The evolution of life cycles or life histories is one of the most important topics in behavioral and population ecology and in evolutionary biology. There is inherent interest in explaining the diversity of life cycles among species: These span a range from rapidly multiplying but short-lived bacteria and yeast to the long life and slow breeding of the wandering albatross (1). However, when we look closely at the characteristics of a life history, we see traits that reflect the evolutionary fitness of organisms: survival of individuals from young to old and the onset, magnitude, and duration of reproduction. When advantages in life-history characteristics are associated with heritable organismal traits, the essence of natural selection is born (2).
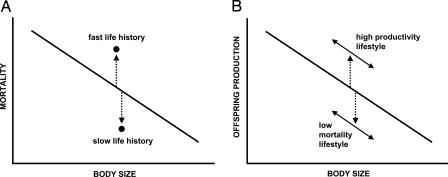
During the past two decades, a paradigm surrounding the evolution of life histories has developed. This paradigm has grown from two research traditions: studies of the diversity of life histories among species and experimental research within a few model species. The first part of the current paradigm is that life histories vary with the body size of species. This is the well known mouse-to-elephant relationship in many traits, and mammals have played a central role in extending this idea from initial physiological traits, such as metabolism, to more ecological traits, such as population densities and life histories (3–5). On the mouse-end of this continuum, we have short life spans and rapid reproduction, and on the elephant-end, we have long lives and slow breeding. However, a change in the tempo of life is not solely reflected by size. When the influence of body size of species is held statistically invariant, a new continuum of faster lives to slower lives can be seen, although the placement of species along this scale differs from that of body size (6–8). Thus, two axes of life-history variation can be identified: a developmental tempo due to the size of organisms (it takes longer to make an elephant than a mouse) and a tradeoff tempo that contrasts a “slow–fast” continuum of life histories that is independent of body size (Fig. 1A).
Fig. 1.
Two views of the evolution of life histories, both of which recognize a primary (first) axis based on body size. (A) The slow–fast continuum, where the second axis of life history reflects long-lived species (slow life histories) compared with short-lived species (high mortality, particularly for juveniles). A tradeoff of survival and reproduction produces low and high levels of reproductive success, respectively. (B) The second axis of life histories reflects different “lifestyles” based on ease of resource acquisition from herbivores and marine mammals (with high production of offspring) to alternative lifestyles (see text) in which mortality is reduced. In the latter, production of offspring is reduced by the tradeoff of survival and reproduction.
The slow–fast continuum is the second part of the current paradigm. It contrasts species that have short lives and rapid reproduction for their body size to longer-lived and slower-reproducing species. For example, bats are small mammals with shorter-than-average lives, but when the body-size axis is statistically removed from life-history data for the mammalian orders, bats are seen to have exceptionally long lives (slow life histories) for their body size (9, 10). Efforts to find further major axes of variation in life histories focused on characteristics such as the precociality of young (9, 11), but tests of these ideas have not documented support (12, 13). Thus, the best evidence to date supports just two important axes of life-history variation in mammals, both having to do with the tempo of life.
What causes variation in the major components of life histories? For body size, a current suggestion is that there is an “optimal size” of species that fill particular niches, such as mammalian herbivores, and other sizes evolve via competition (14) or predation (15) into filling alternative ecological niches. Variation along the slow–fast continuum has been explained by mortality patterns, particularly juvenile mortality (8, 16). The basic idea is that high mortality rates produce species at the fast end of the continuum, whereas low mortality rates favor the slow end of the continuum. In general, there is a tradeoff of reproduction and survival (1 − mortality) so that a range of species from high to low reproduction and mortality is produced. The importance of mortality patterns also has been supported in modeling of life histories (17) and experimental studies of life histories within species (18).
At this point, Sibly and Brown enter the life-history arena with a new view (see ref. 19 in this issue of PNAS): Perhaps it is ease of resource acquisition and subsequent biomass production (in terms of mass of annual reproduction) that produces different types of lifestyles, and life histories follow the lifestyles. Production is easiest when resources are exceptionally abundant, and high levels of reproduction should be favored in such species. They contrast this lifestyle to one based on avoiding predators, either with flying, burrowing, or arboreal lifestyles, with specialized antipredator adaptations, or with growth to exceptional size. Additional lifestyles that combine the influences of moderate resource abundance and somewhat lowered mortality are intermediate, so that considerable variation in life histories is produced. Each lifestyle has the same body-size constraint, so that a range of body sizes occurs within typical lifestyles. Species with abundant food resources should exhibit high productivity of offspring, and species that exhibit reduced mortality via specialized traits or larger size should exhibit lower productivity (Fig. 1B). The idea of lifestyles augments and replaces the “slow–fast continuum” of life histories. This appears to be a niche-based concept that brings the environment into the picture of diversity of life histories more clearly than past efforts, an exciting advance over current thinking. Experimental studies within species of mammalian herbivores support the idea that improved ease of resource acquisition is usually associated with increased production (20, 21).
Sibly and Brown (19) present an impressive interspecific comparison of 637 mammalian species to support their new view. They begin by defining shrews and allies as the historically primitive condition in mammals (and thus average in lifestyle) and showing that groups of mammals with abundant resources have high rates of offspring production (rodent, lagomorph, and ungulate herbivores and marine mammals). Mammals that exhibit lowered mortality through flying (bats), arboreal habits (many primates and tree-dwelling rodents), burrowing (moles and ground-dwelling rodents), and desert-dwelling (some rodents) have relatively lower offspring productivity compared with herbivores. One of the most appealing features of the tests of the “lifestyle” hypothesis is that groupings based on lifestyles appear to have more similar life histories than historical clades (shown by dividing the Order of bats into insect versus nectar and fruit foragers, carnivores into terrestrial and marine, and rodents into folivores and nonfolivores).
The lifestyle hypothesis is based on ease of resource acquisition rather than the level of resource demand.
Life-history theory has been dominated by demographics, the study of the interplay of life-history traits. Demographic studies have lacked a specific focus on environmental influences on life-history traits. The essential link to the environment is provided by Sibly and Brown's (19) new view. The interaction of life-history traits within an environmental context, such as a lifestyle, produces fitness differences. In turn, fitness differences provide the basis for the evolution of both life histories and other organismal traits.
If the lifestyle hypothesis is to become a paradigm in life-history theory, it will have to stand up to more tests. The impressive support that mammal species present in Sibly and Brown's study should reveal individual species that have recently evolved different life-histories from the rest of their clade. These cases should be exceptions that “prove the rule” by reflecting either greater or lesser production in response to shifts in the lifestyles of the exceptional species. In addition, other clades can be tested, such as birds or reptiles, for the predicted associations of lifestyle and life-history traits. It would be particularly interesting to see whether the predicted patterns are found in clades of ectothermic species. The lifestyle hypothesis is based on ease of resource acquisition rather than the level of resource demand. Ectotherms, such as herbivorous turtles and squamates, should exhibit high ease of resource acquisition but with relatively low metabolic resource demand. More complete phylogenetic analyses of Sibly and Brown's (19) data set will also provide a test of their new view, given that phylogenetic comparative analyses can reveal either weaker or stronger patterns than analyses that do not take phylogeny explicitly into account (22, 23).
Footnotes
The author declares no conflict of interest.
See companion article on page 17707.
References
- 1.Stearns SC. The Evolution of Life Histories. New York: Oxford Univ Press; 1992. [Google Scholar]
- 2.Endler JA. Natural Selection in the Wild. Princeton: Princeton Univ Press; 1986. [Google Scholar]
- 3.Peters RH. The Ecological Implications of Body Size. Cambridge, UK: Cambridge Univ Press; 1983. [Google Scholar]
- 4.Calder WA. Size, Function and Life History. Cambridge, MA: Harvard Univ Press; 1984. [Google Scholar]
- 5.Brown JH, Gillooly JF, Allen AP, Savage VM, West GG. Ecology. 2004;85:1771–1789. [Google Scholar]
- 6.Harvey PH, Zammuto RM. Nature. 1985;315:319–320. doi: 10.1038/315319a0. [DOI] [PubMed] [Google Scholar]
- 7.Read AF, Harvey PH. J Zool. 1989;219:329–353. [Google Scholar]
- 8.Promislow DEL, Harvey PH. J Zool. 1990;220:417–437. [Google Scholar]
- 9.Gaillard J-M, Pontier D, Allaine D, Lebreton JD, Trouvilliez J, Clobert J. Oikos. 1989;56:59–76. [Google Scholar]
- 10.Harvey PH, Read AF, Promislow DEL. In: Oxford Surveys in Evolutionary Biology. Harvey PH, Partridge L, editors. Vol. 6. Oxford: Oxford Univ Press; 1989. pp. 13–32. [Google Scholar]
- 11.Stearns SC. Oikos. 1983;41:173–187. [Google Scholar]
- 12.Dobson FS, Oli MK. Écoscience. 2007;14:292–299. [Google Scholar]
- 13.Dobson FS, Oli MK. In: Rodent Societies. Wolff JO, Sherman PW, editors. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 14.Brown JH. Macroecology. Chicago: Univ of Chicago Press; 1995. [Google Scholar]
- 15.Brown JH, Sibly RM. Proc Natl Acad Sci USA. 2006;103:17595–17599. doi: 10.1073/pnas.0608522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Promislow DEL, Haervey PH. Acta Œcol. 1991;12:119–137. [Google Scholar]
- 17.Kozłowski J, Wiener J. Am Nat. 1997;149:352–380. [Google Scholar]
- 18.Reznick D, Butler MJ, Rodd H. Am Nat. 2001;157:126–140. doi: 10.1086/318627. [DOI] [PubMed] [Google Scholar]
- 19.Sibly RM, Brown JH. Proc Natl Acad Sci USA. 2007;104:17707–17712. doi: 10.1073/pnas.0707725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobson FS, Murie JO. Am Nat. 1987;129:382–397. [Google Scholar]
- 21.Boutin S. Can J Zool. 1990;68:203–220. [Google Scholar]
- 22.Jouventin P, Dobson FS. Proc R Soc London Ser B. 2002;269:1955–1961. doi: 10.1098/rspb.2002.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobson FS, Jouventin P. Proc R Soc London Ser B. 2007;274:275–279. doi: 10.1098/rspb.2006.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]