Abstract
At birth, the transition to gas breathing requires the function of endothelial vasoactive agents. We investigated the function of endothelial nitric oxide synthase (eNOS) in pulmonary artery (PA) vessels and endothelial cells isolated from fetal and young (4-wk) sheep. We found greater relaxations to the NOS activator A-23187 in 4-wk-old compared with fetal vessels and that the NOS inhibitor nitro-l-arginine blocked relaxations in both groups. Relaxations in 4-wk vessels were not blocked by an inhibitor of soluble guanylate cyclase, 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one, but were partially blocked by catalase. We therefore hypothesized that activation of eNOS produced reactive oxygen species in 4-wk but not fetal PA. To address this question, we studied NO and superoxide production by endothelial cells at baseline and following NOS stimulation with A-23187, VEGF, and laminar shear stress. Stimulation of NOS induced phosphorylation at serine 1177, and this event correlated with an increase in NO production in both ages. Upon stimulation of eNOS, fetal PA endothelial cells (PAEC) produced only NO. In contrast 4-wk-old PAEC produced superoxide in addition to NO. Superoxide production was blocked by l-NAME but not by apocynin (an NADPH oxidase inhibitor). l-Arginine increased NO production in both cell types but did not block superoxide production. Heat shock protein 90/eNOS association increased upon stimulation and did not change with developmental age. Cellular levels of total and reduced biopterin were higher in fetal vs. 4-wk cells. Sepiapterin [a tetrahydrobiopterin (BH4) precursor] increased basal and stimulated NO levels and completely blocked superoxide production. We conclude that the normal function of eNOS becomes uncoupled after birth, leading to a developmental adaptation of the pulmonary vascular system to produce oxygen species other than NO. We speculate this may be related to cellular production and/or maintenance of BH4 levels.
Keywords: endothelial nitric oxide synthase, nitric oxide, superoxide, pulmonary artery, development
The pulmonary circulation in the near-term fetus is characterized by high pulmonary vascular resistance (PVR) and reduced responsiveness to endothelium-dependent vasodilators. After birth, with initiation of ventilation of the lungs and an increase in alveolar oxygen tension, PVR decreases and pulmonary blood flow increases 8- to 10-fold (1, 7, 15, 28). PVR continues to decrease over the first several months of life until it reaches the low levels normally found in the adult circulation. Recent evidence suggests that the decrease in PVR is regulated by both mechanical and metabolic factors. Among the metabolic factors, reactive oxygen species (ROS), including nitric oxide (NO), have been demonstrated to play a major role in the regulation of pulmonary vascular tone (10). NO is produced from the oxidation of l-arginine to l-citrulline by NO synthase (NOS). Inhibition of NO synthesis increases PVR in late-gestation fetal lambs (1), whereas inhaled NO has the opposite effect (18).
Despite many studies on endothelial nitric oxide synthase (eNOS) activity and NO production, the normal developmental changes in eNOS expression and function in the pulmonary circulation remain incompletely understood. Lung eNOS mRNA and protein expression increase in late gestation and then decrease postnatally in sheep lung parenchyma (13, 17, 25). Similarly, others have reported that endothelium-dependent cGMP production and relaxations increase in the first several weeks of life (22, 26, 31). Therefore, the maturational increases in NO production and eNOS expression appear to parallel the decrease in PVR that occurs during postnatal life, suggesting that the developmental regulation of eNOS gene expression and function plays a key role in changes in pulmonary vascular tone observed after birth.
Recent studies have demonstrated that other mediators, such as endothelium-derived hyperpolarizing factors (EDHFs), are also important in endothelium-dependent vasodilation. Various candidates have been identified as potential EDHFs. For instance, endogenously produced hydrogen peroxide (H2O2) induces hyperpolarization of smooth muscle cells and vasorelaxation of small mesenteric arteries in the mouse (23). Uncoupling of eNOS has been shown to be one of the sources of endogenously produced H2O2. A number of factors may lead to uncoupling of eNOS with subsequent production of ROS. For instance, decreased heat shock protein (HSP) 90 recruitment (12, 27), l-arginine (9, 30), or tetrahydrobiopterin (BH4) (21, 29) availability have all been shown to lead to eNOS uncoupling resulting in the generation of superoxide instead of NO. Pathological conditions such as atherosclerosis and diabetes can lead to increased generation of ROS together with decreased NO production, in part due to eNOS uncoupling (14, 24). However, little is known about whether endogenous production of ROS like H2O2 and superoxide occurs in the normal pulmonary vasculature and, if so, whether this process is developmentally regulated.
In this study, we tested the hypothesis that developmental changes occurred in pulmonary artery (PA) responses to NOS agonists and that activation of eNOS produced ROS such as superoxide in PA isolated from normal juvenile but not fetal sheep.
MATERIALS AND METHODS
All studies were approved by the Animal Care and Use Committee of Northwestern University.
Isolated vessel studies
Vascular relaxations to the NOS agonist A-23187 were performed in isolated PA as previously described (32, 33). In the present study, we utilized fifth-generation PA with inside diameters of <500 μm. Vessel rings were mounted on stainless steel hooks and placed in water-jacketed chambers containing Krebs-Ringer solution. The buffer was maintained at 37°C and aerated with a gas mixture of 94% O2 and 6% CO2 to maintain a pH of 7.40, a Pco2 of 38 Torr, and a Po2 of >500 Torr. Vessels were pretreated for 20 min with 10−5 M indomethacin to prevent the formation of vasoactive prostaglandins and with 10−6 M propranolol to block activation of β-adrenergic receptors. Vessels were preconstricted with a submaximal concentration of norepinephrine (10−6 M). Cumulative concentration-response curves for A-23187 and H2O2 were developed. Some vessels were pretreated with nitro-l-arginine (l-NA, 10−3 M), polyethylene glycol-conjugated superoxide dismutase (PEG-SOD, 75 units), SOD + catalase (1,200 units), l-arginine (10−3 M), or sepiapterin (10−4 M) for 30–60 min before relaxations with A-23187.
Cell culture
Primary cultures of pulmonary artery endothelial cells (PAEC) from sheep were isolated as previously published (35, 38). In brief, the main and branch PA were removed from fetal (136 days) and juvenile (4-wk old) lambs and placed in a sterile 10-cm dish containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 1 g/l glucose. The segment was stripped of adventitia with a sterile forceps. The artery segment was then cut longitudinally to open the vessel, and the endothelial layer was removed by gentle rubbing with a cell scraper. We then placed the endothelium in medium DMEM-H16 containing 10% fetal bovine serum and antibiotics and incubated it at 37°C in 21% O2/5% CO2 balanced with N2. After 5 days, islands of PAEC were cloned to ensure purity. Basic fibroblast growth factor (1 ng/ml, Sigma) was added to the media every other day. When confluent the cells were passaged to maintain them in culture or frozen in liquid nitrogen. Endothelial cell identity was confirmed by morphology, specific uptake of 1,1′-dioctadecyl-1,3,3′,3′-tetramethylindocarbocyanine perchlorate-labeled acetylated low-density lipoprotein (Molecular Probes, Eugene, OR), and positive staining for von Willebrand factor (Dako, Carpinteria, CA) as previously published (35, 37). Ovine PAEC were studied between passages 5 and 8.
Cell treatments
To stimulate NOS we utilized three different activators: a calcium ionophore A-23187 (1 μM), human recombinant vascular endothelial growth factor (VEGF, 50 ng/ml), and laminar shear stress (20 dyn/cm2). We studied eNOS activation in confluent PAEC that were made quiescent by an overnight culture in serum-free media. Laminar shear stress was applied using a cone-plate viscometer that accepts six-well tissue culture plates, as described previously (35, 38). This method achieves laminar flow rates that represent physiological levels of laminar shear stress in the major human arteries, which is in the range of 5–20 dyn/cm2 (Lin) with localized increases to 30–100 dyn/cm2 (8). A-23187 and VEGF exposures were conducted for 5 min, while shear stimulation was conducted for 15 min. To inhibit NOS we utilized the competitive inhibitor nitro-l-arginine methyl ester (l-NAME, 100 μM). To inhibit NAD(P)H oxidases we utilized apocynin (1 mM), a selective and reversible antagonist. Some cells were pretreated with 1 mM of l-arginine, or with overnight exposure to sepiapterin (100 μM), a cell membrane-permeable precursor of BH4.
Determination of cellular superoxide, H2O2, and NO levels by fluorescence
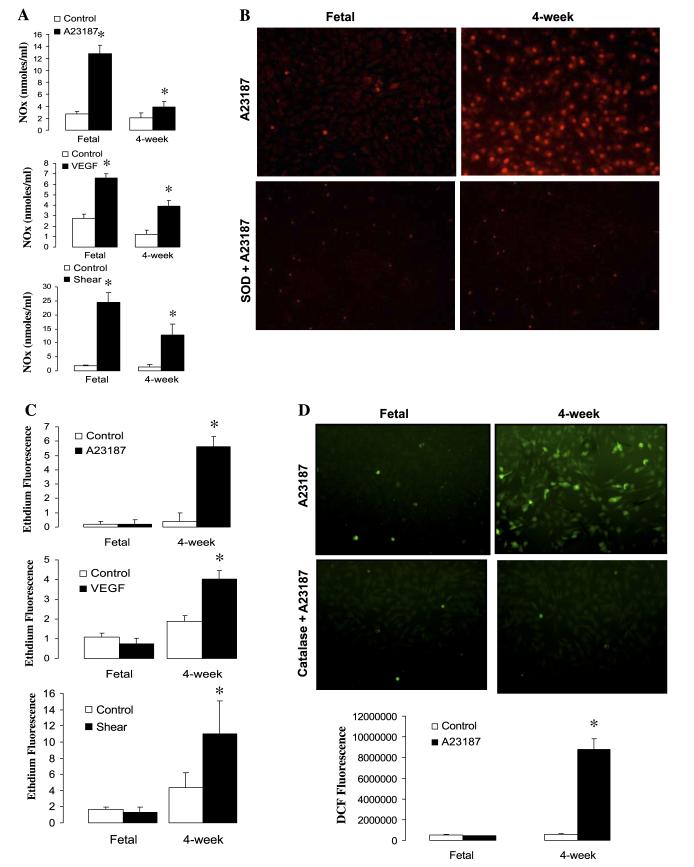
Superoxide, NO, and H2O2 cellular levels were detected with fluoroprobes as described previously (4, 36). In brief, cells seeded onto 24-well or 6-well plates (for shear assays) were made quiescent overnight with serum-free media. After NOS stimulation by various agents as described above, the following fluoroprobes were utilized: dihydroethidium (DHE, 10 μM; Molecular Probes) for the detection of superoxide, diaminofluorescein-2 diacetate (5 μM) for the detection of NO, and dichlorofluorescein-diacetate (DCF-DA, 3 μM) for the detection of H2O2. Fluoroprobes were added to the medium, and the incubation was continued for an additional 15 min. Cells were washed with PBS and imaged under a Nikon Eclipse TE-300 fluorescence microscope. Fluorescence images were captured with a Photometrix digital camera, and the average fluorescence intensities (to correct for differences in cell number) were quantified with Metamorph imaging software (Fryer). The DHE signal was specific for superoxide, because SOD, but not catalase, completely abolished it (see Fig. 3A). Catalase abolished the signal of DCF-DA, showing specificity for H2O2 (see Fig. 3D).
Fig. 3.
A: effect of NOS stimulation on NO production as measured by chemiluminescence in confluent pulmonary artery endothelial cells (PAEC). A-23187 (1 μM), recombinant human (rh) VEGF (50 ng/ml), and laminar shear stress (20 dyn/cm2) increase the production of NO more dramatically in fetal vs. postnatal (4-wk) cells. NOx, nitric oxide products. Data are means ± SD, n = 3–5 separate experiments done in triplicate for each data point. *P < 0.05 vs. control (basal values). B: representative fluorescent images show that A-23187 stimulates an increase in dihydroethidium (DHE) fluorescence in 4-wk but not fetal PAEC. This increase is abolished in the presence of SOD, indicating specificity for superoxide. C: NOS stimulation by A-23187, VEGF, and shear significantly increases superoxide production in 4-wk PAEC relative to fetal cells. Results express the levels of superoxide as relative fluorescent units. Data are means ± SD, n = 3–5 separate experiments done in triplicate for each data point. *P < 0.05 vs. control (basal values). D: effect of NOS stimulation on H2O2 production as measured by dichlorofluorescein (DCF) fluorescence in fetal and 4-wk cells. Representative fluorescent images show that A-23187 stimulates an increase in DCF fluorescence in 4-wk but not fetal PAEC. This increase is abolished in the presence of catalase, indicating specificity for H2O2. Data are means ± SD, n = 3–5 separate experiments done in duplicate for each data point. *P < 0.05 vs. control (basal values).
Analysis of NO products by chemiluminescence
An NO chemiluminescence analyzer (Sievers NOA 280i; Ionics Instruments, Boulder, CO) was used to measure levels of NO products (nitrate, nitrite, and nitrosothiols) in the conditioned media of control and NOS-stimulated PAEC, as previously described (38). The conditioned media were kept at −20°C until analysis. The samples were thawed, and a 5-μl aliquot was injected into the vacuum chamber of the NO analyzer containing 4 ml of 0.1 M vanadium chloride (type III, Sigma) at 90°C to convert nitrates, nitrites, and nitrosothiols back to NO. Liberated NO was driven into the gas phase of the vacuum chamber by bubbling the reaction mixture with nitrogen. Linear curves were obtained by measuring NO liberated by sodium nitroprusside (0.5–50 μM). Background signal produced from the serum-free media alone was subtracted from the sample signals.
Immunoprecipitation of eNOS and Western blotting
PAEC were stimulated with A-23187 or laminar shear stress for the indicated amount of time, washed with cold PBS, and lysed in a commercial lysis buffer (Upstate Biotechnology) plus protease inhibitor cocktail (Sigma). Protein was quantified by the Coomassie-Bradford method, and 300 μg of total protein were immunoprecipitated with 1 μg of monoclonal anti-eNOS (BD Transduction) at 4°C overnight. The next morning, the immunoprecipitate was washed three times with the same lysis buffer containing protease inhibitors. The protein complexes were then released from the agarose beads by adding 100 μl of Laemmli buffer and boiling for 10 min. Immunoprecipitated protein extracts (40 μl) or whole protein extracts (30 μg) in the case of Western blotting were separated on 12% SDS-polyacrylamide gel and electrophoretically transferred to HyBond nitrocellulose membranes (Amersham, Arlington Heights, IL). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T). After 1 h of blocking, the membranes were incubated at 4°C overnight with 1:200 dilution of monoclonal anti-eNOS, anti-HSP90, or phosphoSer1177 eNOS antibody (BD Transduction Laboratories). Membranes were washed 3 × 15 min with TBS-T and then hybridized with anti-mouse horseradish peroxidase antibody for 45 min. After 3 × 15 min washes, bands were visualized with chemiluminescence using a Kodak Digital Science Image Station (NEN, Rochester, NY). Relative phosphoSer1177eNOS was calculated as the densitometric value of phosphoSer1177eNOS divided by β-actin values. HSP90 recruitment was calculated as the densitometric value of HSP90 divided by the densitometric value of total eNOS.
Determination of total and reduced biopterin levels by HPLC
Measurements of cellular pulmonary aortic endothelial cell content were performed by HPLC analysis and a differential oxidation method as described previously (21). BH4 levels were determined by the difference between the acid extraction (total biopterin) and the alkaline extraction (H2B plus biopterin). A C-18 reverse chromatography column (5 μm) was used with a solvent system of 5% methanol-95% water at a flow rate of 1.0 ml per min. Biopterins were detected by fluorescence at 350 nm for excitation and 450 nm for emission.
Statistical analysis
All data are expressed as means ± SD. Comparisons between treatment groups were made by one-way or repeated-measures ANOVA as appropriate. Values of P < 0.05 were considered statistically significant.
RESULTS
Developmental differences on vasorelaxations to NOS activators
We investigated the response of preconstricted PA from late-gestation fetal (136 days) and juvenile (4-wk-old) sheep to A-23187. A-23187 induced nearly full relaxations in juvenile PA, while inducing only a partial vasorelaxation in fetal PA (Fig. 1A). The NOS antagonist l-NA significantly inhibited relaxations in both vessel types (Fig. 1, B and C), as did removal of the endothelium (data not shown). However, pretreatment with the soluble guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ) inhibited relaxations in fetal but not juvenile arteries (Fig. 1, B and C).
Fig. 1.
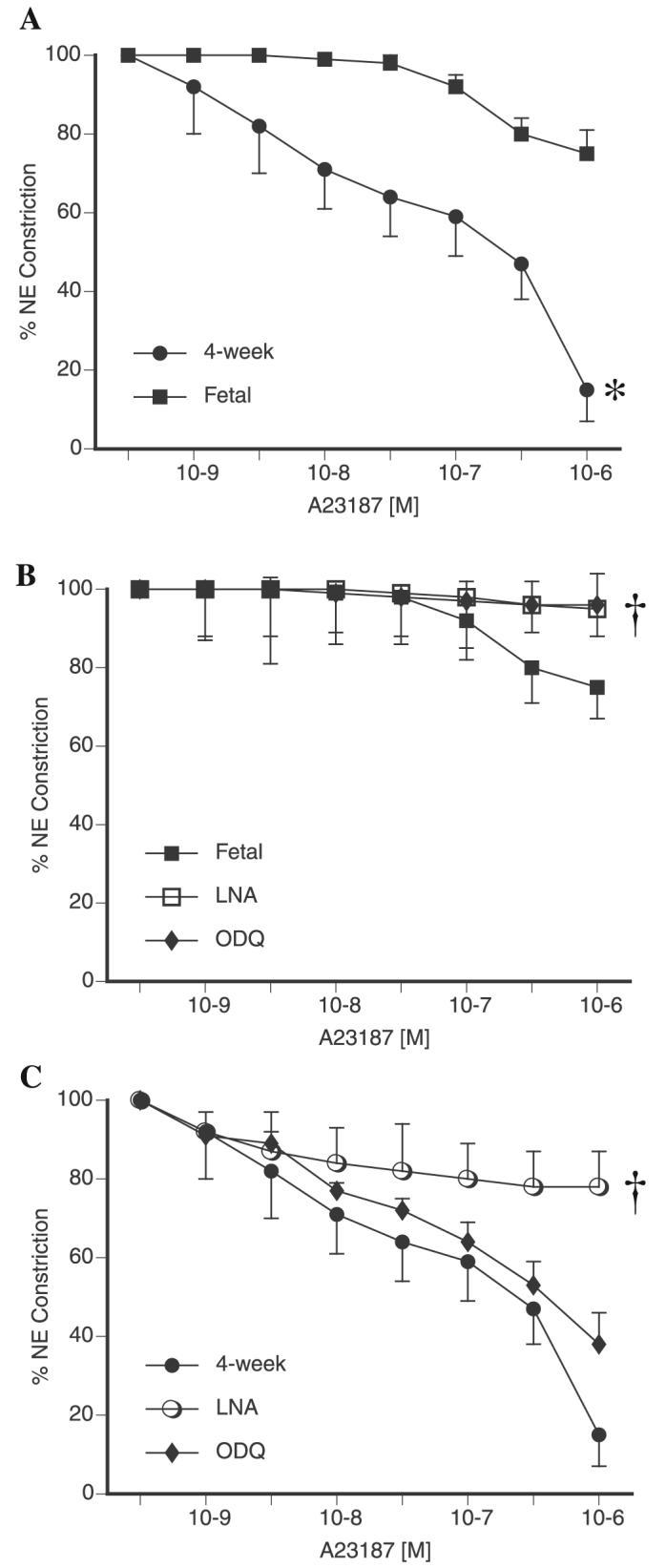
Cumulative relaxation-response curves for the nitric oxide synthase (NOS) activator A-23187 in 5th-generation pulmonary arteries (PA) isolated from near-term fetal and juvenile (4-wk) lamb lungs. All rings were pretreated with indomethacin and propranolol. Relaxations are expressed as percent of a plateau constriction in response to norepinephrine (NE, 100%). A: Comparison of fetal and juvenile PA relaxations to A-23187. Effect of nitro-l-arginine (l-NA) and 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ) on juvenile (B) and fetal (C) PA relaxations to A-23187. Data are means ± SE, n = 4–6 lambs for each data point. *P < 0.05 vs. fetal, †P < 0.05 vs. A-23187 alone.
We performed additional experiments to explore the mechanism of relaxation to A-23187 in juvenile PA. SOD and catalase had no effect on A-23187-induced vasorelaxation in fetal PA (data not shown). In contrast, we observed that SOD did not inhibit vasorelaxation to A-23187 in juvenile PA, but the combination of SOD and catalase did (Fig. 2A). Addition of the ROS scavenger Tiron also blocked A-23187-induced vasorelaxations in juvenile PA (data not shown). These data suggested the involvement of H2O2 in A-23187-induced vasorelaxations. Therefore, we investigated the effect of exogenously added H2O2 in preconstricted PA. As shown in Fig. 2B, H2O2 at micromolar concentrations induced vasorelaxations in both fetal and juvenile PA. Juvenile PA were noted to have greater relaxations to H2O2 than fetal vessels.
Fig. 2.
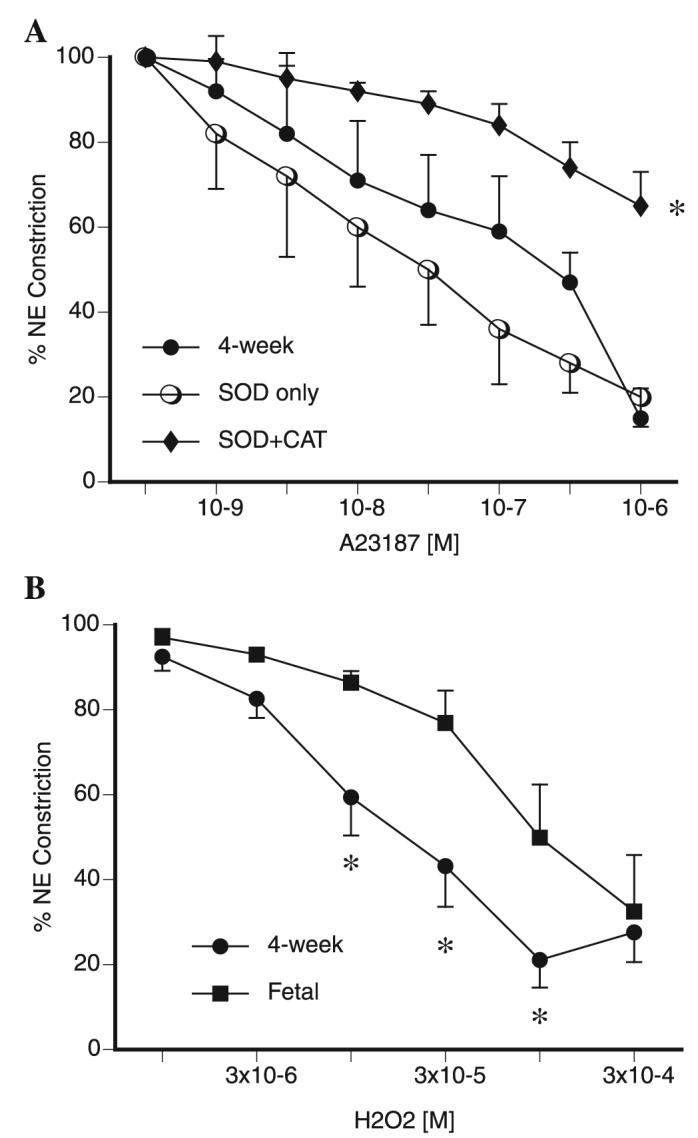
A: effect of addition of superoxide dismutase (SOD) alone and the combination of SOD and catalase (CAT) on cumulative relaxation-response curves for the NOS activator A-23187 in juvenile PA. All rings were pretreated with indomethacin and propranolol. Relaxations are expressed as percent of a plateau constriction in response to NE (100%). Data are means ± SE, n = 4–6 lambs for each data point. *P < 0.05 vs. A-23187 alone. B: cumulative relaxation-response curves for H2O2 in 5th-generation PA isolated from near-term fetal and juvenile (4-wk) lamb lungs. All rings were pretreated with indomethacin and propranolol. Relaxations are expressed as percent of a plateau constriction in response to NE (100%). Data are means ± SE, n = 4 lambs for each data point. *P < 0.05 vs. fetal.
Postnatal PAEC produce superoxide by NOS stimulation
Our vessel data suggested that NOS-dependent vasorelaxations in juvenile PA could be mediated either by a cGMP-independent effect of NO or by another NOS product. To determine whether PA from juvenile sheep produce NO or ROS in response to NOS stimulation, we investigated the production of NO, superoxide, and in some cases H2O2, by NOS in fetal and 4-wk PAEC. For these studies we utilized the following NOS activators: A-23187 (calcium-dependent, receptor-independent activator), VEGF (receptor-dependent activator), and laminar shear stress (physiologically relevant, receptor- and calcium-independent activator) as described under materials and methods. We found that all three NOS activators stimulated the production of NO in both fetal and 4-wk PAEC (Fig. 3A). However, depending on the stimulus, NO production was 1.5- to 3-fold higher in fetal PAEC vs. the 4-wk-old PAEC (Fig. 3A).
In contrast, all three NOS activators induced the production of superoxide in 4-wk-old PAEC but not in fetal PAEC (Fig. 3C). Superoxide is converted to H2O2 by SOD. To confirm that NOS-derived superoxide in 4-wk-old PAEC was converted to H2O2, we analyzed the production of this ROS after NOS stimulation with A-23187. As shown in Fig. 3D, A-23187 increased H2O2 levels in 4-wk-old PAEC, and this effect was abolished by addition of catalase.
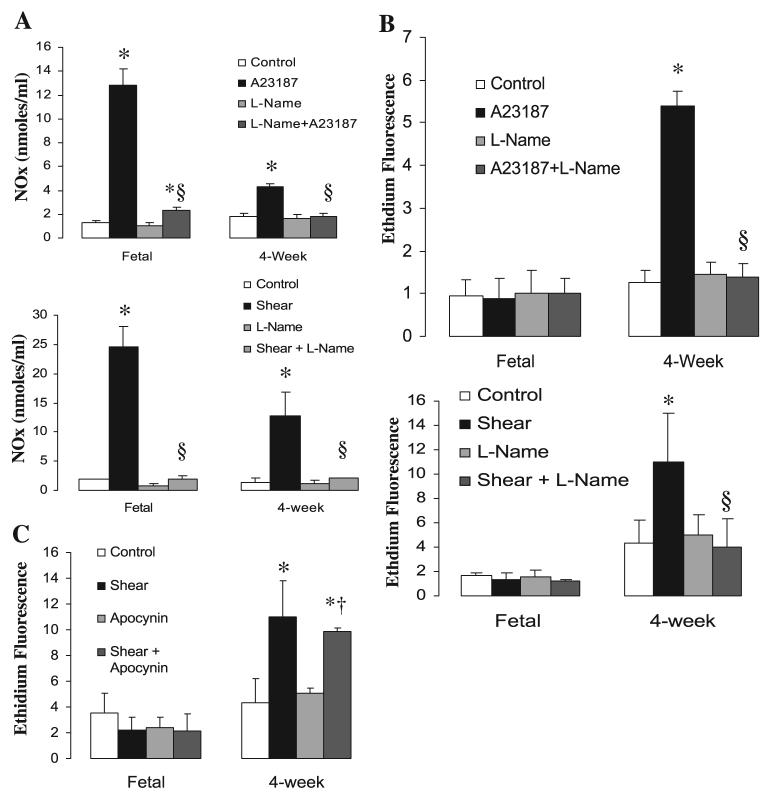
Because A-23187 and shear could potentially stimulate NADPH oxidase in addition to NOS, we determined the source of superoxide in 4-wk PAEC by means of pharmacologic inhibitors. The NOS inhibitor l-NAME completely blocked the increased production of NO in both fetal and 4-wk PAEC in response to A-23187 and shear and blocked superoxide production in juvenile PAEC (Fig. 4, A and B). On the other hand, the NADPH oxidase inhibitor apocynin had no effect on shear-induced superoxide production in 4-wk-old PAEC (Fig. 4C). Similarly, apocynin did not abolish A-23187-induced production of superoxide in juvenile PAEC, indicating that NADPH oxidase is not the main source of superoxide upon A-23187 stimulation (data not shown).
Fig. 4.
A-23187- and shear-mediated increases in NO (A) and superoxide (B) are blocked by the NOS antagonist nitro-l-arginine methyl ester (l-NAME). Superoxide production was inhibited by l-NAME but not affected by pretreatment with the NADPH oxidase inhibitor apocynin. Confluent and quiescent PAEC were treated with various NOS agonists in the presence or absence of l-NAME (100 μM) or apocynin (1 mM) followed by analysis for NO and superoxide production. Data are means ± SD, n = 3–5 separate experiments done in duplicate for each data point. *P < 0.05 vs. control (basal values), †P < 0.05 vs. apocynin alone, §P < 0.05 vs. NOS agonist alone.
NOS uncoupling: role of l-arginine availability or HSP90/NOS association
To investigate potential causes of NOS uncoupling in 4-wk PAEC, we determined the effect of pretreatment with l-arginine on NO/superoxide production by shear. Addition of l-arginine increased stimulated NO levels in both fetal and 4-wk PAEC (Fig. 5A). However, l-arginine did not significantly reduce basal superoxide production in fetal or 4-wk PAEC (Fig. 5B) and induced only a 42% decrease in shear-induced superoxide production in 4-wk-old PAEC. Therefore, superoxide production by eNOS persisted in the presence of saturated levels of l-arginine. We further found that pretreatment with l-arginine had no effect on A-23187 relaxations in fetal or 4-wk PA (data not shown).
Fig. 5.
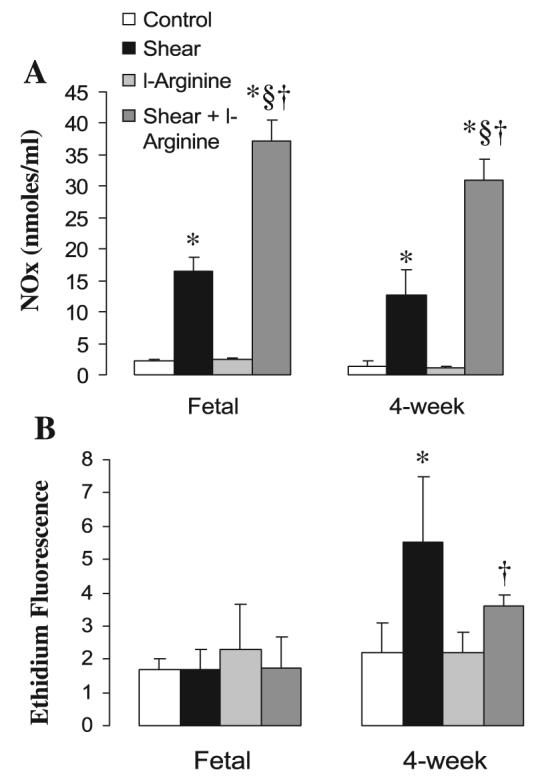
l-Arginine increased production of NO (see Fig. 6A) but did not inhibit production of superoxide (see Fig. 6B) in PAEC following NOS stimulation. Confluent and quiescent PAEC were treated with shear in the presence or absence of l-arginine (1 mM) followed by analysis of NO and superoxide production. Data are means ± SD, n = 3–5 separate experiments done in duplicate for each data point. *P < 0.05 vs. control (basal values), §P < 0.05 vs. shear alone, †P < 0.05 vs. l-arginine alone.
We then investigated basal and stimulated levels of HSP90 in association with eNOS in PAEC by immunoprecipitation. As shown in Fig. 6, A and B, basal levels of HSP90 associated with eNOS did not significantly differ in fetal compared with 4-wk-old PAEC. Stimulation with A-23187 and shear led to significant increases in HSP90 association with eNOS in both groups of PAEC (Fig. 6, A and B). These data correlated well with eNOS phosphorylation at Ser1177 (Fig. 7, A and B). Basal levels of phosphoSer1177 eNOS were higher in fetal than in 4-wk-old PAEC. Similarly, basal levels of total eNOS by Western blot were higher in fetal than in 4-wk-old PAEC (data not shown). However, both fetal and juvenile cells responded to A-23187 and shear with an increase in phosphoSer1177 eNOS (Fig. 7, A and B).
Fig. 6.
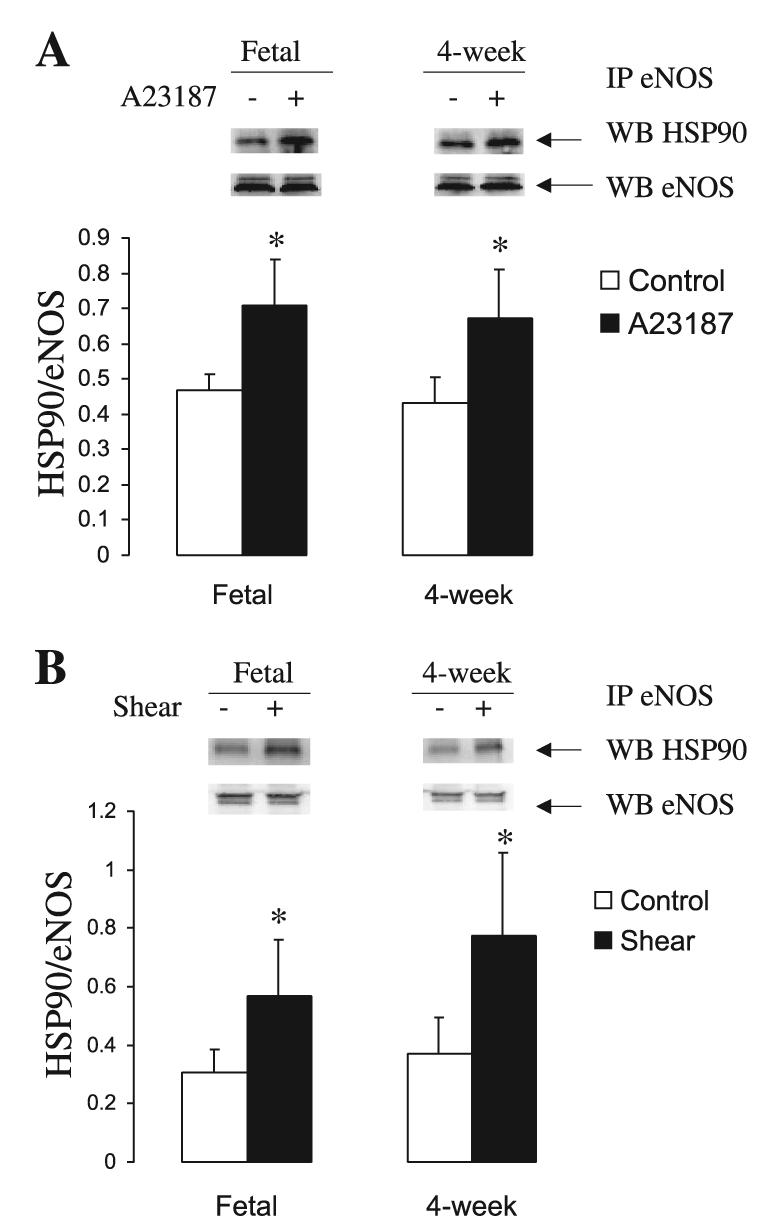
A-23187 and shear induce the recruitment of heat shock protein (HSP) 90 to eNOS complexes in PAEC. Protein lysates from basal and stimulated PAEC were immunoprecipitated (IP) for endothelial NOS (eNOS) and probed for HSP90. A-23187 (A) and shear (B) increases HSP90/eNOS association in both fetal and juvenile PAEC. Results are shown as relative HSP90 associated to eNOS. WB, Western blotting. Bar graphs represent the averages ± SE (n = 5). *P < 0.05 of control (basal values).
Fig. 7.
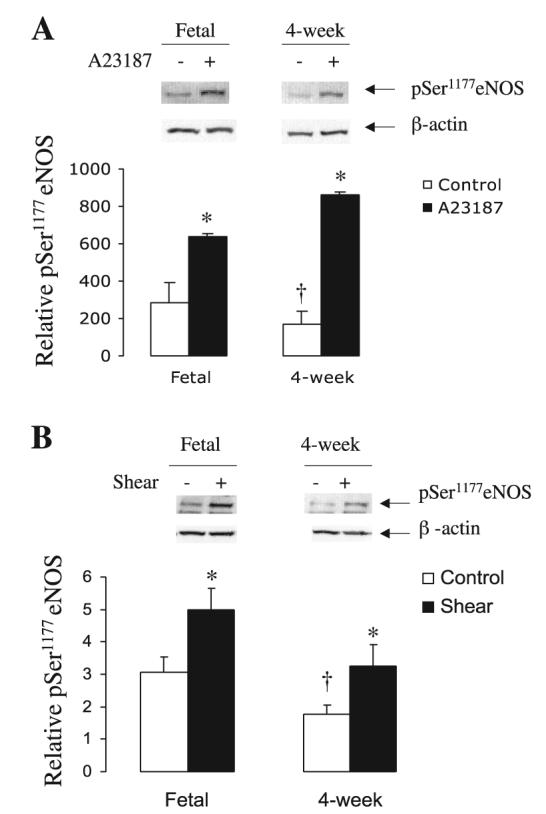
A-23187 and shear increase phosphorylation at Ser1177 of eNOS. Protein lysates from basal and stimulated PAEC were probed for phosphoSer1177 eNOS and β-actin. A-23187 (A) and shear (B) increase phosphorylation of eNOS in both fetal and juvenile PAEC. Results are shown as relative phosphoSer1177 eNOS over β-actin values. Bar graphs represent the averages ± SE (n = 3). *P < 0.05 of control (basal values).
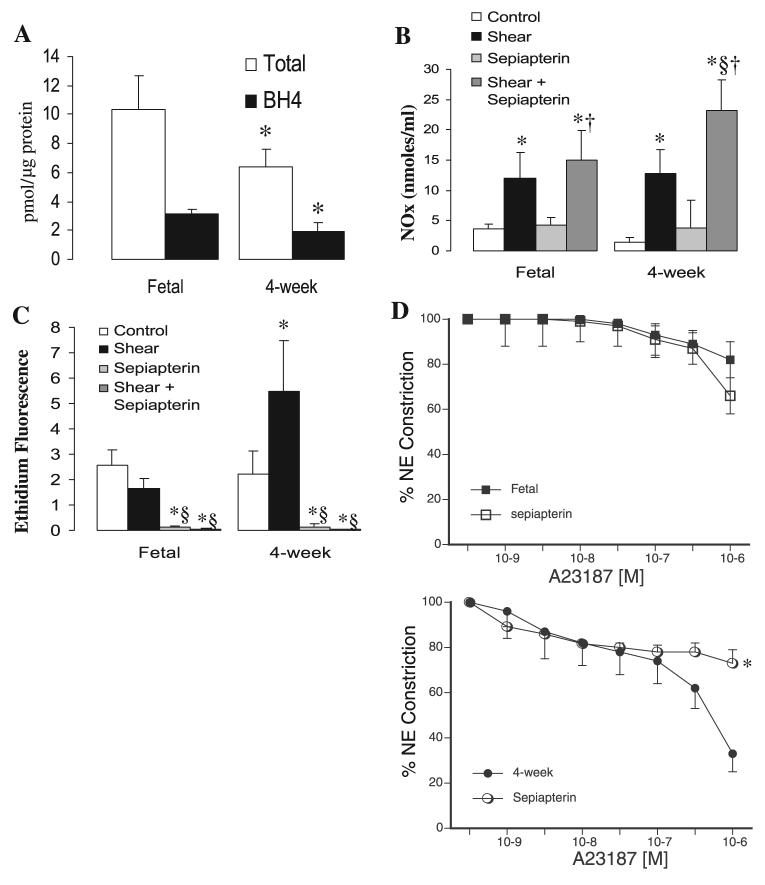
Decreased BH4 levels lead to postnatal NOS uncoupling
The availability and oxidation state of biopterin have been shown to have a significant impact on eNOS function (6, 23). Decreases in BH4 in vivo and in vitro have been shown to induce eNOS uncoupling, leading to decreased NO and increased superoxide production (29). Therefore, we investigated the relative levels of reduced and total biopterin in fetal and juvenile PAEC. We found total and reduced biopterin to be significantly lower in 4-wk compared with fetal PAEC (Fig. 8A). Then, we analyzed the effect of exogenous sepiapterin, a BH4 precursor, on eNOS-derived NO and superoxide. As shown in Fig. 8B, sepiapterin increased shear-stimulated NO production in both types of PAEC, with larger increases noted in the 4-wk cells. Moreover, sepiapterin dramatically decreased basal and shear-induced superoxide production by NOS in postnatal PAEC (Fig. 8C). Similar results were found in A-23187-treated PAEC (data not shown). Finally, sepiapterin pretreatment of isolated PA resulted in significant reductions in relaxations to A-23187 in juvenile but not fetal vessels (Fig. 8D). Relaxations to A-23187 in juvenile and fetal PA were of similar magnitude following pretreatment with sepiapterin.
Fig. 8.
Tetrahydrobiopterin (BH4) availability is a key factor on the uncoupling of eNOS in postnatal PAEC. A: total and reduced biopterin levels were analyzed by HPLC; levels were significantly reduced in 4-wk PAEC. Data are means ± SD (n = 3). B: addition of sepiapterin increases NO production and abolishes superoxide production by PAEC. Confluent and quiescent PAEC were treated with shear in the presence or absence of the BH4 precursor sepiapterin (100 μM) followed by analysis for NO and superoxide production. Data are means ± SD, n = 6 separate experiments done in duplicate for each data point. *P < 0.05 vs. control (basal values), §P < 0.05 vs. shear alone, †P < 0.05 vs. sepiapterin alone. D: effect of addition of sepiapterin on cumulative relaxation-response curves for the NOS activator A-23187 in fetal and juvenile PA. All rings were pretreated with indomethacin and propranolol. Relaxations are expressed as percent of a plateau constriction in response to NE (100%). Data are means ± SE, n = 4–6 lambs for each data point. *P < 0.05 vs. A-23187 alone.
DISCUSSION
In this study, we found maturational differences in the vascular reactivity of PA isolated from fetal and juvenile sheep. Relaxations to the NOS activator A-23187 were greater in PA isolated from juvenile compared with fetal lambs (Fig. 1A). These findings are similar to those reported in previous studies (2, 32). In the current study, vascular relaxations were likely not mediated by cyclooxygenase or lipoxygenase derivatives, as vessels were pretreated with indomethacin. In addition, vascular relaxations were significantly inhibited by the NOS antagonist l-NA. We found that inhibition of soluble guanylate cyclase with ODQ completely inhibited A-23187 relaxations in fetal PA but only partially inhibited relaxations in juvenile PA. Together, these findings suggested that mechanisms other than, or in addition to, generation of NO from eNOS account for the increased vascular relaxation to A-23187 we observed in PA isolated from juvenile lambs.
Previous studies have indicated that vasodilation to NOS agonists in pulmonary and mesenteric arteries is inhibited by catalase, suggesting that H2O2 may be an important effector of vasodilation (5, 23). Similarly, we found that SOD modestly enhanced relaxations to A-23187 in juvenile PA, whereas the addition of catalase inhibited them (Fig. 2A). The SOD enhancement could have been due to increased availability of NO or H2O2 for vasodilation, although the inhibition by catalase indicates that H2O2 was an important mediator of these relaxations. We also found that micromolar additions of H2O2 induced vasorelaxation in both fetal and juvenile PA (Fig. 2B). Together, our vessel data suggested that NOS activation by A-23187 resulted in H2O2-dependent vasorelaxations in juvenile but not fetal PA.
Vascular production of H2O2 occurs primarily from the reaction of superoxide with SOD. Vascular endothelial cells have several enzymatic sources for superoxide production, including eNOS, cyclooxygenases, lipoxygenases, P-450 cytochromes, and NAD(P)H oxidases (11, 16). Because relaxations to A-23187 were inhibited by l-NA in both age groups, our studies in intact PA suggested that NOS is the main source of NO in fetal and superoxide/H2O2 in juvenile vessels. We therefore hypothesized that activation of eNOS produced ROS such as superoxide and H2O2 in 4-wk but not fetal PA. To test this hypothesis, we investigated the effect of NOS activators on in vitro production of NO and superoxide in PAEC isolated from fetal and juvenile (4-wk) lambs.
We observed that fetal PAEC produced NO and not superoxide upon NOS stimulation by A-23187, VEGF, or shear. In contrast, 4-wk PAEC produced mainly superoxide following A-23187 stimulation and both NO and superoxide following VEGF and shear stimulation of NOS. Apocynin, a pharmacologic inhibitor of NADPH oxidase, had no significant effect on superoxide production by shear (Fig. 4C) or A-23187 (data not shown). These results suggest that A-23187 and shear increase superoxide production in juvenile PAEC through activation of eNOS. Therefore, we conclude that a certain degree of “un-coupling” of eNOS occurs in PAEC from normal postnatal lambs, producing a state that favors the production of superoxide.
Uncoupling of eNOS can occur by various mechanisms. Deficiencies of substrates such as oxygen and l-arginine can lead to decreased NO production by eNOS. A recent study demonstrated that eNOS uncoupling is resistant to the addition of l-arginine analogs (34). We found that while l-arginine did not decrease superoxide production in response to NOS agonists, it significantly increased NO production. Our studies suggest that l-arginine supplementation does not reverse post-natal eNOS uncoupling and superoxide production. Although l-arginine did improve the NO/superoxide ratio in postnatal PAEC, it had no effect on relaxations to A-23187 in fetal or 4-wk PA. The effect of various degrees of oxygenation on eNOS-mediated generation of NO or superoxide was not addressed in the current study. However, preliminary data (not shown) suggested that varying the oxygen levels of the buffer did not affect A-23187 relaxations in fetal or 4-wk PA, similar to what has been previously reported in the literature (3, 20, 19). However, considering that both NO and H2O2 are mediators of vasorelaxation whose production requires O2, it is possible that changes in Po2 levels may further modulate changes in vascular tone. Therefore, studies on the developmental changes that occur in response to Po2 sensing are warranted.
Interaction of eNOS with regulatory proteins such as HSP90 has been shown to increase eNOS activity and production of NO, whereas failure of HSP90 association leads to production of superoxide by eNOS (27). HSP90 also increases phosphorylation of eNOS at Ser1177, a necessary step for eNOS activation. We demonstrated that HSP90 recruitment and phosphorylation of eNOS at Ser1177 upon NOS stimulation does not change significantly with age. However, basal levels of phospho-Ser1177 eNOS were higher in fetal compared with juvenile PAEC, suggesting that fetal cells are primed for the production of NO, whereas juvenile PAEC require various stimuli to produce NO.
Finally, we investigated the effect of BH4 on postnatal uncoupling of eNOS. We observed that sepiapterin, a precursor of BH4, dramatically decreased basal and NOS-stimulated superoxide levels while increasing NO levels in postnatal PAEC. Our results suggest that BH4 levels are important in whether eNOS functions in the coupled or uncoupled state during early postnatal life. Moreover, we demonstrated that total and reduced biopterin levels were significantly lower in juvenile compared with fetal PAEC. In vessel baths, we found that pretreatment with sepiapterin reduced A-23187 relaxations in 4-wk PA to levels similar to those observed in the fetal vessels. These findings further support our hypothesis that the enhanced relaxations observed in juvenile PA (Fig. 1A) are due to increased production of superoxide/H2O2. Together, our data strongly suggest that a postnatal reduction in BH4 levels leads to eNOS uncoupling and favors eNOS-mediated generation of superoxide/H2O2 in postnatal PAEC.
Other investigators have demonstrated uncoupled activity of eNOS in isolated endothelial cells by using geldanamycin, an HSP90 antagonist (27) or by utilizing mice with decreased tetrahydrobiopterin levels (6). Under those conditions, endothelium-dependent relaxations were mediated by eNOS production of H2O2 instead of NO. We now show for the first time that production of both NO and H2O2 by eNOS can occur simultaneously in normal PAEC. Furthermore, we demonstrated that the uncoupling of eNOS is not evident in fetal cells but is evident in juvenile cells, indicating that these changes may occur in response to air breathing.
In summary, our data suggest that in PA and PAEC isolated from late-gestation fetal lambs, NOS is primed to produce NO exclusively, an event that is linked to higher levels of total and reduced biopterin. In contrast, in PA and PAEC from 4-wk lambs, eNOS activity is uncoupled. This is due in part to reduced BH4 availability, leading to the production of ROS in addition to NO. H2O2 induces vasorelaxation by acting as an EDHF or by stimulating soluble guanylate cyclase through nonheme site binding. This “developmental switch” of eNOS function could be in response to oxygen and may allow the pulmonary vasculature to respond to the changes that occur with air breathing and its associated increase in Po2. We encourage future investigations on BH4 and antioxidant defense mechanisms during development.
ACKNOWLEDGMENTS
The technical expertise of Sylvia Gugino and Lisa Forquer is gratefully acknowledged.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-54705 (R. H. Steinhorn), HL-67841 (S. M. Black), HL-72123 (S. M. Black), HL-60190 (S. M. Black), and HL-70061 (S. M. Black)
REFERENCES
- 1.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol. 1990;259:H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Chatfield BA, Rodman DM, Hall SL, McMurtry IF. Maturational changes in endothelium-derived relaxing factor activity of ovine pulmonary arteries in vitro. Am J Physiol Lung Cell Mol Physiol. 1991;260:L280–L285. doi: 10.1152/ajplung.1991.260.4.L280. [DOI] [PubMed] [Google Scholar]
- 3.Belik J, Pan J, Jankov RP, Tanswell AK. Bronchial epithelium-associated pulmonary arterial muscle relaxation in the rat is absent in the fetus and suppressed by postnatal hypoxia. Am J Physiol Lung Cell Mol Physiol. 2005;288:L384–L389. doi: 10.1152/ajplung.00309.2004. [DOI] [PubMed] [Google Scholar]
- 4.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs. A role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 5.Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol. 1987;252:H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- 6.Cosentino F, Barker JE, Brand MP, Heale SJ, Werner ER, Tippins JR, West N, Channon KM, Volpe M, Luscher TF. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- 7.Dawes GS, Mott JC, Widicombe JG, Wyatt DG. Changes in the lungs of the newborn lamb. J Physiol. 1953;121:141–162. doi: 10.1113/jphysiol.1953.sp004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewey CF, Bussolari SR, Gimbrone MA, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 9.Fineman JR, Chang R, Soifer SJ. L-Arginine, a precursor of EDRF in vitro, produces pulmonary vasodilation in lambs. Am J Physiol Heart Circ Physiol. 1991;261:H1563–H1569. doi: 10.1152/ajpheart.1991.261.5.H1563. [DOI] [PubMed] [Google Scholar]
- 10.Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol. 1995;57:115–134. doi: 10.1146/annurev.ph.57.030195.000555. [DOI] [PubMed] [Google Scholar]
- 11.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 12.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- 13.Halbower AC, Tuder RM, Franklin WA, Pollock JS, Forstermann U, Abman SH. Maturation-related changes in endothelial nitric oxide synthase immunolocalization in developing ovine lung. Am J Physiol Lung Cell Mol Physiol. 1994;267:L585–L591. doi: 10.1152/ajplung.1994.267.5.L585. [DOI] [PubMed] [Google Scholar]
- 14.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia. 2000;43:1435–1438. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto HS, Teitel D, Rudolph A. Effect of birth-related events on blood flow distribution. Pediatr Res. 1987;22:634–640. doi: 10.1203/00006450-198712000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Katusic ZS. Superoxide anion and endothelial regulation of arterial tone. Free Radic Biol Med. 1996;20:443–448. doi: 10.1016/0891-5849(96)02116-8. [DOI] [PubMed] [Google Scholar]
- 17.Kawai N, Bloch DB, Filippov G, Rabkina D, Suen HC, Losty PD, Janssens SP, Zapol WM, dela Monte S, Bloch KD. Constitutive endothelial nitric oxide synthase gene expression is regulated during lung development. Am J Physiol Lung Cell Mol Physiol. 1995;268:L589–L641. doi: 10.1152/ajplung.1995.268.4.L589. [DOI] [PubMed] [Google Scholar]
- 18.Kinsella JP, McQueston JA, Rosenberg AA, Abman SH. Hemodynamic effects of exogenous nitric oxide in ovine transitional pulmonary circulation. Am J Physiol Heart Circ Physiol. 1992;263:H875–H880. doi: 10.1152/ajpheart.1992.263.3.H875. [DOI] [PubMed] [Google Scholar]
- 19.Konduri GG, Bakhutashvili I, Frenn R, Chandrasekhar I, Jacobs ER, Khanna AK. P2Y purine receptor responses and expression in the pulmonary circulation of juvenile rabbits. Am J Physiol Heart Circ Physiol. 2004;287:H157–H164. doi: 10.1152/ajpheart.00617.2003. [DOI] [PubMed] [Google Scholar]
- 20.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, III, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. doi: 10.1203/01.pdr.0000191136.69142.8c. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy M, Souil E, Sabry S, Favatier F, Vaugelade P, Mercier JC, Dall'Ava-Santucci J, Dinh-Xuan AT. Maturational changes of endothelial vasoactive factors and pulmonary vascular tone at birth. Eur Respir J. 2000;15:158–165. doi: 10.1183/09031936.00.15115800. [DOI] [PubMed] [Google Scholar]
- 23.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller FJ, Gutterman DD, Rios D, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 25.North AJ, Star RA, Brannon TS, Ujiie K, Wells LB, Lowenstein CJ, Snyder SH, Shaul PW. Nitric oxide synthase type I and type III gene expression are developmentally regulated in rat lung. Am J Physiol Lung Cell Mol Physiol. 1994;266:L635–L641. doi: 10.1152/ajplung.1994.266.6.L635. [DOI] [PubMed] [Google Scholar]
- 26.Perreault T, DeMarte J. Maturational changes in endothelium-derived relaxations in newborn piglet pulmonary circulation. Am J Physiol Heart Circ Physiol. 1993;264:H302–H309. doi: 10.1152/ajpheart.1993.264.2.H302. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard KA, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric oxide synthase. J Biol Chem. 2001;276:17621–17624. doi: 10.1074/jbc.C100084200. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph AM. Fetal and neonatal pulmonary circulation. Annu Rev Physiol. 1979;41:383–395. doi: 10.1146/annurev.ph.41.030179.002123. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt HHHW, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. No NO from NO synthase. Proc Natl Acad Sci USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider R, Raff U, Vornberger N, Schmidt M, Freund R, Reber M, Schramm L, Gambaryan S, Wanner C, Schmidt HH, Galle J. L-Arginine counteracts nitric oxide deficiency and improves the recovery phase of ischemic acute renal failure in rats. Kidney Int. 2003;64:216–225. doi: 10.1046/j.1523-1755.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 31.Shaul PW, Farrar MA, Magness RR. Pulmonary endothelial nitric oxide production is developmentally regulated in the fetus and newborn. Am J Physiol Heart Circ Physiol. 1993;265:H1056–H1063. doi: 10.1152/ajpheart.1993.265.4.H1056. [DOI] [PubMed] [Google Scholar]
- 32.Steinhorn RH, Morin FC, III, Gugino SF, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in iso-lated ovine pulmonary arteries and veins. Am J Physiol Heart Circ Physiol. 1993;264:H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 33.Steinhorn RH, Russell JA, Morin FC., III Disruption of cyclic GMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1995;268:H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 34.Stroes S, Hijmering M, van Zandvoort M, Wever R, Rabelink TJ, van Faassen EE. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett. 1998;438:161–164. doi: 10.1016/s0014-5793(98)01292-7. [DOI] [PubMed] [Google Scholar]
- 35.Wedgwood S, Bekker JM, Black SM. Shear stress regulation of endothelial NOS in fetal pulmonary arterial endothelial cells involves PKC. Am J Physiol Lung Cell Mol Physiol. 2001;281:L490–L498. doi: 10.1152/ajplung.2001.281.2.L490. [DOI] [PubMed] [Google Scholar]
- 36.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2005;288:L480–L487. doi: 10.1152/ajplung.00283.2004. [DOI] [PubMed] [Google Scholar]
- 37.Wedgwood S, McMullan DM, Bekker JM, Fineman JR, Black SM. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ Res. 2001;89:357–364. doi: 10.1161/hh1601.094983. [DOI] [PubMed] [Google Scholar]
- 38.Wedgwood S, Mitchell CJ, Fineman JR, Black SM. Developmental differences in the shear stress-induced expression of endothelial NO synthase: changing role of AP-1. Am J Physiol Lung Cell Mol Physiol. 2003;284:L650–L662. doi: 10.1152/ajplung.00252.2002. [DOI] [PubMed] [Google Scholar]