Abstract
Experiments in monkeys demonstrated that many parietal and premotor neurons coding a specific motor act (e.g., grasping) show a markedly different activation when this act is part of actions that have different goals (e.g., grasping for eating vs. grasping for placing). Many of these “action-constrained” neurons have mirror properties firing selectively to the observation of the initial motor act of the actions to which they belong motorically. By activating a specific action chain from its very outset, this mechanism allows the observers to have an internal copy of the whole action before its execution, thus enabling them to understand directly the agent's intention. Using electromyographic recordings, we show that a similar chained organization exists in typically developing children, whereas it is impaired in children with autism. We propose that, as a consequence of this functional impairment, high-functioning autistic children may understand the intentions of others cognitively but lack the mechanism for understanding them experientially.
Keywords: mirror neurons, motor chains, motor acts, goal understanding, motor intention
Humans and monkeys possess a neural system, called the mirror neuron system, that maps visual descriptions of actions done by others onto the observer's motor representations of the same actions (1). In humans, the mirror neuron system has two major components. One is formed by the inferior parietal lobule and the ventral premotor cortex plus the caudal part of Broca's area, the other by the insula and anterior cingulate gyrus (1, 2).
The mirror neuron system does not possess a unique function. Besides its originally proposed role in action understanding, its parieto–frontal component appears to mediate the understanding of intentions of others (3) and imitation (4–7), whereas its insular–cingulate component appears to play a fundamental role in emotion recognition (2, 8).
Recent data obtained in the monkey revealed that the mirror neuron mechanism underlying intention understanding relies on the activation of a specific set of “action-constrained” parietal neurons (9). These neurons discharge in association with specific motor acts but become maximally activated when the coded motor act is embedded into a specific motor action. Thus, for example, action-constrained grasping neurons strongly discharge when grasping a piece of food is followed by bringing it to the mouth, but not when it is followed by placing it into a container. Most interestingly, many action-constrained neurons have mirror properties. These neurons selectively discharge when the observation of motor acts is part of a given action (e.g., grasping for eating but not grasping for placing) (9). Their activation provides, therefore, information on the fact that an individual is grasping, but most importantly also gives clues on why the individual is doing it. Through this mechanism the observer, besides recognizing the observed motor act, is also able to predict what will be the final goal of the action. In other words, the observer is able to understand the intentions behind the agent action.
It has been noted that the functions in which the mirror neuron system appears to be involved are precisely those that are impaired in autism. Hence, the hypothesis that a core symptom of autism, the inability to relate to people in an ordinary way (10–12), depends on a malfunctioning of the mirror neuron system (13–15). Anatomical investigations (16, 17) and evidence coming from neurophysiological [electroencephalogram, magnetoencephalogram, transcranial magnetic stimulation (TMS)] and brain imaging studies support this view (18–21).
The mirror neuron system hypothesis of autism claims that the incapacity to relate to others depends on a deficit of the mirror neurons to respond normally to the observation of actions of others. It might be, however, that the primary deficit is not in the responsiveness of the mirror neurons to the observation of others' action, but in the impaired organization of motor chains underlying action representation. If this hypothesis is correct, one could expect that there is a difference between typically developing (TD) children and children with autism not only in responding to the observation of actions performed by others but also in the organization of their actions. We tested this hypothesis in a series of experiments, in which we recorded the electromyographic (EMG) activity in children with autism and TD children while they were observing actions done by others and when they were executing the same actions.
Results
Experiment 1: Muscle Activation During the Observation of Actions Done by Others in TD Children and Children with Autism.
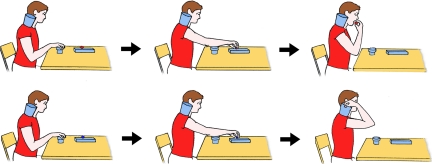
This experiment was run on eight right-handed TD children [four males and four females, ages 5.1–9.1 years; mean age, 6.5 years; mean intelligence quotient (IQ), 104.6 ± 6.6] and on seven right-handed high-functioning children with autism (six males and one female, ages 5.1–9.0 years; mean age, 6.2 years; mean IQ, 98 ± 12.4). The participants were required to watch carefully the experimenter performing two different actions: grasping with the right hand a piece of food placed on a touch-sensitive plate, bringing it into the mouth and eating it, or grasping a piece of paper placed on the same plate and putting it into a container, located on the experimenter's right shoulder (Fig. 1). The two actions were repeated 20 times each in a pseudorandom order. During both actions, the activity of the mouth-opening mylohyoid (MH) muscle of the participants was recorded. The MH activity was the variable used to assess a possible mirror effect.
Fig. 1.
Schematic representation of the tasks of experiments 1 and 2. (Upper) The individual reaches for a piece of food located on a touch-sensitive plate, grasps it, brings it to the mouth, and finally eats it. (Lower) The individual reaches for a piece of a paper located on the same plate, grasps it, and puts into a container placed on the shoulder.
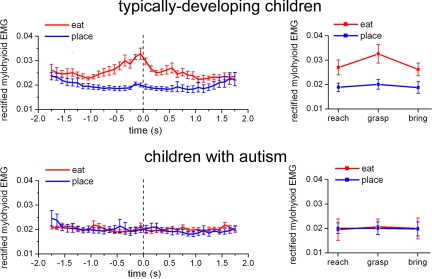
The results are shown in Fig. 2 and supporting information (SI) Table 1. It clearly appears that during the observation of eating action, a marked increase of MH activity was seen in TD children already during the reaching phase. This increase became more evident during grasping and persisted during bringing to the mouth. No increase of MH activity was present during the observation of the placing action. In contrast, the children with autism did not show any MH activation during the observation of eating or placing actions.
Fig. 2.
Time course of the rectified EMG activity of MH muscle during the observation of the bringing-to-the-mouth action (red) and the placing action (blue) in experiment 1. (Left) Vertical bars indicate the SE. All curves are aligned with the moment of object lifting from the touch-sensitive plate (t = 0, dashed vertical line). (Right) Mean EMG activity of MH muscle in the three epochs of the two actions in experiment 1. Vertical bars indicate 95% confidence intervals.
To quantify the observed effects, the two actions were subdivided into three epochs corresponding to the movement phases of reaching, grasping, and bringing to the mouth or putting into the container. The ANOVA performed on the EMG activity of the MH muscle in the two actions and in the three epochs showed a significant interaction between the two factors: Group of subjects × Action type [F(1,13) = 43.442, P < 0.0001] because of a greater activation of MH in TD children while watching the grasp-to-eat action compared with watching the grasp-to-place action throughout the three epochs (P < 0.0001). Fig. 2 Right shows the mean values of MH activity in the two groups (additional results in SI Text A and SI Fig. 5).
These results indicate that TD children show an activation of their MH muscle, that is, of the muscle involved in the final stage of the experimenter's action, already when they observe the experimenter's initial motor act, food reaching. This activation reflects their understanding of the final goal of the observed action. In children with autism this action-understanding motor activation is lacking.
An additional analysis was carried out on the epoch durations of the experimenter's movements, which showed no significant differences between the two groups of participants (SI Text B). To ascertain that TD and children with autism observed the experimenter's actions, an analysis was carried out on video records of all trials that form the experiment database. The results showed that both groups of participants gazed in virtually all trials first at the items that the experimenter was going to grasp and then at the final target of the action, both during eating and placing. Videos showing the behavior of children with autism are presented in SI Movies 1–12.
Experiment 2: Muscle Activation During Action Execution in TD and in Children with Autism.
In this experiment, we investigated the motor activity of MH muscle during the execution of the same two actions (grasping to eat and grasping to place) studied in the first experiment (Fig. 1). The experiment was carried out on eight children with autism and eight TD children. Seven children of the autistic group were the same as in the first experiment. The eighth child was 6.1 years old, male, with an IQ of 93. As a control, a new group of eight TD children was recruited (four males and four females, ages 5.2–11.9 years; mean age, 6.5 years; mean IQ, 104.7 ± 7.7). As in the first experiment, the two groups were matched for age and IQ.
Before the experiment, participants were instructed to grasp and eat the food or to place the piece of paper into the container, according to the stimulus presented. They were also told to perform the movements in a natural way. Again, two touch-sensitive devices signaled the release of the hand from the table and the contact with the grasped object. Trials started with participants resting their right hand on the table. The actions were prompted by the object that was presented (food or paper) without any verbal instruction. The two actions were repeated 20 times each in a pseudorandom order with an intertrial interval of 20 s. All participants performed a brief training session before recordings. The muscle recorded in all participants was, as in the first experiment, the MH muscle. The EMG signals were processed in the same way as in the first experiment.
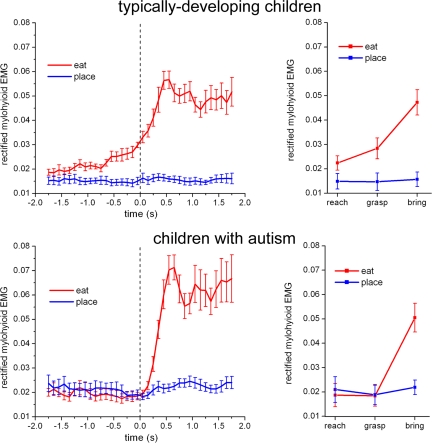
Fig. 3Left shows the time course of the mean EMG signal of the MH muscle in the two experimental conditions (grasping to eat and grasping to place) in TD and children with autism. In TD children, the EMG activity of MH muscle started to increase several hundred ms before the hand grasped the food. It continued to increase during actual grasping, and, as expected, it reached its peak when the individual started to open the mouth. The behavior of the MH muscle found in children with autism was strikingly different. In this group, no activity increase was found during the entire reaching and grasping phases. The muscle became active only during the bringing-to-the-mouth phase.
Fig. 3.
Time course of the rectified EMG activity of MH muscle in experiment 2 during execution of the bringing-to-the-mouth (red) and placing actions (blue). (Left) Other conventions as in Fig. 2. (Right) Mean EMG activity of MH muscle in the three epochs of the two actions in experiment 2. Vertical bars indicate 95% confidence intervals.
A quantitative analysis of the data were carried out by dividing the actions into three epochs: reaching, grasping, and bringing to the mouth or into the container. As in the first experiment, for each child MH activity was rectified, averaged separately in the three epochs for the two actions, and used as dependent variable in the ANOVA. The main finding was a significant interaction [F(2,28) = 4.7525, P < 0.05] among the three factors. Post hoc analysis showed a significant increase of MH muscle activity in the reach (P < 0.05) and grasp (P < 0.0001) epochs of the grasp-to-eat action compared with the grasp-to-place action in TD children. No change in MH muscle activity was present in the reaching and grasping epochs in children with autism (Fig. 3 Right and SI Table 2). In the bringing epoch, EMG activity was significantly higher in the grasping-to-eat action than in the grasping-to-place action in both groups, with no differences between TD and children with autism (for additional results, see SI Text A).
An ANOVA performed on the durations of the epochs of the two actions in the two groups did not reveal any significant difference (SI Text B). A video control of the two actions showed that both groups of participants gazed at the target of the action as soon as the item was placed on the touch-sensitive plate (Video Clips 13–24 in SI). As a control for experiments 1 and 2, we replicated them in TD children by using as stimuli both edible and nonedible items (pieces of paper) to be placed in the mouth. Nonedible items were used to have identical stimuli in the two action tested. The results showed an overlapping behavior of MH muscle in both conditions (SI Text C).
Experiment 3: Muscle Activation During the Execution of Hand/Foot Actions.
Finally, to examine whether children with autism show an impaired motor chain organization in action concerning other effectors than hand and mouth, we carried out a further experiment involving hand/foot actions. The TD and children with autism who participated in this experiment were the same as in experiment 2. They performed two actions. The first consisted of grasping a piece of food with their right hand and placing it into a container covered by a lid that had to be opened by pressing a foot pedal; the second consisted of grasping a paper ball and placing it in an open container, keeping their foot still on the pedal (a schematic of the two actions is shown in SI Fig. 6). The cue for which action had to be performed was provided by the type of object (piece of food for the pedal-controlled container and paper ball for the open container). All participants performed a brief training session before recordings.
During both actions, the EMG activity of the ankle dorsiflexor muscle, tibialis anterior (TA), was recorded and used as the dependent variable for studying the motor act chaining. Throughout the trials, subjects were required to keep the right foot lifted on the pedal, a position requiring a constant contraction of the right TA. The pressing of the pedal corresponded, therefore, to a decrease in the activity of the muscle.
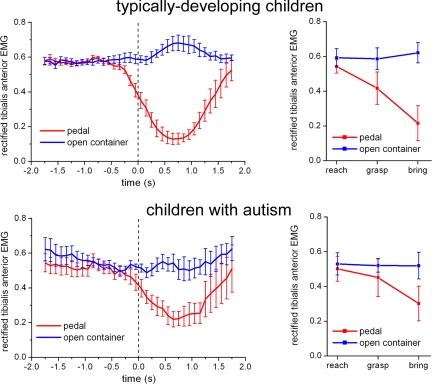
Fig. 4Left shows the mean activity of the TA muscle during the two actions (pedal pressing and non-pedal pressing) in the two groups of participants. As in experiment 2, a preparatory activity, consisting in this case of a decrease in TA EMG activity, was observed well before (≈300–400 ms) food lifting in TD children. In contrast, in children with autism the decrease of TA activity was observed only after food lifting.
Fig. 4.
Time course of the rectified EMG activity of TA muscle in experiment 3 during the execution of the pedal (red) and of the nonpedal actions (blue). (Left) Vertical bars indicate SE. All curves are aligned with the release of the hand from the touch-sensitive plate (t = 0, dashed vertical line). (Right) Mean EMG activity of TA muscle in the three epochs of the two actions in experiment 3. Vertical bars indicate 95% confidence intervals.
As in the previous experiments, the actions were subdivided into three epochs corresponding to the movement phases of reaching, grasping, and bringing the object to the containers. The statistical analysis was made on the rectified EMG activity of the TA muscle averaged between trials of the same action within single subjects. The findings confirmed the result of experiment 2, showing a lack of chaining of single motor acts in children with autism while they were performing a complex action. The ANOVA showed an interaction among the three factors Group × Action × Epoch [F(2,28) = 3.7037, P < 0.05]. Post hoc analysis showed that the mean EMG activity in the TA was suppressed in the pedal-pressing action during the grasping (P < 0.0005) and bringing (P < 0.0001) epochs in the TD children. In contrast, this suppression was significant only in the bringing (P < 0.0001) epoch in children with autism (for additional results, see SI Text A and SI Table 3). An ANOVA performed on the durations of the epochs of the two actions in the two groups did not reveal any significant difference (SI Text B).
Discussion
Chained Organization of Actions in TD Children.
In the monkey, the representation of actions in the parietal and frontal lobe is based on chains of motor acts. These chains are formed by action-constrained neurons that code specific motor acts (e.g., grasping) according to the final goal of the action in which the motor act is embedded. When an individual intends to grasp food to eat it, a motor chain starting with “grasping” neurons and ending with “bringing-to-the-mouth” neurons is selected at the very outset of the action, whereas if the same individual intends to grasp the food for placing it, a different chain is selected (9).
The present data provide strong, although indirect, evidence that a similar organization of motor acts also exists in humans. In TD children, the muscles responsible for the action final goal increase their activity as soon as the action starts. When TD children moved the hand to reach the food (or another object) to bring it to the mouth, there was an increase of the EMG activity of the muscles involved in the mouth opening. This activation was lacking when the child grasped an object to place it into a container. A similar dissociation was found for hand/foot actions.
Another interesting result was found during the observation of actions done by others: when the experimenter grasped food to bringing it to the mouth, there was an immediate activation of the observer's MH muscle, that is, of the muscle that controls the last motor act of the chains. This activation was lacking during the observation of grasping-for-placing. The MH muscle activation provides strong evidence that, during the food-grasping observation condition, the entire grasp-to-eat motor chain became active from the action outset, thus allowing the observer to capture immediately the intention of the agent. Note that this forward activation of sequential motor acts is different from the feedforward mechanism proposed by some authors (e.g., 22) to account for some aspects of mirror neuron functions. According to these authors, a feedforward mechanism could help the observer to perceive the ongoing motor acts via a top–down activation of perceptual areas. The action-chain mechanism shown here is involved not in motor act perception but in the selection of impending motor acts for action execution as well as for understanding the intentions of others.
Overt effector-specific muscle activation during observation of action done by others was previously reported by Berger and Hadley (23) in a setting in which the observer was strongly empathic with the actor. Typically, however, the muscle activation during action observation remains subthreshold, requiring TMS to be demonstrated (24–27). The overt MH muscle activation found in the present work is most likely present because unlike TMS experiments, all run in adults, our experiments were done in children, who possess a less strong inhibitory control of the prefrontal lobe on the mirror system with respect to adults. This interpretation is in line with the commonly observed tendency of children to imitate others.
Action Representation in Children with Autism.
A behavior radically different from that of TD children was found in children with autism both during the execution and the observation of actions done by others. The most striking result was that during the execution of grasping to eat there was no activation of the MH muscle during the reaching and grasping phase. Its activation was found only during bringing to the mouth.
How can these finding be explained? It is obvious that when performing the two required actions, children with autism desired to achieve the requested goal. The problem is how they were able to perform them. The data show that, unlike the TD children, they were unable to organize their action using a forward mechanism. It is likely that, because the tested actions were extremely simple, they could be performed also using other strategies based, for example, on somatosensory and visual information.
The behavior of the MH muscle in children with autism during action observation was also clearly different with respect to TD children. The EMG of this muscle was flat during the observation of the bringing-to-the-mouth action, as during the observation of bringing an object into the container.
What does this absence of activation mean? Our interpretation is that the children with autism lack a full comprehension of the intention of others. This statement needs, however, specification. The term intention is frequently used to indicate the “goal” of a single motor act, that is “what one is doing” (grasping a cup of coffee) and not exclusively “why he is doing it” (for drinking, for placing, or even for throwing it). These two uses of the same term describe two different processes: one is the immediate comprehension of the observed goal-directed motor act, the other is the prediction of the final goal of the whole action. According to some authors, the “what” process does not require necessarily the understanding of the mental state of the agent (e.g., 28), whereas the “why” process should imply it.
There is evidence that children with autism are able to understand the what of a motor act (e.g., 29–31). Note however, that the what of the action can be understood in different ways. In addition to its comprehension caused by a direct matching mechanism (mirror mechanism), the object per se gives semantic cues on what are the actions typically done with it. Furthermore, a mere association between object and some motor acts can give clues to understand what the agent most likely will do. Thus, even if the mirror neuron system is impaired, the recognition of the what might remain intact.
More complex is the situation for the intention defined as the why of a motor action. As far as we know, the why-intention has never been studied in children with autism, mostly because the goal of the motor act was considered “tout-court” the agent intention. Clinical evidence suggests, however, that a deficit in understanding the why of the actions is present in children with autism. An example is the classical finding that although TD children respond to the arm extension toward them with a similar gesture, children with autism fail to do it not understanding the why of the mother's gesture (10).
It is likely, however, that also for the why-intention there could be cues, like those discussed above (semantics, context, habits) that may help an individual to understand why the others are doing what they are doing. However, this type of understanding should be clearly distinguished from that in which the individuals are able to understand the actions of others using their own intentional motor chains. The first type of understanding gives a merely associative, not experiential knowledge, whereas the other allows the individuals to comprehend experientially the intention of others as if that intention was his/her own (see 32). We propose that this deficit in understanding experientially the why of others' action is one of the reasons of the subsequent profound social disabilities characterizing children with autism.
Besides a large psychological literature stressing the communicative deficits in autism there is also a vast literature on anatomical abnormalities and functional impairments of motor system in this syndrome. Anatomical abnormalities have been reported in the cerebellum, the basal ganglia (see 33), and in various cortical structures including the parieto–frontal circuits (16, 34, 35). Functional motor impairments comprise dysfunction of the gait (36–38) as well as deficits in the anticipatory postural adjustment (39).
It is possible that intention comprehension and motor deficits are independent from each other. However, considering the recent evidence that the motor system plays an important role in action and intention understanding and communication (1, 40), it is plausible that damage to a common mechanism is the underlying reason of these deficits in autism. The present data provide evidence in favor of this hypothesis.
Methods
Participants.
Children with autism were recruited in a center of pediatric neuropsychiatry (Azienda Unità Sanitaria Locale 11, Empoli, Italy). The diagnosis had been made by means of the Autism Diagnostic Observation Schedule (ADOS) (41) and Autistic Diagnostic Interview Revised (ADI-R) (42). All participants had an IQ >70, as calculated with the Wechsler Intelligence Scale for Children Revised (WISC-R) (43) and Wechsler Preschool and Primary Scale of Intelligence (WPPSY) (44). Clinical information is shown in SI Table 4.
Recording Apparatus and EMG.
All muscles were recorded using surface electrodes (PG 10S; FIAB SpA, Firenze, Italy). For MH recording, the two electrodes were placed 5 cm apart under the subject's chin, symmetrically to the midline. The TA muscle was recorded with a standard bipolar belly tendon montage. EMG was recorded continuously throughout the experiment. The interval between trials was 20 s by default, but, according to the compliance of young subjects, it could be shortened up to 10 s. The signal was amplified 1,000×, sampled at 1 kHz by means of an analog-to-digital converter 1401 Micro Unit (CED, Cambridge, U.K.), controlled by the Signal software (CED), and stored for offline filtering (bandpass: 30–500 Hz) and further analysis.
Signal Processing.
Trials were discarded whenever the participant was not showing enough attention in the task or when other movements contaminated the recordings (e.g., speaking or swallowing), both online and offline with the help of video recordings (for the number of discarded trials, see SI Table 5). The EMG signal was rectified and averaged within each experimental condition (type of action) aligning all recordings on the moment of object or food lifting, as signaled by the touch-sensitive plate (time = 0). The data points of the averaged recordings were then adjacently averaged into bins of 100 ms. To allow comparison between participants, the individual mean EMG was normalized by dividing all data points by the maximum EMG activity during maximal contraction of the target muscle, recorded in a separate session.
Definition of Movement Epochs.
In all experiments, two touch-sensitive devices, the start button and the touch-sensitive plate, on which the food/object was placed, were linked to an electrical circuit. All trials started with the experimenter's hand resting on the start button. The release of the start button signaled the beginning of the reaching epoch (T1). The contact with the plate on which the object was put signaled the end of the reaching epoch and the start of the grasp epoch (T2). The release from the plate signaled the end of the grasp epoch and the start of the bringing movement (T3). Video recordings were used to assess the time at which the hand reached the final target of the action (mouth or container for experiments 1 and 2 and containers for experiment 3), defined as T4.
Statistical Analysis.
ANOVAs were carried out by using as dependent variable the mean EMG activity in the three epochs of action. First, the median value of the epochs calculated in single trials was obtained for each subject in each action. Then the mean EMG was calculated in each subject by measuring the area under the curve of the normalized mean EMG signal, obtained for each action as described above, in the time interval corresponding to the median value of the epochs. In all experiments, between-subjects ANOVAs for repeated measures were applied, structured with three factors: Group (two levels: TD children, children with autism), Action type (two levels: eating and placing), and Epoch of the action (three levels: reaching, grasping, and bringing). Post hoc analyses were made with Bonferroni-corrected multiple t tests. Significance levels were set at P = 0.05. A Greenhouse–Geisser (G-G) correction was applied whenever ANOVA assumptions were violated.
Supplementary Material
Acknowledgments
We thank our colleagues at the Department of Neuroscience, and in particular Fabian Chersi and Pier Francesco Ferrari, for comments on the manuscript. This work was supported by European Union Contract 012738, Neurocom, by Progetti Ricerca Interesse Nazionale (PRIN) 2004 and 2006 (to G.R.), by Fondo Investimenti Ricerca Base (FIRB) RBNE018ET9, and by the Fondazione Monte Parma (FMP). M.F-D. was supported by Fondazione Cassa di Risparmio di Ferrara and S.B. by FMP.
Abbreviations
- EMG
electromyographic/electromyogram
- IQ
intelligence quotient
- MH
mylohyoid
- TA
tibialis anterior
- TD
typically developing
- TMS
transcranial magnetic stimulation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706273104/DC1.
References
- 1.Rizzolatti G, Craighero L. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 2.Gallese V, Keysers C, Rizzolatti G. Trends Cogn Neurosci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishitani N, Hari R. Proc Natl Acad Sci USA. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 6.Nishitani N, Schürmann M, Amuts K, Hari R. Physiology. 2005;20:60–69. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- 7.Buccino G, Vogt S, Ritzl A, Fink G, Zilles K, Freund H, Rizzolatti G. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- 8.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 9.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Science. 2005;308:622–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 10.Kanner L. Nervous Child. 1943;2:217–250. [Google Scholar]
- 11.Baron-Cohen S, Leslie AM, Frith U. Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 12.Frith U. Autism: Explaining the Enigma. 2nd Ed. Oxford: Blackwell; 2003. [Google Scholar]
- 13.Williams JHG, Whiten A, Suddendorf T, Perrett DI. Neurosci Biobehav Rev. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran VS, Oberman LM. Sci Am. 2006;295:62–69. doi: 10.1038/scientificamerican1106-62. [DOI] [PubMed] [Google Scholar]
- 15.Gallese V. Brain Res. 2006;1079:15–24. doi: 10.1016/j.brainres.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 16.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Cereb Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- 17.Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthélémy C, Mouren MC, Artiges E, Samson Y, et al. NeuroImage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. Cognitive Brain Res. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Nishitani N, Avikainen S, Hari R. Ann Neurol. 2004;55:558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- 20.Theoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H. Pascual-Leone A Curr Biol. 2005;15:84–85. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson M, Knoblich G. Psychol Bull. 2005;131:460–473. doi: 10.1037/0033-2909.131.3.460. [DOI] [PubMed] [Google Scholar]
- 23.Berger SM, Hadley SW. Am J Psychol. 1975;88:263–276. [PubMed] [Google Scholar]
- 24.Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- 25.Gangitano M, Mottaghy FM, Pascual-Leone A. NeuroReport. 2001;12:1489–1492. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- 26.Aziz-Zadeh L, Maeda F, Zaidel E, Mazziotta J, Iacoboni M. Exp Brain Res. 2002;144:127–131. doi: 10.1007/s00221-002-1037-5. [DOI] [PubMed] [Google Scholar]
- 27.Borroni P, Montagna M, Cerri G, Baldissera F. Brain Res. 2005;1065:115–124. doi: 10.1016/j.brainres.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Pacherie E, Dokic Cognitive Systems Res. 2006;7:101–112. [Google Scholar]
- 29.Aldridge MA, Stillman RD, Bower TGR. Dev Sci. 2001;4:220–232. [Google Scholar]
- 30.Carpenter M, Pennington BF, Rogers SJ. J Autism Dev Disord. 2001;31:589–599. doi: 10.1023/a:1013251112392. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton AF, Brindley RM, Frith U. Neuropsychologia. 2007;45:1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Merleau-Ponty M. Phenomenology of Perception. London: Routledge; 1945. [Google Scholar]
- 33.Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain Res Bull. 2003;61:57–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Müller R-A, Kleinhans N, Kemmotsu N, Pierce K, Courchesne E. Am J Psychiatry. 2003;160:1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- 35.Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. NeuroImage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nayate A, Bradshaw J, Rinheart NJ. Brain Res Bull. 2005;67:327–334. doi: 10.1016/j.brainresbull.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Rinehart NJ, Tonge BJ, Iansek R, McGinley J, Brereton AV, Enticott PG, Bradshaw JL. Dev Med Child Neurol. 2006;48:819–824. doi: 10.1017/S0012162206001769. [DOI] [PubMed] [Google Scholar]
- 38.Teitelbaum P, Teitelbaum O, Nye J, Frayman J, Maurer RG. Proc Natl Acad Sci USA. 1998;95:13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz C, Barthelemy C, Assaiante C. Neurosci Lett. 2003;348:17–20. doi: 10.1016/s0304-3940(03)00644-x. [DOI] [PubMed] [Google Scholar]
- 40.Iacoboni M, Dapretto M. Nat Rev Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 41.Lord C, Rutter M, Di Lovore PC, Risi S. Autism Diagnostic Observation Schedule. Firenze: Organizazzioni Speciali; 2005. [Google Scholar]
- 42.Rutter M, Le Couteir A, Lord C. Autistic Diagnostic Interview Revised. Firenze: Organizazzioni Speciali; 2005. [Google Scholar]
- 43.Wechsler D. Wechsler Intelligence Scale for Children Revised. 3rd Ed. Firenze: Organizazzioni Speciali; 1995. [Google Scholar]
- 44.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Firenze: Organizazzioni Speciali; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.