the WT. Fold decrease after addition of chloramphenicol (stability experiments) was determined by direct scanning and analyzing bands with ImageJ software (National Institutes of Health, Bethesda, MD).
Protein Purification.
The following proteins were purified essentially as described: RNA polymerase (38), His-tagged σ32 (18), GroEL, GroES (39), DnaK, DnaJ, and GrpE (40). Misfolded proteins were removed from chaperone preparations as described (16). σ32 (untagged) was purified by using pQE30-Xa vector, as suggested by the manufacturer (Qiagen, Valencia, CA). The protein was affinity purified as a His-tagged protein and treated with protease Xa to cleave off the His tag, leaving the intact σ32 with no additional amino acid attached. Xa was removed by using Xa removal resin. Fluorescently labeled σ32 was prepared by purifying His-tagged L118C σ32 from a slyD mutant strain (41). The L118C mutation allows specific labeling at 118 because σ32 does not have any endogenous cysteines and SlyD is a contaminant labeled by the fluorophore. Alexa Fluor 488 C5-maleimide (Molecular Probes, Eugene, OR) was used to label σ32 according to the manufacturer's instructions.
Fluorescence Anisotropy.
Fluorescence data were collected on an ISS K2 multifrequency phase fluorometer running on Vinci software. For GroEL binding, 100 nM labeled σ32 was incubated in 50 mM Tris, pH 7.5/100 mM KCl/0.002% Tween 20/0.2 mM β-mercaptoethanol/10% (vol/vol) glycerol. For RNA polymerase binding, 10 nM labeled σ32 was incubated in 50 mM Tris, pH 7.5/500 mM KCl/5% glycerol/0.05% Nonidet P-40/0.001% Tween 20/0.01 mM β-mercaptoethanol. For DnaK binding, 100 nM N-terminal FITC-labeled peptide QRKLFFNLRKTKQ (Tufts University Core Facility, Boston MA), previously shown to bind to DnaK, was incubated in 50 mM Tris, pH 7.5/100 mM KCl/10% (vol/vol) glycerol (42). In each case, competition experiments were performed by using either unlabeled WT σ32 or unlabeled I54N σ32. The equilibrium binding constant was then calculated (43).
Surface Plasmon Resonance.
Data were collected by using a Biacore 1000. Untagged WT or I54N σ32 was flowed over an nitrilotriacetic acid chip containing immobilized His-tagged DnaJ in 10 mM Hepes, pH 8.3/500 mM NaCl/350 mM EDTA/0.05% Tween 20. Each binding experiment used duplicate samples of at least four different concentrations of σ32 spanning at least 27× change in concentration. Data were analyzed by using a 1:1 binding with drifting baseline model and BiaEvaluation software.
In Vitro Transcription.
Multiround in vitro transcription was carried out as reported previously (16), and single-round transcription was done as described (44).
Supplementary Material
Acknowledgments
We thank B. Bukau (Zentrum für Molekulare Biologie, Heidelberg) and R. Young (Texas A&M University, College Station, TX) for strains; M. Kanemori (Kanazawa University, Kanazawa, Japan) for plasmids; I. Wilder for assistance; D. Mullins and A. Kelly for help with anisotropy; P. Hwang, J. Wilbur, and R. Fletterick for help with Biacore; and members of the Gross laboratory for discussion. T.Y. thanks Kazuhiro Nagata, Chieko Wada, and Hirotada Mori, in whose laboratories part of this work was done. This work was supported by National Institutes of Health Grant GM36278 (to C.A.G.) and a National Science Foundation Graduate Research Fellowship (to E.G.).
Abbreviations
- hsp
heat shock protein
- IPTG
isopropyl β-d-thiogalactoside.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708819104/DC1.
References
- 1.Morimoto RI. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 2.Gross CA. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd Ed. Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Washington, DC: Am Soc Microbiol; 1996. pp. 1382–1399. [Google Scholar]
- 3.Yura T, Kanemori M, Morita MT. In: Bacterial Stress Responses. 2nd Ed. Storz G, Hengge-Arronis R, editors. Washington, DC: Am Soc Microbiol; 2000. pp. 3–18. [Google Scholar]
- 4.Bukau B, Horwich AL. Cell. 1998;92:351–356. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 5.Hartl FU, Hayer-Hartl M. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 6.Craig EA, Gross CA. Trends Biochem Sci. 1991;16:135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- 7.Bukau B. Mol Microbiol. 1993;9:671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 8.Connolly L, Yura T, Gross CA. In: Molecular Chaperones and Folding Catalysts: Regulation, Cellular Function, and Mechanism. Bukau B, editor. Harwood: Amsterdam; 1999. pp. 13–33. [Google Scholar]
- 9.Nollen EA, Morimoto RI. J Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- 10.Grossman AD, Straus DB, Walter WA, Gross CA. Cell. 1984;38:383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- 11.Landick R, Vaughn V, Lau ET, VanBogelen RA, Erickson JW, Neidhardt FC. Cell. 1984;38:175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- 12.Yura T, Tobe T, Ito K, Osawa T. Proc Natl Acad Sci USA. 1984;81:6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straus DB, Walter WA, Gross CA. Genes Dev. 1989;3:2003–2010. doi: 10.1101/gad.3.12a.2003. [DOI] [PubMed] [Google Scholar]
- 14.Kanemori M, Mori H, Yura T. J Bacteriol. 1994;176:4235–4242. doi: 10.1128/jb.176.14.4235-4242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomoyasu T, Ogura T, Tatsuta T, Bukau B. Mol Microbiol. 1998;30:567–581. doi: 10.1046/j.1365-2958.1998.01090.x. [DOI] [PubMed] [Google Scholar]
- 16.Guisbert E, Herman C, Lu CZ, Gross CA. Genes Dev. 2004;18:2812–2821. doi: 10.1101/gad.1219204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamer J, Bujard H, Bukau B. Cell. 1992;69:833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- 18.Liberek K, Galitski TP, Zylicz M, Georgopoulos C. Proc Natl Acad Sci. 1992;89:3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamer J, Multhaup G, Tomoyasu T, McCarty JS, Rudiger S, Schonenfeld HJ, Schirra C, Bujard H, Bukau B. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 20.Blaszczak A, Georgopoulos C, Liberek K. Mol Microbiol. 1999;31:157–166. doi: 10.1046/j.1365-2958.1999.01155.x. [DOI] [PubMed] [Google Scholar]
- 21.Herman C, Prakash S, Lu CZ, Matouschek A, Gross CA. Mol Cell. 2003;11:659–669. doi: 10.1016/s1097-2765(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 22.Horikoshi M, Yura T, Tsuchimoto S, Fukumori Y, Kanemori M. J Bacteriol. 2004;186:7474–7480. doi: 10.1128/JB.186.22.7474-7480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatsuta T, Tomoyasu T, Bukau B, Kitagawa M, Mori H, Karata K, Ogura T. Mol Microbiol. 1998;30:583–593. doi: 10.1046/j.1365-2958.1998.01091.x. [DOI] [PubMed] [Google Scholar]
- 24.Straus DB, Walter WA, Gross CA. Nature. 1987;329:348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 25.Joo DM, Ng N, Calendar R. Proc Natl Acad Sci USA. 1997;94:4907–4912. doi: 10.1073/pnas.94.10.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obrist M, Narberhaus F. J Bacteriol. 2005;187:3807–3813. doi: 10.1128/JB.187.11.3807-3813.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorenson MK, Ray SS, Darst SA. Mol Cell. 2004;14:127–138. doi: 10.1016/s1097-2765(04)00150-9. [DOI] [PubMed] [Google Scholar]
- 28.Campbell EA, Tupy JL, Gruber TM, Wang S, Sharp MM, Gross CA, Darst SA. Mol Cell. 2003;11:1067–1078. doi: 10.1016/s1097-2765(03)00148-5. [DOI] [PubMed] [Google Scholar]
- 29.Anthony JR, Newman JD, Donohue TJ. J Mol Biol. 2004;341:345–360. doi: 10.1016/j.jmb.2004.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell EA, Greenwell R, Anthony JR, Wang S, Lim L, Das K, Sofia HJ, Donohue TJ, Darst SA. Mol Cell. 2007;27:793–805. doi: 10.1016/j.molcel.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wild J, Walter WA, Gross CA, Altman E. J Bacteriol. 1993;175:3992–3997. doi: 10.1128/jb.175.13.3992-3997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLennan N, Masters M. Nature. 1998;392:139. doi: 10.1038/32317. [DOI] [PubMed] [Google Scholar]
- 33.Nishihara K, Kanemori M, Kitagawa M, Yanagi H, Yura T. Appl Environ Microbiol. 1998;64:1694–1699. doi: 10.1128/aem.64.5.1694-1699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. appendices 1–3. [Google Scholar]
- 35.Datsenko KA, Wanner BL. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis HM, Yu D, DiTizio T, Court DL. Proc Natl Acad Sci USA. 2001;98:6742–6746. doi: 10.1073/pnas.121164898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1972. pp. 352–355. [Google Scholar]
- 38.Sharp MM, Chan CL, Lu CZ, Marr MT, Nechaev EW, Merritt EW, Severinov K, Roberts JW, Gross C. Genes Dev. 1999;13:3015–3026. doi: 10.1101/gad.13.22.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fenton WA, Kashi Y, Furtak K, Horwich AL. Nature. 1994;371:614–619. doi: 10.1038/371614a0. [DOI] [PubMed] [Google Scholar]
- 40.Suh WC, Burkholder WF, Lu CZ, Zhao X, Gottesman ME, Gross CA. Proc Natl Acad Sci USA. 1998;95:15223–15228. doi: 10.1073/pnas.95.26.15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roof WD, Horne SM, Young KD, Young R. J Biol Chem. 1994;269:2902–2910. [PubMed] [Google Scholar]
- 42.McCarty JS, Rudiger S, Schonfeld HJ, Schneider-Mergener J, Nakahigashi K, Yura T, Bukau B. J Mol Biol. 1996;256:829–837. doi: 10.1006/jmbi.1996.0129. [DOI] [PubMed] [Google Scholar]
- 43.Vinson VK, De La Cruz EM, Higgs HN, Pollard TD. Biochemistry. 1998;37:10871–10880. doi: 10.1021/bi980093l. [DOI] [PubMed] [Google Scholar]
- 44.Nonaka G, Blankschien M, Herman C, Gross CA, Rhodius VA. Genes Dev. 2006;20:1776–1789. doi: 10.1101/gad.1428206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.