Abstract
Biological nitrogen fixation, the conversion of atmospheric N2 to NH3, is an essential process in the global biogeochemical cycle of nitrogen that supports life on Earth. Most of the biological nitrogen fixation is catalyzed by the molybdenum nitrogenase, which contains at its active site one of the most complex metal cofactors known to date, the iron–molybdenum cofactor (FeMo-co). FeMo-co is composed of 7Fe, 9S, Mo, R-homocitrate, and one unidentified light atom. Here we demonstrate the complete in vitro synthesis of FeMo-co from Fe2+, S2−, MoO42−, and R-homocitrate using only purified Nif proteins. This synthesis provides direct biochemical support to the current model of FeMo-co biosynthesis. A minimal in vitro system, containing NifB, NifEN, and NifH proteins, together with Fe2+, S2−, MoO42−, R-homocitrate, S-adenosyl methionine, and Mg-ATP, is sufficient for the synthesis of FeMo-co and the activation of apo-dinitrogenase under anaerobic-reducing conditions. This in vitro system also provides a biochemical approach to further study the function of accessory proteins involved in nitrogenase maturation (as shown here for NifX and NafY). The significance of these findings in the understanding of the complete FeMo-co biosynthetic pathway and in the study of other complex Fe-S cluster biosyntheses is discussed.
Keywords: FeMo-co, nitrogen fixation, nif, Fe-S proteins
Biological nitrogen fixation, the conversion of atmospheric N2 to NH3, is an essential process of the global biogeochemical cycle of nitrogen that supports life on Earth (1). Biological nitrogen fixation provides an alternative solution to the heavy use of chemically synthesized nitrogen fertilizers and, thus, is important to achieve sustainable agricultural practices (2). The major part of biological nitrogen fixation is catalyzed by the molybdenum nitrogenase enzyme according to the following reaction: N2 + 8e− + 16 MgATP + 8 H+ → 2 NH3 + H2 + 16 MgADP + 16 Pi (3). Remarkably, this reaction produces H2 as a by-product and has regained attention over the last few years as a promising source of clean energy (4). The molybdenum nitrogenase has two component proteins: dinitrogenase (NifDK) and dinitrogenase reductase (NifH). Dinitrogenase carries at its active site one of the most complex biological metalloclusters known to date, the iron–molybdenum cofactor (FeMo-co), composed of seven Fe, nine S, one Mo, one R-homocitrate, and one light unidentified atom (5, 6).
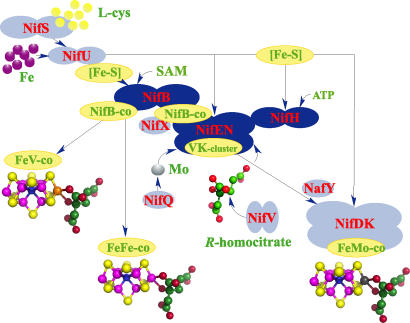
The systematic phenotypic analyses of Azotobacter vinelandii and Klebsiella pneumoniae strains with mutations in nitrogen fixation (nif) genes and the subsequent biochemical analyses have led to the identification of at least 11 genes (nifUS-BQ-ENX-V-H-Y and nafY) encoding proteins involved in FeMo-co biosynthesis and its insertion into a FeMo-co-deficient apo-NifDK protein (7–9). Fig. 1 depicts an integrative model of the current understanding of the pathway for FeMo-co biosynthesis. It is important to emphasize that this model is mostly based on the ability to isolate nif mutant strains. Several nif genes were shown to be essential for FeMo-co synthesis in vivo (e.g., nifB, nifU, nifS, nifH, nifN, nifE, and nifV), whereas others seemed to be required only under certain metabolic conditions (e.g., nifQ, nifX, nifY, and nafY).
Fig. 1.
Current model for FeMo-co biosynthesis and insertion into apo-NifDK. The model shows in dark blue the core components of the FeMo-co biosynthesis pathway: NifB, NifEN, and NifH (this work). Simple [Fe-S] clusters (most likely [2Fe-2S] and [4Fe-4S]), which can be chemically reconstituted on NifB, are converted into NifB-co precursor in a reaction that requires SAM and reducing conditions. Presumably, NifS and NifU would be responsible for the synthesis of those “NifB-co precursors” on NifB in vivo (8, 9). NifB-co is a common precursor for the biosynthesis of FeMo-co and the cofactors for the alternative nitrogenases, iron–vanadium cofactor and iron-only cofactor. NifEN binds NifB-co and converts it into the VK cluster. Incorporation of Mo and R-homocitrate into the VK cluster and final assembly of FeMo-co require NifH and MgATP. The resulting FeMo-co is inserted into apo-NifDK to activate it. Analogous reactions are hypothesized to occur during the maturation of alternative nitrogenases. The model shows in light blue the other Nif/Naf proteins known to participate in FeMo-co biosynthesis and insertion into apo-NifDK. NifS, NifU, NifQ, and NifV provide physiologically relevant forms of Fe, S, Mo, and R-homocitrate. NifX and NafY may assist NifB, NifEN, and NifDK on FeMo-co precursor trafficking among them and may serve as a reservoir of precursors giving some buffering capacity to the pathway.
The identification and isolation of in vivo synthesized FeMo-co precursors, such as NifB cofactor (NifB-co) and the VK cluster† (10–12), entailed very important progress and allowed the in vitro reconstitution of the late steps of the FeMo-co biosynthetic pathway in reactions that contained only purified Nif proteins, providing direct biochemical evidence to support those steps in the current model.
Much less is known about the early steps of the FeMo-co biosynthetic pathway, especially at the interface with the pathway for the biosynthesis of more simple [2Fe-2S] and [4Fe-4S] clusters, which involves the activities of the cysteine desulfurase NifS and the scaffolding protein NifU (13, 14). Pioneering studies on nitrogenase maturation by Dean and colleagues (reviewed in ref. 13) uncovered the existence of a complex cellular machinery involved in the assembly of ubiquitous [2Fe-2S] and [4Fe-4S] clusters that, at present, is recognized to be very similar for a diversity of Fe-S proteins in all organisms. It is generally accepted that the biosynthesis of the complex FeMo-co uses common [2Fe-2S] and/or [4Fe-4S] cluster as precursors, but support from direct biochemical evidence is currently lacking.
Genetic evidence has revealed the critical function of the nifB gene product for the in vivo accumulation of NifB-co and the VK cluster. The NifB protein has been recently purified and used to activate apo-NifDK when added to cell-free extracts of a NifB-deficient A. vinelandii mutant strain. This NifB-dependent in vitro activation of apo-NifDK required ferrous iron, sulfide, S-adenosyl methionine (SAM), molybdate, sodium dithionite (DTH), Mg-ATP, and R-homocitrate (15). It is still unknown whether other gene products (in combination with NifB) are required for the synthesis of NifB-co.
All currently known proteins that are critical for FeMo-co biosynthesis are available in purified form. This fact has given us the unprecedented opportunity to attempt to reconstitute in vitro the complete FeMo-co biosynthesis pathway. The present study establishes a minimal and composition-defined reaction mixture showing that FeMo-co can be synthesized in vitro from Fe, S, Mo, and R-homocitrate with only purified NifB, NifEN, and NifH proteins. This demonstration provides direct biochemical evidence to confirm the current model for FeMo-co biosynthesis and apo-NifDK activation.
Results
In Vitro Activation of apo-NifDK Using Purified Nif Proteins.
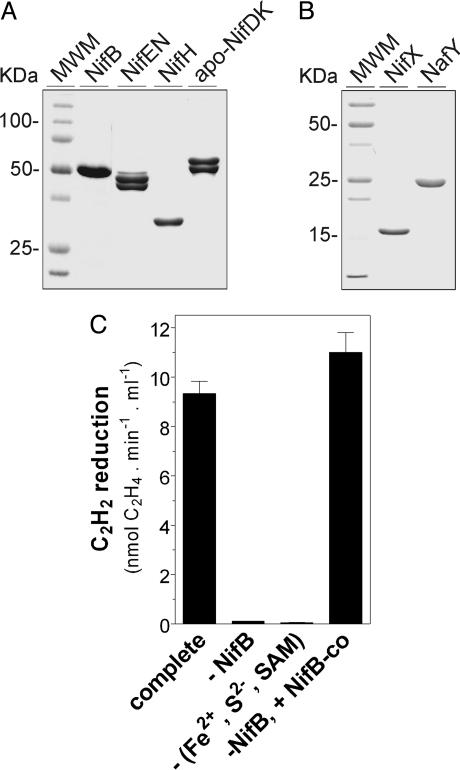
In the initial set of experiments, we preincubated NifB with a 50-fold molar excess of each (NH4)2Fe(SO4)2 (or FeSO4), Na2S, and SAM. Then, NifX, DTH, R-homocitrate, Na2MoO4, Mg-ATP, NifEN, NafY, NifH, and apo-NifDK were sequentially added. After allowing 35 min for apo-NifDK activation, we determined the activity of reconstituted NifDK by the acetylene reduction assay. As shown in Fig. 2C, this complete reaction resulted in the NifB- and (Fe2+, S2−, and SAM)-dependent activation of apo-NifDK. Remarkably, NifB, Fe2+, S2−, and SAM completely substituted for the requirement of NifB-co, the metabolic product of NifB activity, indicating that efficient synthesis of this FeMo-co precursor had been achieved in vitro. It is apparent that Fe2+, S2−, and SAM were spontaneously assembled on NifB to build precursors required for NifB-co biosynthesis. This assembly might be assisted by NifU and NifS in vivo. It was hypothesized that NifB-co would accumulate on the NifB protein in a mutant strain having the FeMo-co biosynthetic pathway interrupted at the level of NifEN (10). Although it is still possible that this is the case in vivo, our data indicate that NifB as purified from an A. vinelandii ΔnifHDK-TY-ENX strain does not contain bound NifB-co. Seven independent preparations of NifB purified from a ΔnifHDK-TY-ENX A. vinelandii strain and two independent preparations of NifB purified from A. vinelandii wild type showed minimal differences in Fe content, specific activity, requirement of Fe2+, S2, and SAM for activity, and UV-visible spectra (data not shown).
Fig. 2.
Purification of A. vinelandii Nif/Naf proteins and in vitro activation of apo-NifDK with purified Nif proteins. (A) SDS/PAGE analysis of purified NifB, NifEN, NifH, and apo-NifDK proteins on a 12% polyacrylamide gel. (B) SDS/PAGE analysis of purified NifX and NafY on a 14% polyacrylamide gel. (C) NifB-dependent in vitro activation of apo-NifDK. The complete reaction (NifB-dependent assay) contained NifB, NifEN, NifH, NifX, NafY, apo-NifDK, FeSO4, Na2S, SAM, Na2MoO4, R-homocitrate, Mg-ATP, and DTH. In the NifB-co-dependent assay, purified NifB-co substitutes for NifB, FeSO4, Na2S, and SAM. Data are the mean and standard deviation of two to four independent assays.
NifDK protein activated in vitro by the NifB-dependent assays also presented N2 to NH3 reduction activity, the physiological reaction catalyzed by nitrogenase. In vitro activated NifDK exhibited a ratio of specific activities for NH3 production to C2H4 production very similar to that of purified wild-type NifDK enzyme (Table 1). The fact that this ratio was remarkably different from that of the alternative nitrogenases, especially the Fe-only nitrogenase (ratio of 1.35) (16, 17), strongly supports that most of apo-NifDK activation was due to synthesis and insertion of FeMo-co.
Table 1.
Substrate-reducing activities of in vitro activated apo-NifDK
| Assay | NifDK specific activity, nmol·min−1·mg−1 |
Ratio | |
|---|---|---|---|
| C2H4 formed | NH3 formed | ||
| In vitro activated apo-NifDK* | 197 ± 25 | 64 ± 1 | 0.32 |
| NifDK (standard conditions)† | 1,800 ± 25 | 600 ± 30 | 0.33 |
| NifDK (in vitro activation conditions)‡ | 1,234 ± 80 | ND | ND |
ND, not determined.
*In vitro activated apo-NifDK using excess apo-NifDK (NifB-dependent reaction).
†Activities according to ref. 33.
‡NifDK, purified under the same conditions used for apo-NifDK, substitutes for apo-NifDK in the NifB-dependent reaction. These data provide an estimate of the maximum NifDK activity expected for 100% in vitro reconstituted apo-NifDK.
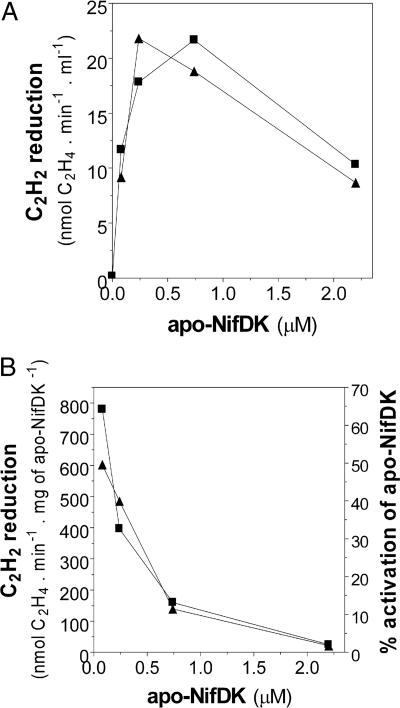
Addition of increasing amounts of apo-NifDK to the reaction mixture was carried out to estimate the percentage of apo-NifDK maturated in vitro. The activities obtained were compared with the specific activity of purified wild-type NifDK as measured in the NifB-dependent reaction conditions. The specific activity of NifDK in the current reaction conditions (1,234 ± 79 nmol C2H4 formed per min−1·mg−1 NifDK) was set to 100%. Addition of apo-NifDK was required for C2H2-reducing activity (Fig. 3A), ruling out any possibility of observing activity arising from sources other than reconstituted NifDK. Fig. 3B shows in vitro apo-NifDK to holo-NifDK conversions of at least 63% and 48% for the NifB-co- and NifB-dependent reactions, respectively, when the level of apo-NifDK was limiting the maximal C2H2 reduction activity of the assay. Saturating amounts of apo-NifDK (which did not result in maximal in vitro activated NifDK specific activity) allowed a higher absolute level of in vitro apo-NifDK activation (Fig. 3A) and were subsequently used for further analysis. The specific activity of NifB in these reactions was 0.5 mg of activated NifDK per h−1·mg−1 of NifB with an apparent Kcat of 0.23·h−1.
Fig. 3.
Effect of apo-NifDK concentration on the specific activity of reconstituted NifDK. Reactions were set essentially as those in Fig. 2C, but the concentration of apo-NifDK present in the assay varied from 0 to 2.4 μM. ▲, NifB-dependent reactions; ■, NifB-co-dependent reactions. (A) Plot of C2H2 reduction activity versus apo-NifDK concentration in the assay. (B) Specific activity of in vitro activated NifDK versus apo-NifDK concentration in the assay, as calculated from data in A. The percentage of apo-NifDK activated in the assay is indicated on the right axis. The specific activity of purified NifDK determined under the same reaction conditions (1,234 nmol C2H4 formed per min−1·mg−1) represents 100% activation.
The Minimal Defined System for in Vitro Activation of apo-NifDK.
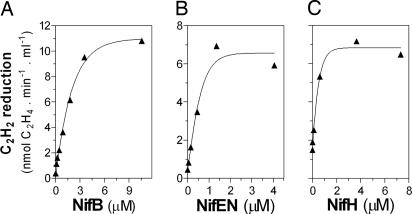
An interesting aspect of performing complete in vitro reconstitution of FeMo-co biosynthesis pathway using only purified proteins is the possibility of asking whether or not a particular protein component has a direct participation in the assembly of FeMo-co and which minimal combination of components is sufficient to achieve complete FeMo-co synthesis. Regarding the protein components, we observed two different groups. In one group, NifB, NifEN, and NifH‡ were essential to achieve complete in vitro FeMo-co synthesis (Fig. 4).
Fig. 4.
NifB, NifEN, and NifH are essential for in vitro activation of apo-NifDK. Shown is the effect of NifB, NifEN, or NifH concentration on in vitro apo-NifDK activation. The NifB-dependent assays were conducted in the absence of NifX and NafY (see Experimental Procedures) with the following modifications: in A the concentration of NifB in the assay was increased from 0 to 11 μM, in B the concentration of NifEN in the assay was increased from 0 to 4.5 μM, and in C the concentration of NifH in the assay was increased from 0 to 7.5 μM. In C, apo-NifDK activation was terminated by addition of 0.3 mM (NH4)2MoS4 before the addition of excess NifH for the determination of NifDK activity. The scale of activity axes in B and C are the same. Representative experiments are shown.
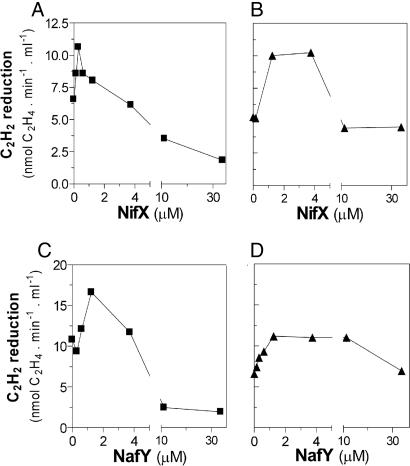
In the other group, the carrier proteins NifX and NafY were not essential for complete FeMo-co synthesis. The addition of either NifX or NafY individually to the in vitro FeMo-co synthesis assay produced a modest but consistent increment of 1.5- to 2.0-fold of apo-NifDK activation (Fig. 5 B and D). This modest stimulation is in line with the fact that mutant strains lacking NifX or NafY exhibit normal NifDK activities when grown diazotrophically under standard laboratory conditions (18). Further addition of NafY or NifX resulted in lower levels of apo-NifDK activation. NifX and NafY have been shown to bind FeMo-co precursors or FeMo-co, possibly providing protection to these clusters during cofactor biosynthesis (8, 9). Thus, the effect of excess NifX or NafY in the reactions, albeit not completely understood, can be explained by an in equilibrium precursor binding-delivery mode, as has been shown for the exchange of NifB-co and the VK cluster between NifX and NifEN (12, 19).
Fig. 5.
Effect of NifX and NafY on the in vitro activation of apo-NifDK. Shown is the effect of NifX (A and B) or NafY (C and D) in the NifB-co-dependent (A and C) or in the NifB-dependent (B and D) reactions. The NifB-co-dependent or NifB-dependent assays were supplemented with 0 to 30 μM NifX (A and B) or 0 to 30 μM NafY (C and D). A representative experiment is shown for each titration experiment.
The results presented in Fig. 5 suggest that NifX and NafY were not required for NifB-co synthesis but for its processing and/or trafficking during apo-NifDK activation. The addition of both NifX and NafY, at stimulating concentrations, produced an additive effect on the activation of apo-NifDK (22.2 ± 2.8 nmol C2H4 formed per min−1·ml−1) that is consistent with these proteins having nonredundant functions in FeMo-co biosynthesis. Besides binding FeMo-co, NafY has been shown to bind to apo-NifDK and to generate a hexameric α2β2γ2 apo-enzyme§ (20, 21).
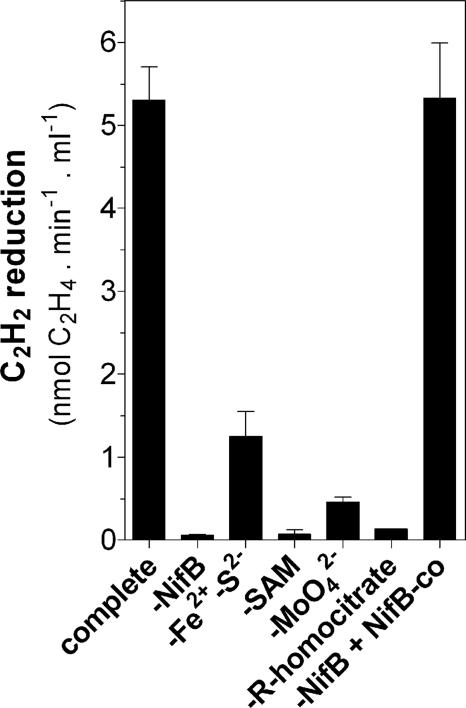
Fig. 6 shows substrate requirements for complete FeMo-co synthesis and activation of apo-NifDK in a mixture containing only NifB, NifEN, NifH, and apo-NifDK as protein components. NifB, Fe2+, S2−, and SAM were required in the complete reaction mixture but not in the NifB-co-dependent reaction, indicating that NifB-dependent in vitro NifB-co synthesis was taking place. The requirement for R-homocitrate and especially for molybdate (Fig. 6) indicates that most of the observed activation of apo-NifDK was due to the complete synthesis of FeMo-co, which is in line with the ratio of specific N2 and C2H2 reduction activities exhibited by the in vitro activated apo-NifDK (see Table 1). Thus, the minimal system for the complete in vitro FeMo-co synthesis and activation of apo-NifDK can be defined as having the following components: NifB, NifEN, and NifH proteins plus Fe2+, S2−, SAM, Mg-ATP, DTH, R-homocitrate, and molybdate.
Fig. 6.
Substrate requirements for in vitro activation of apo-NifDK. FeMo-co synthesis and apo-NifDK activation reactions (containing NifB, NifEN, NifH, and apo-NifDK as protein components) were conducted in the absence of one or more substrates at a time. A reaction containing all substrates (complete reaction) and a NifB-co-dependent reaction for FeMo-co synthesis and apo-NifDK activation were carried out as controls. Acid-washed vials were used to show the Mo dependency of the reaction. Data are the average and standard deviation of two to four independent determinations.
FeMo-co synthesis and apo-NifDK activation were achieved in vitro by using as substrates inorganic Fe2+ and S2− or purified FeMo-co precursors generated in vivo. Apo-NifDK to holo-NifDK conversions were 48% when starting synthesis from inorganic Fe2+ and S2− (Fig. 3B), 63% starting from purified NifB-co (Fig. 3B), and 89% starting from purified NifEN/VK cluster complex (22). These data permit us to indirectly estimate the efficiency of precursor synthesis during the individual steps of in vitro FeMo-co synthesis, which are the equivalent to (i) an amount of NifB-co (synthesized from Fe2+ and S2−) that activates at least 76% of apo-NifDK in the assay and (ii) the equivalent to an amount of VK cluster (synthesized from NifB-co) that activates at least 71% of apo-NifDK in the assay [see supporting information (SI) Fig. 7].
It should be noted that no attempt to search for or to include a likely source for the central atom of FeMo-co was made during this work. If we assume that the presence in FeMo-co of a central atom is essential to the acetylene- and N2-reducing activities of NifDK, then the source of this atom must be found within the components of the reaction mixture described in this study.
Discussion
The purification of most of the proteins known to participate in FeMo-co biosynthesis provided an unprecedented opportunity to obtain direct biochemical evidence to support and extend the current working model for FeMo-co biosynthesis and apo-NifDK activation. In this work we report in vitro activation of 48% of apo-NifDK present in a composition-defined reaction mixture containing pure NifB, NifX, NifEN, NafY, NifH, and apo-NifDK as protein factors, along with Na2MoO4, R-homocitrate, (NH4)2Fe(SO4)2, Na2S, SAM, Mg-ATP, and DTH (Fig. 3). Because no carry-over of in vivo-generated precursors bound to protein factors was evident through this work, these results indicate that all relevant chemistry essential for the assembly of FeMo-co and its insertion into apo-NifDK takes place among these components.
NifB, NifEN, and NifH are shown to be essential and sufficient for the conversion of Fe2+, S2−, molybdate, and R-homocitrate into FeMo-co in a complex reaction that also requires Mg-ATP, SAM, and DTH (Fig. 6). The essentiality of NifB, NifEN, and NifH (compared with the accessory character of NafY and NifX) was not surprising because it matches previous genetic evidence obtained from mutating the corresponding genes in A. vinelandii and other diazotrophs (7, 8). However, it is remarkable because it shows that the reconstitution in vitro of this complex metabolic pathway roughly mimics the pathway occurring in vivo.
Although the genetic–biochemical approach has been powerful in identifying key nif gene products and their arrangement in the pathway of FeMo-co biosynthesis, it did not rule out the possibility that other nif or non-nif gene products were also required for this trait. This report gives strong support to the current genetic–biochemical model for FeMo-co biosynthesis and indicates that no yet-unidentified protein or group of proteins has essential direct participation in FeMo-co assembly in vitro.
The demonstration that NifB, NifEN, and NifH are sufficient for a significant activation of apo-NifDK from Fe2+, S2−, molybdate, and R-homocitrate (13% activation) strongly suggests that within a complex multiprotein machinery for the biosynthesis of FeMo-co there is a catalytic core composed of NifB, NifEN, and NifH that might be responsible for all of the critical biochemical transformations of Fe, S, Mo, and R-homocitrate that lead to mature FeMo-co (Fig. 1). Other proteins (minimally NifU, NifS, NifX, NafY, NifQ, and NifV) might provide the physiological substrates and accessory support to the catalytic core. Because FeMo-co and its precursors are extremely unstable and sensitive to oxidation (10, 18), the in vitro activation of apo-NifDK in the absence of carrier proteins suggests that NifB, NifEN, NifH, and apo-NifDK might be in close proximity or even forming a multiprotein complex during the synthesis of FeMo-co. There is evidence that the A. vinelandii NifEN protein forms a stable complex with the L127Δ variant of NifH (23). It is also known that the genes encoding NifB and NifN are fused in a single ORF in several nitrogen-fixing Clostridium strains (24). Although these observations point to the existence of a multiprotein complex composed of NifB, NifEN, and NifH, copurification experiments performed during the course of this work have not provided any evidence of its existence (data not shown).
The proposal of a minimal catalytic core for the assembly of FeMo-co does not underestimate the function of the accessory proteins. As discussed above, an efficiency of 48% in the in vitro conversion of apo-NifDK to NifDK indicates that no critical factor is missing. However, the estimated apparent Kcat for NifB-dependent in vitro activation of apo-NifDK (0.23·h−1) is unlikely to reflect the in vivo efficiency of the pathway. This stimulates new research to integrate more accessory proteins into the in vitro reconstituted pathway. As demonstrated here for NifX and NafY, the in vitro reconstitution of the catalytic core of the pathway represents a platform onto which the activity of other Nif proteins can be analyzed in vitro as a complementary and powerful tool to confirm and refine current knowledge on FeMo-co biosynthesis.
It is expected that the availability of a minimal defined reaction for the in vitro synthesis of FeMo-co from Fe2+, S2−, Mo, and R-homocitrate would enormously facilitate and focus future research to advance in the structural and mechanistic aspects of the pathway, especially those involved in the early steps of FeMo-co assembly, which are shared by the alternative nitrogenase cofactor biosynthetic pathways.
Recent progress has been made in understanding the assembly of another heavily studied complex [Fe-S] cluster, the H-cluster located at the active site of the [FeFe] hydrogenase. In vitro biochemical complementation of Escherichia coli cell-free extracts expressing either apo-HydA or the accessory proteins HydE, HydF, and HydG led to reconstitution of hydrogenase activity (25). Despite the structural differences between FeMo-co and the H-cluster (26), it is apparent that their biosynthetic pathways share some common features, including the participation of specific SAM radical proteins and nucleotide-hydrolyzing enzymes (27). Thus, new insights in one of the pathways might help advancing in the other as well. For the longer term, understanding how complex [Fe-S] are biochemically assembled not only will complement the knowledge on the ecologically and geochemically relevant reactions that are catalyzed by these cofactors, but also has the potential to constitute the basis for the bio-inspired design of potent catalyst for the industrial production of H2 and other commodities.
Experimental Procedures
In Vitro FeMo-co Synthesis and Activation of apo-NifDK.
NifB, NifEN, NifH, apo-NifDK, NifX, and NafY protein preparations were at least 95% pure as analyzed by SDS/PAGE (Fig. 2 A and B). In the in vitro FeMo-co biosynthesis system, the addition of R-homocitrate and Na2MoO4 substitutes for NifV and NifQ activities, respectively (10). Thus, the requirements for NifQ and NifV were not tested in this work.
The complete NifB-dependent FeMo-co synthesis and apo-NifDK activation assay contained 3.0 μM NifB, 3.0 μM NifX, 1.5 μM NifEN, 1.2 μM NafY, 3.0 μM NifH, and 0.6 μM apo-NifDK in 22 mM Tris·HCl buffer (pH 7.5), 3.5% glycerol, 7.5 μM Na2MoO4, 0.175 mM R-homocitrate, 0.125 mM FeSO4, 0.125 mM Na2S, 0.125 mM SAM, 3 mM Na2S2O4, and an ATP-regenerating mixture (1.23 mM ATP, 18 mM phosphocreatine, 2.2 mM MgCl2, 3 mM Na2S2O4, and 40 μg of creatine phosphokinase). For the experiments shown in Figs. 4 A and C and 5 B and D, 5 mg·ml−1 BSA, 0.42 mM (NH4)2Fe(SO4)2, 0.42 mM Na2S, and 0.88 mM SAM were used with no observable change in apo-NifDK activation.
The NifB-co-dependent FeMo-co synthesis and apo-NifDK activation assay contained NifB-co (8.5 μM Fe), 1.5 μM NifEN, 3.0 μM NifH, and 0.6 μM apo-NifDK in 22 mM Tris·HCl buffer (pH 7.5), 3.5% glycerol, 7.5 μM Na2MoO4, 0.175 mM R-homocitrate, 3 mM Na2S2O4, and an ATP-regenerating mixture (1.23 mM ATP, 18 mM phosphocreatine, 2.2 mM MgCl2, and 40 μg of creatine phosphokinase). For the experiments shown in Fig. 5 A and C, 5 mg·ml−1 BSA was included in the reaction mixture.
Reactions were carried out at two different scales in total volumes of 200 μl or 600 μl inside 3.6-ml or 9-ml stoppered vials, respectively, for 35 min at 30°C under an argon atmosphere to allow for the FeMo-co synthesis and insertion reactions. Extensive vacuum and gas exchange was performed to saturate the mixture with either argon or nitrogen. No difference in activation was observed in either case. The resulting activation of apo-NifDK present in the reaction mixtures was routinely analyzed by the acetylene reduction assay after addition of excess NifH and ATP-regenerating mixture (28).
Details.
Additional methods are detailed in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Susan O'Connor for excellent technical assistance; Mary Wildermuth and Marc Strawn for assistance with high-performance liquid chromatography; Dennis Dean (Virginia Tech, Blacksburg, VA) for providing A. vinelandii strains DJ884, DJ995, and DJ1143; Juan Imperial for stimulating discussions; and Paul Ludden for discussions and support. This work was supported by National Institute of General Medical Sciences/National Institutes of Health Grant GM-35332 (to Paul Ludden).
Abbreviations
- FeMo-co
iron–molybdenum cofactor
- NifB-co
NifB cofactor
- DTH
sodium dithionite
- SAM
S-adenosyl methionine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
NifB-co, the metabolic product of NifB activity, is an isolatable [Fe-S] cluster of unknown structure that serves as precursor to FeMo-co. NifB-co has also been proposed to be a precursor of the iron–vanadium and iron-only cofactors of the alternative nitrogenases (29). The VK cluster is a precursor to FeMo-co that accumulates on NifEN in a ΔnifH mutant strain and is thought to represent an intermediate after NifB-co in FeMo-co synthesis.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703050104/DC1.
We interpret the background C2H2-reducing activity at zero addition of NifH in Fig. 4 as the result of incomplete inhibition of apo-NifDK by tetrathiomolybdate. Under optimal inhibitory conditions, a residual 7% of activatable apo-NifDK remains in the assay (30). The fraction of apo-NifDK that remains competent for FeMo-co insertion is activated by FeMo-co synthesized during the phase of NifDK activity determination. In this particular case, all components for FeMo-co synthesis (including NifH) are present in this phase of the activity assay.
References
- 1.Falkowski PG. Nature. 1997;387:272–275. [Google Scholar]
- 2.Oldroyd GE. Science. 2007;315:52–53. doi: 10.1126/science.1137588. [DOI] [PubMed] [Google Scholar]
- 3.Igarashi RY, Seefeldt LC. Crit Rev Biochem Mol Biol. 2003;38:351–384. doi: 10.1080/10409230391036766. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai H, Masukawa H. Mar Biotechnol. 2007;9:128–145. doi: 10.1007/s10126-006-6073-x. [DOI] [PubMed] [Google Scholar]
- 5.Chan MK, Kim J, Rees DC. Science. 1993;260:792–794. doi: 10.1126/science.8484118. [DOI] [PubMed] [Google Scholar]
- 6.Einsle O, Tezcan FA, Andrade SL, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 7.Ludden PW, Rangaraj P, Rubio LM. In: Catalysts for Nitrogen Fixation: Nitrogenases, Relevant Chemical Models, and Commercial Processes. Smith BE, Richards RL, Newton WE, editors. The Netherlands: Kluwer Academic, Dordrecht; 2004. pp. 219–253. [Google Scholar]
- 8.Dos Santos PC, Dean DR, Hu Y, Ribbe MW. Chem Rev. 2004;104:1159–1174. doi: 10.1021/cr020608l. [DOI] [PubMed] [Google Scholar]
- 9.Rubio LM, Ludden PW. J Bacteriol. 2005;187:405–414. doi: 10.1128/JB.187.2.405-414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah VK, Allen JR, Spangler NJ, Ludden PW. J Biol Chem. 1994;269:1154–1158. [PubMed] [Google Scholar]
- 11.Hu Y, Fay AW, Ribbe MW. Proc Natl Acad Sci USA. 2005;102:3236–3241. doi: 10.1073/pnas.0409201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez JA, Igarashi RY, Soboh B, Curatti L, Dean DR, Ludden PW, Rubio LM. Mol Microbiol. 2007;63:177–192. doi: 10.1111/j.1365-2958.2006.05514.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DC, Dean DR, Smith AD, Johnson MK. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 14.Lill R, Muhlenhoff U. Annu Rev Cell Dev Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- 15.Curatti L, Ludden PW, Rubio LM. Proc Natl Acad Sci USA. 2006;103:5297–5301. doi: 10.1073/pnas.0601115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider K, Gollan U, Drottboom M, Selsemeier-Voigt S, Muller A. Eur J Biochem. 1997;244:789–800. doi: 10.1111/j.1432-1033.1997.t01-1-00789.x. [DOI] [PubMed] [Google Scholar]
- 17.Chisnell JR, Premakumar R, Bishop PE. J Bacteriol. 1988;170:27–33. doi: 10.1128/jb.170.1.27-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah VK, Brill WJ. Proc Natl Acad Sci USA. 1977;74:3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah VK, Rangaraj P, Chatterjee R, Allen RM, Roll JT, Roberts GP, Ludden PW. J Bacteriol. 1999;181:2797–2801. doi: 10.1128/jb.181.9.2797-2801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paustian TD, Shah VK, Roberts GP. Biochemistry. 1990;29:3515–3522. doi: 10.1021/bi00466a014. [DOI] [PubMed] [Google Scholar]
- 21.Rubio LM, Rangaraj P, Homer MJ, Roberts GP, Ludden PW. J Biol Chem. 2002;277:14299–14305. doi: 10.1074/jbc.M107289200. [DOI] [PubMed] [Google Scholar]
- 22.Soboh B, Igarashi RY, Hernandez JA, Rubio LM. J Biol Chem. 2006;281:36701–36709. doi: 10.1074/jbc.M606820200. [DOI] [PubMed] [Google Scholar]
- 23.Rangaraj P, Ryle MJ, Lanzilotta WN, Goodwin PJ, Dean DR, Shah VK, Ludden PW. J Biol Chem. 1999;274:29413–29419. doi: 10.1074/jbc.274.41.29413. [DOI] [PubMed] [Google Scholar]
- 24.Chen JS, Toth J, Kasap M. J Ind Microbiol Biotechnol. 2001;27:281–286. doi: 10.1038/sj.jim.7000083. [DOI] [PubMed] [Google Scholar]
- 25.McGlynn SE, Ruebush SS, Naumov A, Nagy LE, Dubini A, King PW, Broderick JB, Posewitz MC, Peters JW. J Biol Inorg Chem. 2007;12:443–447. doi: 10.1007/s00775-007-0224-z. [DOI] [PubMed] [Google Scholar]
- 26.Drennan CL, Peters JW. Curr Opin Struct Biol. 2003;13:220–226. doi: 10.1016/s0959-440x(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 27.Posewitz MC, King PW, Smolinski SL, Zhang L, Seibert M, Ghirardi ML. J Biol Chem. 2004;279:25711–25720. doi: 10.1074/jbc.M403206200. [DOI] [PubMed] [Google Scholar]
- 28.Shah VK, Brill WJ. Biochim Biophys Acta. 1973;305:445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- 29.Bishop PE, Joerger RD. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:109–125. [Google Scholar]
- 30.Shah VK, Ugalde RA, Imperial J, Brill WJ. J Biol Chem. 1985;260:3891–3894. [PubMed] [Google Scholar]
- 31.Christiansen J, Goodwin PJ, Lanzilotta WN, Seefeldt LC, Dean DR. Biochemistry. 1998;37:12611–12623. doi: 10.1021/bi981165b. [DOI] [PubMed] [Google Scholar]
- 32.Schmid B, Ribbe MW, Einsle O, Yoshida M, Thomas LM, Dean DR, Rees DC, Burgess BK. Science. 2002;296:352–356. doi: 10.1126/science.1070010. [DOI] [PubMed] [Google Scholar]
- 33.Barney BM, Igarashi RY, Dos Santos PC, Dean DR, Seefeldt LC. J Biol Chem. 2004;279:53621–53624. doi: 10.1074/jbc.M410247200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.