Abstract
We examined pollen grains and starch granules from a large number of modern populations of teosinte (wild Zea spp.), maize (Zea mays L.), and closely related grasses in the genus Tripsacum to assess their strengths and weaknesses in studying the origins and early dispersals of maize in its Mesoamerican cradle of origin. We report new diagnostic criteria and question the accuracy of others used previously by investigators to identify ancient maize where its wild ancestor, teosinte, is native. Pollen grains from teosinte overlap in size with those of maize to a much greater degree than previously reported, making the differentiation of wild and domesticated maize in palynological studies difficult. There is presently no valid method for separating maize and teosinte pollen on a morphological basis. Starch grain analysis, a recently developed tool of archaeobotany, appears to be of significant utility in distinguishing the seeds of teosinte from maize. We propose that the differences in starch grain morphology and size between wild and domesticated maize defined in this study may be associated with domestication genes in Zea that have been documented in the starch biosynthesis pathway. As previously reported, phytoliths effectively discriminate the female reproductive structures of Tripsacum, teosinte, and maize. Multiproxy microfossil studies of archaeological and paleoecological contexts appear to be effective tools for investigating the earliest stages of maize domestication and dispersals.
Documenting the antiquity of maize domestication and early dispersals is a topic of intense interest to scholars from a number of disciplines. The wild ancestor of maize is a species of teosinte, Zea mays ssp. parviglumis, native to the Río Balsas watershed of tropical southwestern Mexico (1). There are three other species and two subspecies of teosinte with highland, midelevational, and lowland representatives spread widely from northern Mexico to western Nicaragua (2). Therefore, to study the history of maize in Mesoamerica the possible occurrence of teosinte must be taken into account and identification criteria must be applied to plant remains that can effectively discriminate wild from domesticated Zea. Both macrobotanical (cobs, kernels, etc., recoverable mainly from archaeological sites) and microbotanical approaches (pollen and phytoliths, retrievable from lakes and swamps in addition to archaeological contexts) have been used to identify maize in Mesoamerica (3–12). Macrofossil analysis is most effective in arid highland zones where plant remains of this type are well preserved. Palynological studies of lake cores can be more widely applied because, in contrast to macrofossils, pollen survives well in sediments of this type from humid, lower elevation environments (5–12). Phytoliths, microscopic pieces of silica formed in plant cells, remain well preserved in most archaeological and paleoecological settings over long periods of time. They have long been used to document ancient maize in the Americas (12–17), but they have been little applied in areas of Mesoamerica where wild Zea is native (6, 12).
A promising approach not yet applied to the question of maize domestication where wild maize is native is starch grain analysis. Starch grains, found in cellular organelles known as amyloplasts, are the major areas in which plants store their carbohydrates or energy. They occur in large numbers in storage organs such as seeds, roots, and rhizomes, and these types of grains, called reserve starches, occur in a diverse array of forms that can be diagnostic to the genus and even species (18–27). Archaeological applications in southern Central America and South America have shown that the grains survive for long periods of time on stone implements used to process plants, allowing various aspects of prehistoric agriculture, including maize, to be documented (15, 23–31). The utility of starch analysis for identifying maize in its geographic area of origin has not yet been investigated.
This paper examines the promise, potential importance, and pitfalls of distinguishing teosinte, maize, and the closest wild relatives of the genus Zea, members of the genus Tripsacum, by using pollen, starch grain, and phytolith analysis. The complementarity of these microfossils and major strengths and weaknesses of each are examined. Large modern reference collections are used to compare and contrast microfossil morphology and size in wild and domesticated species [see supporting information (SI) Materials and Methods].
Results
Identifying Maize and Teosinte by Using Pollen Grains.
Previous research has indicated that considerable overlap in mean and maximum pollen diameter as well as in a value called the axis/pore ratio (long axis length divided by the diameter of the pore present on the grains) occurs between teosinte and maize (11, 32). In samples mounted in silicone oil, the reported size range in teosinte was from ≈48–87 μm in length, with an average size varying from 56 to 79 μm. The results were based on a limited number of samples usually comprising a single to a few specimens of each species and subspecies. Balsas teosinte, the wild ancestor of maize, was especially poorly represented. Published reports indicate that modern maize pollen mounted in silicone oil can be as small as ≈58 μm to upwards of 120–130 μm in length (11, 32). In most traditional land races from Mexico studied, maximum pollen diameter did not exceed 100 μm, and in about half of them maximum diameter was 90 μm. The average size of Mexican maize pollen varies between ≈70 and 106 μm (11, 32, 33) (SI Table 4).
Direct comparisons of teosinte and maize pollen mounted in glycerine/glycerine jelly and silicone oil are few, but available data indicate size in the former may routinely exceed that in the latter by ≈10–30% (11). With relation to the utility of axis/to/pore ratios, available data indicate a considerable degree of overlap between teosinte and maize pollen, making the value of this attribute highly doubtful (31). With regard to the differentiation of Zea from the closely related genus Tripsacum, previous studies have shown that Tripsacum pollen is smaller and that morphological criteria are also effective for discriminating the two genera (5, 6, 32, 34). These points are discussed in more detail below.
Investigations of Pollen Size.
Based on the results just summarized, a convention arose among palynologists working in Mexico and other areas of Mesoamerica where teosinte is native (e.g., northeastern and northwestern Guatemala and now western Nicaragua) that when fossil pollen grains mounted in silicone oil have a long axis diameter >90 μm they can be identified as maize (7, 8, 35). Our data, based on the examination of a much larger number of teosinte samples than studied previously, indicate that for many teosinte varieties that grow over a geographically widespread area of Mesoamerica, this criterion is not secure (see SI Table 5 for passport information on these plants). When mounted in silicone oil, Balsas teosinte consistently has pollen grains that exceed 90 μm in 50-grain counts and some specimens of this species have grains >100 μm in 50-grain counts (Table 1 contains a summary of the data; SI Table 6 contains the pollen measurements from all of the samples studied). When additional 50- to 100-grain scans of the samples were undertaken, grains measuring between 102 and 108 μm were observed in three different Balsas populations collected in Guerrero and the Valle de Bravo, Michoacán. Balsas teosinte isn't the only example of overlap with maize in the 90- to 100-μm size range, and larger. Two different collections of Zea luxurians, one from Jutiapa, Guatemala, and the other from Nicaragua, have grains >90 μm in the first 50-grain scan and grains reaching maximum diameters of 102 and 108 μm, respectively, in the next 100-grain scan (Table 1; SI Table 6). In the first 50-grain scan, examples of Zea mexicana representing the races Chalco, Central Plateau, and Nobogame have pollen grains >90 μm, and in a specimen of Chalco, grains as large as 114-μm long were recorded. In Zea perennis, grains >90 μm were also observed. The current standard of identifying maize pollen on the basis of grains >90 μm in maximum diameter does not provide a valid diagnostic criterion. Zea mays ssp. huehuetenangensis is the only teosinte that conformed to expectations in having grains <90 μm, but only one collection was studied. The fact that such large grains were routinely recorded in the teosinte samples indicates there is a good probability the grains will enter fossil records. SI Fig. 2a contains a summary illustration of pollen size overlap in teosinte and maize.
Table 1.
Pollen size in microns in teosinte and Tripsacum
| Species | Mounting medium | Range | ×̄(Mean) | SD | n |
|---|---|---|---|---|---|
| Z. mays ssp. parviglumis | (S) | 60–104* | 75 | 6.1 | 400 |
| (G) | 48–109* | 85 | 8.1 | 400 | |
| Z. mays ssp. huehuetenangensis | (S) | 59–77 | 66 | 4.6 | 50 |
| (G) | 75–104 | 90 | 6.2 | 50 | |
| Z. mays ssp. mexicana | (S) | 53–114 | 73 | 6.9 | 350 |
| (G) | 48–137 | 96 | 10.3 | 350 | |
| Z. luxurians | (S) | 59–97* | 75 | 6.2 | 200 |
| (G) | 66–132 | 93 | 9.8 | 200 | |
| Z. perennis | (S) | 61–83* | 70 | 5.9 | 50 |
| (G) | 80–130 | 106 | 11.3 | 50 | |
| Tripsacum dactyloides | (S) | 35–67* | 49 | 3.7 | 250 |
| (G) | 47–77* | 63 | 4.0 | 250 | |
| T. lanceolatum | (S) | 27–64* | 42 | 6.1 | 150 |
| (G) | 34–78 | 53 | 6.4 | 150 | |
| T. latifolium | (S) | 28–50 | 38 | 2.0 | 150 |
| (G) | 35–70 | 50 | 4.9 | 150 | |
| T. maizar | (S) | 38–53* | 43 | 3.2 | 50 |
| (G) | 42–70 | 55 | 6.0 | 50 | |
| T. pilosum | (S) | 34–80* | 57 | 13.4 | 100 |
| (G) | 46–101 | 70 | 2.8 | 100 |
Fifty grains were measured from each specimen of each taxon studied. (S), silicone oil; (G), glycerine.
*Larger grains were observed in extended scanning of samples (see text). Grains as small as 48 μm in length were observed in Z. mexicana mounted in silicone.
As reported (32), the axis/pore ratio of pollen grains has little diagnostic value. The ratios of teosinte grains mounted in silicone oil demonstrate near-total overlap with maize in mean, minimum, and maximum values (SI Table 6). As previous studies have also indicated, pollen mounted in glycerine was prone to swelling, often making them considerably larger than grains mounted in silicone (Table 1, SI Table 6, and SI Fig. 2b). We commonly recorded increases of between 10% and 40% in mean and maximum size in glycerine samples; in some cases the increase approached or exceeded 50%. Grains reached a maximum length of >130 μm in Zea mexicana, Zea luxurians, and Zea perennis. Data are few on maize pollen size in glycerine, but it is likely that, as with silicone oil, a substantial degree of overlap would occur between teosinte and maize, making the differentiation of the two difficult. With regard to the possible discrimination of maize and teosinte by using morphological attributes, our studies of teosinte surface texture and subexine characteristics agree with those of others (32) in indicating there are no discernible differences between wild and domesticated Zea.
Separating Tripsacum from Teosinte and Maize by Using Pollen Size and Morphology.
The results of our analysis indicate that pollen of most species of Tripsacum is smaller than that of teosinte and maize (Table 1). With the exception of Tripsacum pilosum, pollen mounted in silicone oil did not exceed a maximum diameter of 69 μm (a grain of this size was observed in the extended 50-grain scan of one specimen of Tripsacum dactyloides) and a mean of 54 μm, similar to results reported from previous investigations. In T. pilosum, grains reached a maximum diameter of 82 μm (observed in the extended scan) and a mean of 66 μm, the largest reported for the genus. Tripsacum pollen was also larger in glycerine than in silicone oil. Very large grains 101-μm long were found in T. pilosum mounted in glycerine. This species, therefore, has to be taken into account when size is used to discriminate the genus from Zea (see SI Fig. 2 a and b for a summary illustration of size overlap when Tripsacum is compared with teosinte and maize).
A number of investigators have noted that significant morphological differences exist between pollen of Tripsacum and Zea (5, 32, 34, 36). Our results are in accord with these studies. Under both standard light and phase-contrast microscopy, the surface sculpturing of Tripsacum grains is coarsely scabrate to verrucate, whereas in maize and teosinte, the surface is granular to nearly smooth (psilate). Under phase contrast, structures called intertectile columellae beneath the exine of grains strongly tend to be clumped in all species of Tripsacum, whereas in Zea, they are more uniformly distributed, sometimes exhibiting a characteristic mottling (SI Fig. 3 a and b). Morphological criteria, therefore, appear to effectively discriminate the two genera.
The Utility of Starch Grains in Differentiating Maize, Teosinte, and Tripsacum.
Maize and other grass kernels produce substantial quantities of starch grains. They often have morphological characteristics unique to the Poaceae. Previous examinations of starch grain size and morphology in the northern U.S., southern Central America, and South America, where teosinte does not occur, indicate that maize can be distinguished from native wild grasses, allowing identification of maize in starch grain assemblages recovered from archaeological stone tools, pottery, and sediments (23, 28, 29, 31, 37). In maize, starch grains commonly ranged from ≈8 to 25 μm in maximum length (19, 20, 23, 28, 29). In most wild, non-Zea grasses studied by ourselves and others, grain size ranges from ≈3 to 11 μm in mean length and 2–18 μm in maximum length. In many species, including the putative early Mexican cultivar Setaria parvifolia (Poiert) (formerly S. geniculata) (38), maximum grain size reaches only 6–9 μm (refs. 23, 28, and D.R.P., unpublished data). A few grass species have starch grains as large as in maize, but in each case their morphological characteristics appear to distinguish them from maize (28, 29) (SI Fig. 4 a and b).
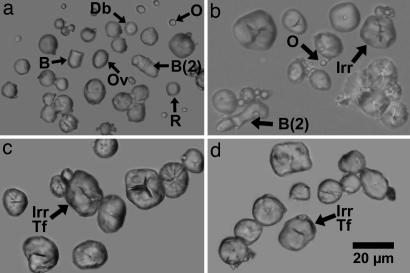
To apply starch-grain analysis to questions of maize domestication and spread in Mexico, detailed comparative data are needed on size and morphology in maize and wild Zea. We examined starch grains from most teosinte taxa and two species of Tripsacum (see Materials and Methods). They were compared with 12 races of Latin American maize, including 9 from Mexico (SI Table 5). Table 2 contains the size data generated from our analyses of teosinte. It should first be noted that starch content in Balsas and other teosintes is typically poor. There is far more oil than starch (e.g., in Balsas teosinte there are ≈50 oil droplets for every starch grain), and repeated sampling of teosinte seeds had to be done to achieve starch grain numbers adequate for analysis. These differences can be seen in Fig. 1 a–d; whereas in maize, each figure shows the starch occurring in a single (Reventador) or two (Bolita) high-power microscope fields, in teosinte, grains from seven (Chalco) to 10 (Balsas) microscope fields were combined to adequately illustrate the starch assemblage features. Starch grains in Balsas teosinte have a mean length of from 8.8 to 9.5 μm and a range of maximum length of from 4 to 18 μm. Other teosintes demonstrate similar size characteristics with the exception of a specimen of Zea luxurians that had a mean length of 11.9 μm, the largest recorded in our study. Individual grains with a maximum length >18 μm were observed only in Zea diploperennis and Z. mexicana (two specimens of Chalco and one of Central Plateau, where single grains measuring 22-μm (Chalco), 20-μm (Central Plateau), and 28-μm long (Chalco) were observed in extended scans of the microscope slides).
Table 2.
Starch grain size in microns in teosinte and maize
| Plant | ×̄ Length | Range | SD | n |
|---|---|---|---|---|
| Teosinte | ||||
| Balsas | ||||
| I81/Doebley | 9.5 | 6–18 | 2.1 | 350 |
| Ejutla-Cobian/Doebley | 8.9 | 4–18 | 1.8 | 350 |
| BK-site-1/Doebley | 8.8 | 4–14 | 1.7 | 350 |
| P1384063/Doebley | 9.1 | 4–14 | 1.7 | 150 |
| Huehuetenango | ||||
| G-120/Doebley | 9.5 | 6–18 | 1.7 | 300 |
| Central plateau | ||||
| Puga-11066/Doebley | 8.9 | 4–12 | 1.9 | 100 |
| Doebley-625 | 9.0 | 4–18 | 2.2 | 300 |
| NMNH-740005 | 6.5 | 2–20 | 4.4 | 50 |
| Nobogame | ||||
| Beadle-1974/Doebley | 9.8 | 4–20 | 1.4 | 300 |
| Chalco | ||||
| ID-401/Doebley | 10.0 | 4–14 | 2.0 | 100 |
| Doebley-479 | 10.7 | 6–22 | 1.6 | 300 |
| Doebley-481 | 10.6 | 4–16 | 2.1 | 300 |
| NMNH-2982425 | 5.0 | 2–28 | 4.2 | 50 |
| Z. luxurians | ||||
| G-5/Doebley | 10.2 | 6–14 | 2.3 | 50 |
| G-42/Doebley | 8.0 | 4–12 | 1.8 | 150 |
| G-38/Doebley | 9.8 | 6–18 | 1.8 | 300 |
| G-36/Doebley | 11.9 | 6–16 | 2.4 | 150 |
| NMNH-30919 | 5.2 | 3–15 | 2.9 | 50 |
| Z. diploperennis | ||||
| 1190/Doebley | 9.6 | 6–20 | 1.5 | 150 |
| Maize | ||||
| Argentine popcorn | 13.4 | 6–18 | 3.2 | 50 |
| Zapalote | 11.6 | 6–18 | 2.9 | 150 |
| Bolita | 11.1 | 6–20 | 1.5 | 150 |
| Confite Morocho | 14.4 | 6–22 | 3.4 | 100 |
| Maiz Ancho | 12.5 | 6–20 | 2.4 | 150 |
| Reventador | 15.3 | 6–24 | 2.7 | 150 |
| Nal-tel | 11.4 | 6–18 | 2.5 | 150 |
| Harinoso de Ocho | 13.5 | 6–22 | 2.1 | 150 |
| Jala | 15.8 | 6–26 | 4.9 | 50 |
| Tabloncillo | 12.6 | 6–20 | 3.2 | 150 |
| Pepitilla | 12.9 | 6–24 | 2.6 | 150 |
| Dzit Bacal | 12.9 | 6–20 | 2.4 | 150 |
Fig. 1.
Starch grains from teosinte and maize. (a) Starch grains from Zea mays ssp. parviglumis. It can be seen that in contrast to maize (c and d) the majority of grains are oval to round, not irregular, and bell-shaped grains are present. The tiny spheres are oil droplets. Letters next to grains indicate the following: B, bell-shaped; Db, with a double border on the edge; Ov, oval in shape; B (2), two bell-shaped grains joined together as they were formed in the amyloplast; R, round in shape; o, oil droplet. Most of the oil droplets that occurred with the starch grains were not included in the figure. (b) Starch grains from Zea mays ssp. mexicana (Chalco teosinte). This race of teosinte has a greater proportion of grains that are more like those in maize, but differences are still apparent in morphology when compared with maize. Irr, irregular in shape. Most of the oil droplets that occurred with the starch grains were not included in the figure. (c) Starch grains from the maize race Reventador. As is typical of maize, many grains are irregular, and oval, round, and bell-shaped grains are absent or nearly so. In this race, many grains also have transverse fissures. Irr, irregular in shape; Tf, transverse fissure. (d) Starch grains from the maize race Bolita.
Maize has a much higher starch content than teosinte, and starch grains in maize are consistently larger (Table 2). Mean size ranges from 11.1 to 15.8 μm and maximum length ranges from 4 to 26 μm. Most races have an average length of >12.5 μm, and individual grains commonly reach or exceed 20 μm in maximum length. Therefore, it appears that size can be of considerable utility for distinguishing maize and teosinte. We studied four plants from two different species of Tripsacum. Starch content was sometimes very low, and as in many wild grasses, grain size is considerably smaller than in maize (SI Table 7).
To investigate the utility of morphological characteristics, we developed a typology based on 22 different shape and surface features that we defined in wild and domesticated Zea (see SI Materials and Methods for details on how we chose the attributes). We examined each plant and scored starch assemblages for the presence and frequency of the various features. Table 3 provides a list of the traits that appear to demonstrate the greatest differences between teosinte and maize. SI Table 8 provides the same information on all of the teosinte and maize samples studied. As can be seen, oval grains are a significant component of teosinte assemblages, occurring most frequently in Balsas teosinte (Fig. 1a), but they were observed in low frequency in just one race of maize, Argentine popcorn. Round and distinctive kinds of elongated bell-shaped grains are considerably more common in teosinte, especially again in race Balsas, than in maize (Fig. 1 a and b). In contrast, grains called “irregular” constitute the dominant ≥83%) proportion of nearly every race of maize studied. These types have no definable shape, because they vary in form when they are rotated, and they have features such as rough and/or bulging surfaces with compression facets (Fig. 1 c and d). They occur in teosinte (Fig. 1b) but in lower proportions than they do in maize. Another significant difference between maize and teosinte is that of all the grains in each that exhibit compression facets, maize often has a high number of clearly “defined” (deep) ones, whereas in Balsas, Huehuetenango, Z. diploperennis, and Zea luxurians, most grains have compression facets that are less deep (they are called “slight” in Table 3) (Fig. 1 a–d). Another difference is that in Balsas teosinte much more often than in maize, grains have a continuous double border (Fig. 1a).
Table 3.
Starch grain characteristics in a representative sample of maize and teosinte
| Starch morphology | Teosinte |
Maize |
||||||
|---|---|---|---|---|---|---|---|---|
| Balsas | Chalco | Central plateau | Z. luxurians | Bolita | Reventador | Nal-tel | Tabloncillo | |
| Shape | ||||||||
| Round | 44 (32–55) | 33 (16–58) | 11 (2–20) | 13 | 5 | 1 | 14 | 3 |
| Oval | 15 (11–19) | 6 (2–10) | 12 (12–13) | 0 | 0 | 0 | 0 | 0 |
| Bell | 10 (2–16) | 3 (0–5) | 7 (5–8) | 4 | 1 | 3 | 0 | 0 |
| Irregular | 30 (27–33) | 58 (40–73) | 70 (61–78) | 83 | 94 | 95 | 86 | 97 |
| Hilum | ||||||||
| Cavity | 24 (15–33) | 12 (6–18) | 9 (9–10) | 11 | 12 | 1 | 7 | 5 |
| Compression facets | ||||||||
| Slight | 58 (54–63) | 47 (35–64) | 44 (42–45) | 48 | 48 | 5 | 50 | 17 |
| Defined | 29 (21–35) | 50 (36–63) | 56 (55–58) | 52 | 52 | 95 | 50 | 83 |
| Fissures | ||||||||
| Transverse | 16 (12–19) | 14 (12–18) | 20 (11–30) | 12 | 15 | 41 | 11 | 22 |
| Total with fissure | 38 (37– 40) | 47 (41–54) | 37 (25–48) | 25 | 25 | 55 | 31 | 31 |
Numbers are percentages of the different types of grains and surface features. Numbers in parentheses are the ranges for percentages of each attribute found in different populations studied.
Attributes such as the number and types of fissures present on grains do not generally appear to demonstrate significant differences when teosinte and maize are compared. An important exception is with a type of fissure called “transverse” that cuts across the greater part of the breadth of the grain (Fig. 1 c and d). More than half of the maize races studied exhibited higher percentages of grains with transverse fissures than were observed in Balsas and other teosintes. In the maize race Reventador, 41% of the grains had this kind of fissure. Very similar in wild and domesticated maize are characteristics of the hilum (the botanical center of the grain), such as whether it is located in a centric position and is closed or open. However, Balsas and Nobogame teosinte have a distinctive cavity at the hilum much more often than does maize.
Balsas teosinte consistently displays the greatest differences from maize in the characteristics that best distinguish teosinte and maize. Other kinds of teosinte generally have higher frequencies of irregular grains than Balsas. However, only in Nobogame and Z. luxurians do proportions of irregular grains (81% and 83%, respectively) come near levels of those found in maize, which are almost always ≥85%, and other features such as the presence of oval and bell-shaped grains and/or low proportions of grains with defined compression facets can serve to distinguish the teosintes from maize. Nonetheless, because single specimens were studied of Nobogame, Huehuetenango, and Z. diploperennis more work is needed before it can be stated with confidence that they can be distinguished from maize.
Race Balsas appears to be the least variable in this analysis when different populations of the same races are compared, whereas Chalco is the most variable. For example, the percentage of irregular grains and grains with defined compression facets in Chalco varied from 40% to 73% and 36–63%, respectively. It is noteworthy that Chalco primarily occurs in and on the edges of maize fields, and it is considered to be the teosinte race that hybridizes the most frequently with maize (2). Balsas teosinte grows apart from, and hybridizes infrequently with, maize (2). Possible effects on teosinte starch grain characteristics resulting from hybridization with maize remain to be studied in more detail.
Differences in starch-grain morphology were also observed among the races of maize studied (see also SI Figs. 5 and 6 for additional examples of starch grains from maize). Considerably more work is needed to robustly assess the potential of starch grain studies for identifying races or racial complexes. With regard to differentiating the genus Tripsacum, our studies indicate that size and morphological contrasts between Tripsacum and teosinte and maize starches are considerable (see SI Materials and Methods for details and SI Fig. 7). Tripsacum starch should not be confused with that of maize in the archaeological record.
In summary, our results indicate that morphological attributes of starch grains can be of significant utility in separating teosinte from maize in the archaeological record. Because in all maize races studied, there are grains that cannot be distinguished from teosinte, making such kinds of precise identifications will require adequate archaeological sample sizes (more than a few grains) and the analysis of starch grain assemblage characteristics. For example, with a representative sample size, the investigator can assess aspects such as: (i) whether irregular grains dominate the starch grain assemblage, (ii) whether oval and bell-shaped grains are absent or rare, (iii) whether grains with transverse fissures are common, and (iv) whether grains with defined compression facets are conspicuous in the assemblage. A positive answer to these questions would indicate maize presence. If grain size in these assemblages was larger than in teosinte (e.g., ≥12.5 μm mean length and 18 μm maximum length) an identification of maize would be supported on the basis of both morphology and size. Because starch grains in Balsas teosinte consistently show the greatest contrasts with maize, it appears that the use of starch grains to document the early history of maize in its postulated homeland, the Balsas River Valley, will be a productive endeavor.
Use of Phytoliths to Distinguish Maize, Teosinte, and Tripsacum.
In brief, a number of different investigators have studied and compared phytoliths from teosinte, maize, and Tripsacum. It has been shown that phytoliths formed in the glumes, rachids, and cupules of the fruitcases and cobs distinguish the three taxa and that different kinds of silica bodies formed in leaves separate maize from Tripsacum and maize from some teosintes, including race Balsas (for a detailed discussion and illustrations, see refs. 12 and 17). In this study, we examined fruitcases from 12 samples of three different Tripsacum species from Mexico and Guatemala (SI Table 5). As in previous studies, phytoliths diagnostic of the genus and that were clearly distinguishable from maize and teosinte were isolated from each specimen. The genetic locus teosinte glume architecture 1 (tga1) controls phytolith formation in Zea fruitcases and cobs (17, 39). Genetic control over phytolith formation in Tripsacum has not been investigated, but it is likely that a gene related to tga1 is involved.
Conclusions
We have found contrasting degrees of utility for the discrimination of microscopic remains of maize, teosinte, and Tripsacum in ancient sedimentary records. Starch grains and phytoliths appear to be more useful than pollen in discriminating wild from domesticated maize. This is not surprising because human selection pressure would have been directed primarily to the plant organs that were consumed to improve food quality, yield, and ease of food preparation. It is already known that a major domestication gene, teosinte glume architecture 1, underlies phytolith formation and morphology in wild and domesticated Zea, and is responsible for the considerable differences between them. Starch grain domestication genes affecting yield and other properties of the grains have recently been identified (40). As discussed below, it is reasonable to posit that these genes may also have exerted important effects on starch grain morphology and size.
The investigation of much larger samples of teosinte than studied previously enabled the establishment of new and more accurate pollen size parameters for teosinte. It is apparent that size standards commonly used for identifying maize pollen in Mexico and other regions of Mesoamerica are not reliable. There is a large zone of overlap in teosinte and maize when both the mean and range of pollen size are considered, and pollen >90 μm in maximum length, previously thought to be diagnostic of maize, is common in teosinte when mounted in silicone oil. Only maize pollen near the largest extreme of its size distribution can be securely identified when mounted in silicone oil. Individual pollen grains of this size, >110–115 μm in length, are rarely recovered from ancient contexts, not surprising in view of the fact that they are rare in modern traditional maize races. It should be mentioned again that grain length reached 114 μm in a sample of Chalco teosinte; thus, in some regions, pollen of even that size cannot be unequivocally identified as maize, at least not until more is known about the possible effects of maize/teosinte hybridization on pollen size.
It does not appear that pollen average length is a significantly stronger diagnostic marker, even assuming that sufficient numbers of the usually uncommon Zea grains can be recovered to construct a reliable average size. Mean length for pollen mounted in silicone oil in different populations of teosinte studied here ranged from 73 to 82 μm in Zea parviglumis, 63–79 μm in Z. mexicana, and 69–79 μm in Z. luxurians (SI Table 6). It was 70 μm in the single Zea perennis specimen studied. Mean pollen length in traditional maize varieties is commonly between 70 and 84 μm (SI Table 4) (11, 32); thus, the overlap is considerable. Mean sizes reported for early Mexican Zea pollen grains identified as maize are well within the range for teosinte (36). These caveats for identifications of grains mounted in silicone oil will probably also apply to grains mounted in glycerine and glycerine jelly.
The present-day geographical distribution of teosinte is fairly well understood, and the various species and subspecies of wild Zea often have disjunct and restricted distributions (2). For example, race Nobogame is found in one valley in Chihuahua state, and Z. diploperennis occurs in small numbers in a localized area of Jalisco state. Just a few clusters of Z. luxurians are known from small portions of southeastern Guatemala and western Nicaragua. No teosinte has been found in the Caribbean watershed of lowland Mesoamerica, the Isthmus of Tehuantepec, the Yucatan Peninsula, and northern Guatemala, and teosinte does not now occur below ≈400–500 m in the possibly crucial Central Balsas region because the climate is too hot there. The problem is that present-day biogeography and abundance may not be a good predictor of the past. For example, Z. luxurians once grew in southeastern Honduras where it now appears to be extinct (41). Possible differences in the past distribution of teosinte have to be taken into account, especially when sites dating to the Late Pleistocene and early Holocene (≈12,000 to 8,000 B.P.) are considered. This is the period during which human populations first intensively used teosinte and turned it into maize. Cooler than present Pleistocene temperatures resulted in 800–1,200 m downward shifts of vegetation throughout Mesoamerica (6, 42) and may well have caused Balsas and other teosintes (e.g., Chalco) to descend into lower lying regions and be widespread below 400–500 m above sea level, where they do not now occur. Our knowledge of vegetational reassortment after the ice age ended at 10,000 B.P. tells us that it probably would have taken a few thousand years for the plants to assume the elevational distributions and habitat preferences they are best adapted to now (6, 42). Furthermore, we know next to nothing about how intensive human modification of landscapes during the pre-Columbian era and after may have altered the natural abundance and distribution of teosinte.
These significant uncertainties relating to pollen records can be addressed by carrying out multiproxy microfossil studies. In paleoecological contexts, it will be important to have phytolith data, which should often enable investigators to assess whether teosinte or maize, or both, were present when Zea pollen is identified or not recovered (6, 12). Phytoliths can identify the remains of maize from both leaves and cobs, detect teosinte fruitcase remains, and rule out, or provide evidence consistent with, leaf phytolith decay from a number of teosinte races, including Balsas (6, 12, 17). As various researchers have noted, when signals of slash-and-burn cultivation are registered in pollen records by way of increases in early successional plant taxa and charcoal, the likelihood that associated Zea pollen represents maize becomes greater, especially if predisturbance horizons lack Zea grains (5). Interpretations like these will be stronger if complementary phytolith evidence indicating maize presence and human interference with vegetation is available (12).
It appears that phytoliths and starch grains acting as complementary sources of information will be of significant utility in archaeological records, where both of these microfossils are likely to occur. Judging from research conducted outside of Mesoamerica (15, 23, 26, 28–31) starch grains from maize kernels should be retrievable in good quantities from specialized stone tools that were used by early farmers to process plants and turn them into food. Also recoverable from stone tools and associated sediments are the diagnostic phytoliths from teosinte fruitcases and maize cobs (16, 17). The phytoliths derive from chaff that was still adhered to the kernels when they were removed from the cob and processed. Thus, phytolith and starch grain data from the same stone tool can provide mutually supporting information on two different parts of the same, consumed plant structure.
The finding in this study of significant morphological differences in starches from teosinte, maize, and Tripsacum is not surprising, given that investigators have long been aware that starch grain morphology can be diagnostic at low taxonomic levels and that archaeobotanists are documenting significant distinctions in the starch of other domesticated plants and their closely related wild species (25, 26). The factors underlying the differences between teosinte and maize in starch grain morphology and size are not well understood. However, it may be of considerable significance that starch grain domestication genes in maize that exert control over attributes such as pastiness and yield have been identified, and that the genes are thought to have been early targets of human selection during maize domestication and subsequent improvement (40, 43). Pastiness, in part a reflection of starch amylopectin qualities, has to do with the suitability for making porridge and tortillas; e.g., it is difficult, if not impossible, to make tortillas out of teosinte. It is possible that selection for increased yield also resulted in larger starch grains and that changes in amylopectin qualities (e.g., increased stickiness) led to the propensity of maize starch grains to develop deep compression facets when they are packed together in amyloplasts during their formation. The degree to which starch grain size and morphology in teosinte and maize reflect these and other genetic factors is being explored through analyses of starch grain attributes in hybrids made between maize and teosinte and their back-crosses that have been genotyped at the relevant loci.
Materials and Methods
To extract starch grains from the seeds of maize, teosinte, and Tripsacum, specimens were cut in half with a razor blade. The inside of the kernel was scraped, and the residue was mounted on a microscope slide in water and examined with polarized and unpolarized light at a magnification of ×400. Pollen and phytoliths were extracted from modern plant material by using standard techniques (17) (see also SI Materials and Methods). A factor that has sometimes limited the comparability of pollen results is that preparation methods in use have varying effects on pollen size. Pollen mounted in glycerine and glycerine jelly has a propensity to swell and become larger, whereas grains mounted in silicone oil retain their truer size characteristics (11, 32). To provide the broadest possible comparisons, we mounted pollen from each sample in both glycerine and silicone and measured the grains between 1 and 15 days after the slides were made.
Supplementary Material
Acknowledgments
Bruce Smith and two reviewers provided helpful comments on the article. This work was supported by the Smithsonian Tropical Research Institute and the Smithsonian National Museum of Natural History.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708736104/DC1.
References
- 1.Matsuoka Y, Vigouroux Y, Goodman MM, Sanchez J, Buckler E, Doebley J. Proc Natl Acad Sci USA. 2002;99:6080–6084. doi: 10.1073/pnas.052125199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukunaga K, Hill J, Vigouroux Y, Matsuoka Y, Sanchez JS, Liu K, Buckler ES, Doebley J. Genetics. 2005;169:2241–2255. doi: 10.1534/genetics.104.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benz B. Proc Natl Acad Sci USA. 2001;98:2104–2106. doi: 10.1073/pnas.98.4.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piperno DR, Flannery KV. Proc Natl Acad Sci USA. 2001;98:2101–2103. doi: 10.1073/pnas.98.4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope KO, Pohl MED, Jones JG, Lentz DL, von Nagy C, Vega FJ, Quitmyer IR. Science. 2001;292:1370–1373. doi: 10.1126/science.292.5520.1370. [DOI] [PubMed] [Google Scholar]
- 6.Piperno DR, Moreno JE, Iriarte J, Holst I, Lachniet M, Jones JG, Ranere AJ, Castanzo R. Proc Natl Acad Sci USA. 2007;104:11874–11881. doi: 10.1073/pnas.0703442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dull RA. Quat Res. 2004;61:159–167. [Google Scholar]
- 8.Dull RA. In: Histories of Maize. Staller J, Tykot R, Benz B, editors. San Diego: Elsevier; 2006. pp. 357–365. [Google Scholar]
- 9.Sluyter A. Glob Planet Change. 1997;14:127–146. [Google Scholar]
- 10.Goman M, Byrne R. Holocene. 1998;8:83–89. [Google Scholar]
- 11.Ludlow-Wiechers B, Alvarado JL, Aliphat M. Biotica. 1983;8:235–258. [Google Scholar]
- 12.Pohl M, Piperno DR, Pope KO, Jones JG. Proc Natl Acad Sci USA. 2006;104:6870–6875. doi: 10.1073/pnas.0701425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozarth SR. Plains Anthropol. 1993;38:279–286. [Google Scholar]
- 14.Boyd M, Surette C, Nicholson BA. J Arch Sci. 2006;33:1129–1140. [Google Scholar]
- 15.Iriarte J, Holst I, Marozzi O, Listopad C, Alonso E, Rinderknecht A, Montaña J. Nature. 2004;432:614–617. doi: 10.1038/nature02983. [DOI] [PubMed] [Google Scholar]
- 16.Pearsall DM, Chandler-Ezell K, Chandler-Ezell A. J Arch Sci. 2003;30:611–627. [Google Scholar]
- 17.Piperno DR. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists. Lanham, MD: AltaMira; 2006. [Google Scholar]
- 18.Whistler RL, Bemiller JN, Paschall EF. Starch:Chemistry and Technology. Orlando, FL: Academic; 1984. [Google Scholar]
- 19.Reichert ET. The Differentiation and Specificity of Starches in Relation to Genera, Species, etc. Washington, DC: Carnegie Institution of Washington; 1913. [Google Scholar]
- 20.Seidemann J. Stärke-Atlas. Berlin: Paul Parey; 1966. [Google Scholar]
- 21.Ugent D, Verdun M. Phytologia. 1983;53:351–363. [Google Scholar]
- 22.Ugent D, Pozorski S, Pozorski T. Econ Bot. 1986;40:78–102. [Google Scholar]
- 23.Zarillo S, Kooyman B. Am Antiquity. 2006;71:473–499. [Google Scholar]
- 24.Fullagar R, Field J, Denham T, Lentfer C. J Arch Sci. 2006;33:595–614. [Google Scholar]
- 25.Perry L, Dickau R, Zarrillo S, Holst I, Pearsall DM, Piperno DR, Berman MJ, Cooke RG, Rademaker K, Ranere AJ, et al. Science. 2007;315:986–988. doi: 10.1126/science.1136914. [DOI] [PubMed] [Google Scholar]
- 26.Piperno DR. In: Documenting Domestication. Zeder MA, Bradley DG, Emshwiller E, Smith BD, editors. Berkeley, CA: Univ of California Press; pp. 46–67. [Google Scholar]
- 27.Torrence R, Barton HJ. Ancient Starch Research. Walnut Creek, CA: Left Coast Press; 2006. [Google Scholar]
- 28.Piperno DR, Ranere AJ, Holst I, Hansell P. Nature. 2000;407:894–897. doi: 10.1038/35038055. [DOI] [PubMed] [Google Scholar]
- 29.Pearsall DM, Chandler-Ezell K, Zeidler JA. J Arch Sci. 2004;31:423–442. [Google Scholar]
- 30.Perry L, Sandweiss DH, Piperno DR, Rademaker K, Malpass MA, Umire A, de la Vera K. Nature. 2006;440:76–79. doi: 10.1038/nature04294. [DOI] [PubMed] [Google Scholar]
- 31.Dickau R, Ranere AJ, Cooke RG. Proc Natl Acad Sci USA. 2006;104:3651–3656. doi: 10.1073/pnas.0611605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitehead DR, Langham EJ. Bull Torrey Bot Club. 1965;92:7–20. [Google Scholar]
- 33.Baltazar BM, de Jesus Sanchez-Gonzalez J, de la Cruz-Larios L, Schoper JG. Theor Appl Genetics. 2005;110:519–526. doi: 10.1007/s00122-004-1859-6. [DOI] [PubMed] [Google Scholar]
- 34.Anchukaitis KJ, Horn SP. Paleogeogr Paleoclimatol Paleoecol. 2005;221:35–54. [Google Scholar]
- 35.Sluyter A. Palynology. 1997;21:35–39. [Google Scholar]
- 36.Sluyter A, Dominguez G. Proc Natl Acad Sci USA. 2006;103:1147–1151. doi: 10.1073/pnas.0510473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry L. J Arch Sci. 2004;31:1069–1081. [Google Scholar]
- 38.Austin DF. Econ Bot. 2006;60:143–158. [Google Scholar]
- 39.Dorweiler JE, Doebley J. Am J Bot. 1997;84:1313–1322. [PubMed] [Google Scholar]
- 40.Whitt SR, Wilson LM, Tenaillon MI, Gaut BS, Buckler ES., IV Proc Natl Acad Sci USA. 2002;99:12959–12962. doi: 10.1073/pnas.202476999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iltis HH. Econ Bot. 2000;54:7–42. [Google Scholar]
- 42.Metcalfe SE. Ann Mo Bot Gard. 2006;93:258–273. [Google Scholar]
- 43.Jaenicke V, Buckler ES, Smith BD, Gilbert MTP, Cooper A, Doebley J, Paabo S. Science. 2003;302:1206–1208. doi: 10.1126/science.1089056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.