Abstract
Mucosally induced immunological tolerance is an attractive strategy for preventing or treating illnesses resulting from untoward inflammatory immune reactions against self- or non-self-antigens. Oral administration of relevant autoantigens and allergens has been reported to delay or suppress onset of clinical disease in a number of experimental autoimmune and allergic disorders. However, the approach often requires repeated feeding of large amounts of tolerogens over long periods and is only partly effective in animals already systemically sensitized to the ingested antigen such as in animals already harboring autoreactive T cells, and thus presumably also in humans with an autoimmune disease. We have recently shown that oral administration of microgram amounts of antigen coupled to cholera toxin B subunit (CTB), can effectively suppress systemic T cell reactivity in naive as well as in immune animals. We now report that feeding small amounts (2–20 μg) of human insulin conjugated to CTB can effectively suppress beta cell destruction and clinical diabetes in adult nonobese diabetic (NOD) mice. The protective effect could be transferred by T cells from CTB-insulin-treated animals and was associated with reduced lesions of insulitis. Furthermore, adoptive co-transfer experiments involving injection of Thy-1,2 recipients with diabetogenic T cells from syngeneic mice and T cells from congenic Thy-1,1 mice fed with CTB-insulin demonstrated a selective recruitment of Thy-1,1 donor cells in the peripancreatic lymph nodes concomitant with reduced islet cell infiltration. These results suggest that protection against autoimmune diabetes can be achieved by feeding minute amounts of a pancreas islet cell autoantigen linked to CTB and appears to involve the selective migration and retention of protective T cells into lymphoid tissues draining the site of organ injury.
Keywords: cholera toxin, oral tolerance, nonobese diabetic
Oral administration of antigens is a long-recognized method for inducing systemic immunological tolerance (1, 2), and has been proposed as an approach for the treatment or prevention of allergic (3–5) and autoimmune diseases (6–13). This approach, however, requires administration of massive amounts of antigens or prolonged feeding of small amounts of antigens, which are then only effective in rather narrow dose ranges. Further, oral tolerance is especially difficult to induce in an already immunologically sensitized host (14–18), such as in patients harboring autoreactive T cells.
The nonobese diabetic (NOD) mouse spontaneously develops an autoimmune disease sharing immunological and histopathological similarities with type I insulin-dependent diabetes mellitus in humans (19–22). Prolonged feeding of large (milligram) amounts of heterologous insulin has been shown to delay the onset of diabetes in NOD mice (23). However, the therapeutic potential of such treatment in humans appears to be limited by the dose and frequency of autoantigen administration and by the short duration of the protection.
We have previously shown that feeding even a single dose of minute amounts (microgram) of antigens conjugated to the receptor-binding nontoxic B subunit moiety of cholera toxin (CTB), can markedly suppress systemic T cell-mediated inflammatory reactions in naive as well as in immune animals (24). More recently, oral administration of a myelin autoantigen conjugated to CTB has been shown to protect animals against experimentally induced allergic encephalomyelitis, even when given after disease induction (25). We now report that feeding adult female NOD mice with small doses of heterologous insulin conjugated to CTB (CTB-insulin) prevents beta cell infiltration by diabetogenic T cells and protects animals against spontaneous autoimmune diabetes.
MATERIALS AND METHODS
Mice.
NOD mice were bred under standard conditions at the Animal Care Facility of Faculty Alexis Carrel (Lyon, France). In this colony, spontaneous diabetes starts as early as 12 weeks of age, reaching an incidence of 55–60% in females and 15–20% in males by 30 weeks of age. Additional female NOD mice were purchased from Taconic Farms. In the latter colony, diabetes starts at approximately 15 weeks and reaches an incidence of 80% by 30 weeks. Congenic NOD-NON Thy-1,1 were obtained from Ed Leiter (Jackson ImmunoResearch) (26). Diabetes was characterized by polydipsia, weight loss, glycosuria as assessed by urine chemstrips (Bayer, Germany), and persistent hyperglycemia, which was determined with blood glucose chemstrips (Lifescan, Mountain View, CA).
Antigens.
CTB was purified by a combination of hexametaphosphate precipitation and Sephadex G-75 gel filtration from the culture filtrate of a mutant strain of Vibrio cholerae deleted of the CT genes and complemented with a recombinant overexpression plasmid encoding CTB (27). Recombinant human insulin (Novo-Nordisk, Baegsvaerd, Denmark) was conjugated to CTB with N-succinimidyl (3-(2-pyridyl)dithio)propionate (SPDP, Pharmacia) as bifunctional coupling reagent (28). The resulting CTB-insulin (CTB-INS) conjugates were purified by gel filtration and characterized for their receptor-binding and serological reactivity by a solid-phase ELISA using immobilized GM1 ganglioside as capture system and enzyme-linked antibodies to CTB and to insulin as detection systems, respectively (29). The purified conjugates were found to contain, on average, 20% (wt/wt) of insulin. The same coupling and purification protocols were employed to obtain a CTB-ovalbumin (CTB-OVA) conjugate. In all protocols, the doses of CTB-INS or CTB-OVA given represent the amount of insulin or ovalbumin in such conjugates.
Induction of Oral Tolerance.
NOD females, 8 weeks old, were fed once with either 0.2 μg, 2 μg, or 20 μg of recombinant human insulin conjugated to CTB; 50 μg of unconjugated insulin; or 100 μg of CTB given in a volume of 400 μl of 0.35 M NaHCO3 by gastric intubation with an 18-gauge stainless steel feeding needle. In some experiments, animals were fed five consecutive doses of CTB-INS (2 μg and 40 μg), unconjugated insulin (10 μg and 100 μg), or CTB-OVA (2 μg), administered every other week starting at 8–10 weeks of age.
Cell Preparation and Adoptive Transfers.
Eight-week-old female NOD mice were fed once with 10 μg of CTB-INS, unconjugated INS, CTB-OVA, or CTB alone. One week later, animals were sacrificed and their spleen was aseptically removed. Splenocytes were dispersed in Hanks’ balanced salt solution by pressing the spleens through a stainless steel mesh. After lysis of red blood cells with ammonium chloride, splenocytes were depleted of B cells by panning on plastic dishes coated with a rabbit anti-mouse IgG (H+L) antibodies (Biosys, Compiègne France). Flow cytometric analyses showed that the resulting cell suspensions comprised more than 90% CD3+ cells (nominal T cells). Five million splenic T cells from mice fed either CTB-insulin, CTB-OVA, free INS, or CTB alone were mixed with 5 × 106 splenic T cells from 20-week-old diabetic NOD females. The resulting cell mixture was injected i.v. into 8- to 10-week-old irradiated (750 rads) NOD males (30).
Histological Procedures.
Pancreas specimens were excised and either directly frozen in liquid nitrogen or immersed in Bouin’s fixative prior to paraffin embedding. Five-micrometer sections were prepared from paraffin-embedded specimens and stained with haematoxylin and eosin (31). The severity of insulitis was evaluated by two observers and using the following scoring system: 0, islet free of inflammation; 1, peri-islet infiltrate; 2, insulitis with <40% of the islet infiltrated; and 3, complete infiltration of the islet. All determinations were made on at least 25 islets for each specimen.
Frozen sections were stained by indirect immunofluorescence using rat monoclonal antibody to murine Thy-1,2 (diluted 1:50) as primary reagent and phycoerythrin-labeled anti-rat IgG (H+L) (Tebu, Le Perray en Yvelines, France) as secondary reagent. Alternate sections were exposed to FITC-labeled rat monoclonal antibody to mouse Thy-1,1 (Cedarlane Laboratories) (diluted 1:50).
Flow Cytometry.
One week after oral administration of a single dose of 10 μg CTB-INS or CTB-OVA, T cells from the spleens of Thy-1,1+ NOD female mice were mixed with T cells prepared from the spleens of 20-week-old female Thy-1,2+ diabetic NOD mice and injected into irradiated Thy-1,2+ NOD males. The percentage of donor Thy-1,1+ T cells in the spleen, mesenteric, and pancreatic lymph nodes of recipient NOD males was determined 7, 15, and 30 days after cell transfer by cytofluorometric analysis using FITC-labeled rat monoclonal antibodies to Thy-1,1 (clone CL005F; Cedarlane Laboratories) or Thy-1,2 (clone 30H12; Biosys, Compiègne, France) (32).
Statistical Analyses.
Differences in prevalence of diabetes between experimental groups were analyzed with 2 × 2 contingency tables using the Fisher exact test. The efficacy of diabetes transfer was compared between animal groups using a two-tailed Wilcoxon rank sum test. Insulitis scores were compared by Student’s t test for unpaired samples.
RESULTS
Oral Administration of CTB-Insulin Conjugate Suppresses Spontaneous Diabetes in NOD Mice.
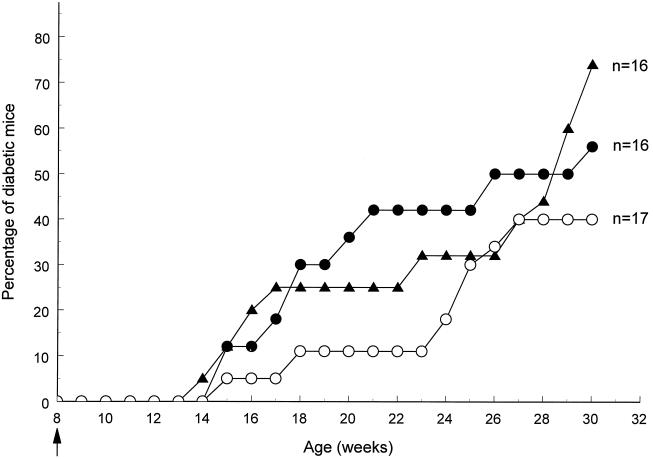
NOD females were fed once at 8 weeks of age with either 0.2 μg, 2 μg, or 20 μg of insulin conjugated to CTB or with 100 μg of CTB, and animals were monitored for diabetes onset. As shown in Fig. 1, onset of diabetes was delayed in animals fed a single dose of CTB-INS conjugate in comparison to control animals. For the whole period between 17 and 24 weeks of age the incidence of diabetes was significantly lower in the CTB-INS (20 μg)-fed group than in the control group, which was fed CTB alone. Thus, at 23 weeks of age (i.e., 15 weeks after treatment), only 2 out of 17 (12%) mice fed insulin that was conjugated to CTB had developed diabetes, as compared with 7 out of 16 (44%) control mice that were fed CTB alone [protective efficacy (PE) = 73%; P = 0.04]. In contrast, mice fed insulin (50 μg) alone had only marginally lower disease incidence over the same time period, with 6 out of 16 animals (38%; PE = 14%; P > 0.10) having developed diabetes by week 23. Thereafter, this difference progressively vanished. Feeding mice with lower doses of CTB-INS had intermediate but not statistically significant effects on diabetes incidence; 2 out of 8 (25%) mice fed 2 μg CTB-INS and 3 out of 8 (37.5%) mice fed 0.2 μg developed diabetes by week 23 (data not shown).
Figure 1.
Suppression of spontaneous autoimmune diabetes after oral treatment with CTB-insulin conjugate. NOD females were treated at 8 weeks of age with a single oral administration of 20 μg of insulin conjugated to CTB (○), insulin alone (▴), or CTB alone (•). Data are combined from two independent experiments.
We also examined the effect of repeated oral administrations of CTB-conjugated insulin on disease development. In the first experiment, only 2 out of 7 (28%) mice treated with 2 μg CTB-INS, given at 8, 10, 12, 14, and 16 weeks of age, had developed disease (PE = 60%; P = 0.04) by 29 weeks, whereas 5 out of 7 (71%) mice fed with a control (CTB-OVA) conjugate had developed diabetes. Feeding mice five consecutive 10-μg doses of free insulin (amounting to a total of 50 μg insulin) had no effect on disease development (data not shown). A second experiment involving larger numbers of animals from another NOD mouse colony yielded similar results. Thus, only 4 out of 35 (11%) mice fed 40 μg of CTB-conjugated insulin given on five consecutive occasions had developed diabetes by 20 weeks of age (PE = 78%; P < 0.001) as compared with 15 out of 30 (50%) sham-treated control mice (Table 1). By 25 weeks of age, 11 out of 35 (31%) animals fed CTB-INS had developed diabetes, as compared with 20 out of 30 control animals (PE = 54%; P < 0.01). Repeated oral treatment with unconjugated INS (5 × 100 μg) had no appreciable effect on diabetes incidence (Table 1).
Table 1.
Suppression of diabetes after repeated oral treatment with low doses of CTB-conjugated insulin
| Feeding | Age, weeks
|
||||
|---|---|---|---|---|---|
| 10 | 15 | 20 | 25 | 30 | |
| CTB-INS | 0/35 | 0/35 | 4/35 | 11/35 | 20/35 |
| P = 0.0009 | P = 0.006 | P = 0.06 | |||
| INS | 0/30 | 3/30 | 9/30 | 16/30 | 22/30 |
| P = 0.18 | P = 0.42 | P = 0.76 | |||
| Sham | 0/30 | 3/30 | 15/30 | 20/30 | 24/30 |
Female NOD mice were fed with CTB-INS (40 μg), unconjugated INS (100 μg), or buffer alone, on weeks 10, 12, 14, 16, and 18. Data are expressed as number of diabetic mice per total number of animals.
T Cells from CTB-Insulin-Fed Mice Abrogate Transfer of Disease by Diabetogenic T Cells.
To determine whether the protective effects of oral administration of CTB-insulin were associated with the generation of protective T cells, adoptive co-transfer experiments were performed. Splenic T cells from mice fed 1 week earlier with a single dose of 2 μg of CTB-INS or CTB-OVA were transferred together with splenic T cells from diabetic mice into irradiated NOD male recipients. As shown in Fig. 2A, only 1 out of 7 male NOD recipients co-injected with diabetogenic T cells and T cells from CTB-insulin-fed animals had developed diabetes. In marked contrast, all eight animals co-injected with diabetogenic T cells and T cells from CTB-OVA-fed mice had developed diabetes 3 weeks after cell co-transfer (PE = 86%; P < 0.001).
Figure 2.
T cells from NOD mice that were fed CTB-insulin conjugate abrogate adoptive transfer of autoimmune diabetes. A depicts the incidence curves of diabetes in mice co-injected with 5 × 106 T cells from the spleens of diabetic females and 5 × 106 T cells from the spleens of 8-week-old females fed a single dose of 2 μg of CTB-conjugated insulin (○) or 2 μg of CTB-conjugated ovalbumin (•). The degree of insulitis was determined histologically on pancreas specimens obtained 34 days after co-transfer and is expressed as mean percentages of infiltrated islets ± SD (B).
Histological analyses of pancreata from mice co-injected with diabetogenic cells and T cells from CTB-INS-fed animals disclosed markedly less severe insulitis lesions as compared with specimens from control recipients injected with diabetogenic cells and T cells from CTB-OVA-fed donors (Fig. 2B). Thus, 34 days after transfer, almost half (48.0 ± 7.5%) of the islets were still intact in NOD male recipients of T cells from CTB-INS-fed donors as compared with 7.8 ± 1.7% in recipients of T cells from animals fed with the CTB-OVA control conjugate (P < 0.001) (Fig. 2B). In the latter situation, most of the islets (63.5 ± 7.8%; n = 8) were severely infiltrated (grade 3) as compared with recipients of T cells from CTB-INS-fed donor mice (11.7 ± 4.8%; P < 0.001) (Fig. 2B).
Homing of Protective T Cells.
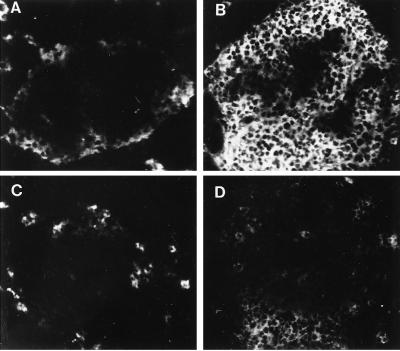
To determine whether protective T cells generated by oral CTB-INS could migrate preferentially toward the pancreas, co-transfer experiments using two strains of donor NOD mice congenic at the Thy-1 locus were performed. Thy-1,1+ T cells from mice fed with CTB-INS or CTB-OVA were injected together with Thy-1,2+ diabetogenic T cells into irradiated Thy-1,2 NOD males. Flow cytometric analyses of lymph nodes from recipients of T cells from CTB-INS fed Thy-1,1+ mice disclosed an early and transient recruitment of donor T cells in pancreatic lymph nodes but not in the mesenteric lymph nodes (Table 2), and also not in the spleen (not shown). Thus, 7 days after transfer, the frequency of Thy-1,1+ T cells was significantly higher in pancreatic lymph nodes of recipients of diabetogenic T cells co-injected with splenic T cells from CTB-INS-fed mice as compared with that of similar recipients co-injected with T cells from CTB-OVA-fed donors. Thereafter, the percentages of Thy-1,1+ T cells in pancreatic and mesenteric lymph nodes as well as in the spleen were comparable between both groups of mice. On the other hand, immunohistological analyses of pancreas specimens from recipient NOD males collected 30 days after transfer disclosed an accumulation of donor Thy-1,1+ T cells around or within the islets with very few infiltrating diabetogenic Thy-1,2+ T cells (Fig. 3). In marked contrast, most of the islets in recipients of T cells from Thy-1,1+ animals fed with CTB-OVA were heavily infiltrated with Thy-1,2+ T cells and harbored very few if any Thy-1,1+ cells (Fig. 3). Taken together, these observations indicate that oral treatment with CTB-INS generates T cells capable to home selectively to the pancreas and to interfere with the migration of diabetogenic T cells.
Table 2.
T cells from CTB-insulin-fed mice migrate to the pancreatic lymph nodes of congenic NOD male recipients
| Organ | Days | Feeding
|
|
|---|---|---|---|
| CTB-INS | CTB-OVA | ||
| PLN | 7 | 38 ± 4% (P < 0.03) | 19 ± 8% |
| 15 | 20 ± 4% | 19 ± 8% | |
| 30 | 12 ± 1% | 11 ± 2% | |
| MLN | 7 | 15 ± 2% | 12 ± 3% |
| 15 | 15 ± 2% | 11 ± 5% | |
| 30 | 13 ± 1% | 14 ± 4% | |
Flow cytofluorometric analyses of pancreatic (PLN) and mesenteric (MLN) lymph nodes from NOD male recipients were performed at the indicated times to determine the percentages of donor Thy-1, 1+ T cells after co-transfer of 5 × 106 Thy-1,2+ T cells from 20-week-old syngeneic diabetic females and 5 × 106 Thy-1,1+ T cells from congenic mice fed one dose (2 μg) of CTB-conjugated insulin (CTB-INS) or ovalbumin (CTB-OVA). Data are expressed as mean percentages of Thy-1,1+ cells ± SD determined for each time point on 3–6 animals per experimental group.
Figure 3.
T cells from CTB-insulin-fed NOD mice suppress beta cell infiltration by diabetogenic T cells. Immunofluorescence staining of pancreatic islets from irradiated NOD males 30 days after co-injection of syngeneic Thy-1,2+ T cells from 20-week-old diabetic female mice with Thy-1,1+ T cells from congenic NOD mice that were fed CTB-insulin (A, C) or CTB-ovalbumin (B, D). Immunostainings were performed with monoclonal antibodies to Thy-1,2+ (A, B) and Thy-1,1+ (C, D). Original magnifications ×250.
DISCUSSION
T cell-mediated beta cell destruction in the pancreas is thought to be the major mechanism leading to autoimmune diabetes in NOD mice (19, 22, 33, 34).
In this study, we demonstrate that a single administration of a specific beta cell autoantigen, insulin, linked to CTB, the nontoxic mucosa-binding moiety of cholera toxin, suppresses diabetes in NOD mice even when given at as late as 8 weeks of age (i.e., well after weaning and at a time when all animals have evidence of insulitis). Further, oral CTB-INS treatment was effective at doses 500–5,000 times lower than those previously required to treat young (weaning) NOD mice with unconjugated insulin (23). Partial protection (48%) has previously been reported after feeding NOD mice at as early as 5 weeks of age with 1 mg of porcine insulin administered twice weekly for 5 weeks and weekly thereafter, whereas lower doses were ineffective (23). The present study demonstrates that feeding minute amounts of insulin conjugated to CTB can significantly reduce the incidence of diabetes even when given as a single dose and that protection tends to be more durable when using five consecutive doses of conjugate. Taken together, these findings are in keeping with our previous observations (24, 25) indicating that CTB can serve as a powerful transmucosal delivery system for inducing peripheral tolerance.
The ability of T cells from CTB-INS-fed animals to abrogate transfer of diabetes by diabetogenic T cells supports the notion that active suppression was induced. However, the possibility that anergic T cells were generated by this treatment, thereby competing with diabetogenic T cells for recognition of the same autoantigen-presenting cells (35) or for locally produced cytokines, cannot be ruled out at that stage. Using similar co-transfer experiments, we have previously shown that T cells from donor mice repeatedly fed with high (1 mg) but not lower doses of unconjugated insulin were able to reduce the incidence of transferred disease apparently without effect on the degree of islet cell infiltration (31). This apparent discrepancy between clinical and histological pictures was interpreted as the result of a possible replacement of diabetogenic T cells by suppressor T cells within the islet cells of protected animals. At variance with the latter observations, the present study demonstrates that animals protected against clinical disease by adoptive transfer of T lymphocytes from mice orally tolerized with much lower doses of insulin conjugated to CTB also have much less severe insulitis lesions with substantial numbers of intact islets. The latter observation is in keeping with the results of our recent study in a rat model of inducible autoimmune encephalomyelitis, in which feeding tiny doses of myelin autoantigen linked to CTB protects animals against clinical disease and also suppresses central nervous system infiltration (25). Taken together, these observations suggest that the mechanisms governing induction of tolerance by feeding CTB-linked antigens may partly differ from those implicated by conventional protocols of tolerance induction (36) and may involve modifications of the migratory behavior of inflammatory cells. Assuming that protective T cells induced by oral CTB-INS treatment, unlike diabetogenic cells and regulatory cells, do not migrate into the islets, they may still leave the gut and, via the blood, reach pancreatic lymph nodes where they could interfere with the recruitment and migration of inflammatory leukocytes into the pancreas. The fact that pancreatic lymph nodes are major sites of activation of diabetogenic T cells (37), together with the finding that transferred T cells from donor mice orally tolerized with CTB-INS migrate to pancreatic lymph nodes in recipients, is compatible with such a mechanism. Further studies on the expression of adhesion molecules on protective T cells and on diabetogenic T cells could be valuable.
The present study reopens the question regarding the role of insulin as one possible target autoantigen in autoimmune destruction of beta cells. The observations that overt diabetes is often preceded by autoantibodies reactive to insulin (20) and that islet-infiltrating cells isolated from NOD mice are enriched in insulin-specific T cells (21) suggest that an immune response to this autoantigen may be involved in the process of beta cell destruction. Additional support to this interpretation has recently been provided by the finding that insulin-specific T cells are present in high frequency within islet cell infiltrates and that insulin-specific T cell clones have the capacity to transfer diabetes (38). The observation that feeding NOD mice with insulin conjugated to CTB protects against diabetes does not imply however that insulin is the dominant autoantigen. As shown in other experimental systems, regulatory T cells generated by repeated feeding of an antigen can secrete antigen-nonspecific suppressive cytokines when recruited and thus reexposed to the fed antigen in the target organ, thereby antagonizing the proinflammatory activity of bystander T cells with a distinct antigen specificity (36). It will thus be worthwhile to compare the protective efficacy of conjugates of CTB linked to other beta cell constitutants (39, 40) or peptides (41).
We conclude that oral treatment with small doses of insulin conjugated to CTB can protect NOD mice from autoimmune diabetes and prevent diabetogenic T cells from infiltrating islets and attacking beta cells in the pancreas. These findings may impact on the development of preventive and therapeutic vaccines against type 1 diabetes.
Acknowledgments
We thank Mrs. A. Durand, R. Enselman, A. Stefanutti, and R. Velasco for valuable help. This work was supported by the Institut National de la Santé et de la Recherche Médicale (France), the Swedish Medical Research Council (contracts 16X-03382 and 24X-08320), and Zymogenetics (Seattle).
ABBREVIATIONS
- NOD
nonobese diabetic
- CT
cholera toxin
- CTB
CT B subunit
- INS
insulin
- OVA
ovalbumin
References
- 1.Wells H G. J Infect Dis. 1911;8:147–171. [Google Scholar]
- 2.Chase M W. Proc Soc Exp Biol. 1946;61:257–259. doi: 10.3181/00379727-61-15294p. [DOI] [PubMed] [Google Scholar]
- 3.Wortmann F. Allergol Immunopathol. 1977;5:15–26. [PubMed] [Google Scholar]
- 4.Rebien W, Puttonen E, Maasch H J, Stix E, Wahn U. Eur J Pediatr. 1982;138:341–344. doi: 10.1007/BF00442513. [DOI] [PubMed] [Google Scholar]
- 5.Holt P G, McMenamin C. Clin Exp Allergy. 1989;19:255–262. doi: 10.1111/j.1365-2222.1989.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson H S G, Staines N A. Clin Exp Immunol. 1986;64:581–586. [PMC free article] [PubMed] [Google Scholar]
- 7.Nagler-Anderson C, Bober L A, Robinson M E, Siskind G W, Thorbecke G J. Proc Natl Acad Sci USA. 1986;83:7443–7446. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitar D M, Whitacre C C. Cell Immunol. 1988;112:364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- 9.Higgins P J, Weiner H L. J Immunol. 1988;140:440–445. [PubMed] [Google Scholar]
- 10.Nussenblatt R B, Caspi R R, Mahdi R, Chan C C, Roberge R, Lider O, Weiner H L. J Immunol. 1990;144:1689–1695. [PubMed] [Google Scholar]
- 11.Wank Z Y, Qiao J, Link H. J Neuroimmunol. 1993;44:209–214. doi: 10.1016/0165-5728(93)90045-z. [DOI] [PubMed] [Google Scholar]
- 12.Weiner H L, Mackin G A, Matsui M, Orav E J, Khoury S J, Dawson D M, Hafler D A. Science. 1993;259:1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 13.Trentham D E, Dynesius-Trentham R A, Orav E J, Combitchi D, Lorenzo C, Sewell K L, Hafler D A, Weiner H L. Science. 1993;261:1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 14.André C, Bazin H, Heremans J F. Digestion. 1973;9:166–175. doi: 10.1159/000197442. [DOI] [PubMed] [Google Scholar]
- 15.Thomas H C, Parrot D M V. Immunology. 1974;27:631–639. [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson D G, Vaz N M, Rowlings L A, Lynch J M. J Immunol. 1979;122:2261–2266. [PubMed] [Google Scholar]
- 17.Titus R, Chiller J M. Int Arch Allergy Appl Immunol. 1981;65:323–328. [PubMed] [Google Scholar]
- 18.Thompson H S G, Staines N A. Immunol Today. 1990;11:396–399. doi: 10.1016/0167-5699(90)90158-6. [DOI] [PubMed] [Google Scholar]
- 19.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Exp Anim. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 20.Palmer J P, Asplin C M, Clemons P, Lyen K, Tatpati O, Raghu P K, Paquette T L. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 21.Wegmann D R, Norbury-Glaser M, Daniel D. Eur J Immunol. 1994;27:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 22.Bach J F. Endocr Rev. 1994;15:516–542. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z J, Davidson L, Eisenbarth G, Weiner H L. Proc Natl Acad Sci USA. 1991;88:10252–10256. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J B, Holmgren J, Czerkinsky C. Proc Natl Acad Sci USA. 1994;91:10795–10799. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J B, Rask C, Olsson T, Holmgren J, Czerkinsky C. Proc Natl Acad Sci USA. 1996;93:7196–7201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prochazka M, Serreze D V, Worthen S M, Leiter E H. Diabetes. 1989;38:1446–1454. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- 27.Lebens M, Johansson S, Osek J, Lindblad M, Holmgren J. Bio/Technology. 1993;11:1574–1578. doi: 10.1038/nbt1293-1574. [DOI] [PubMed] [Google Scholar]
- 28.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svennerholm A M, Holmgren J. Curr Microbiol. 1989;1:19–23. [Google Scholar]
- 30.Wicker L S, Miller B J, Mullen Y. Diabetes. 1986;35:855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]
- 31.Bergerot I, Fabien N, Maguer V, Thivolet C. J Autoimmun. 1994;7:655–663. doi: 10.1006/jaut.1994.1050. [DOI] [PubMed] [Google Scholar]
- 32.Fabien N, Bergerot I, Maguer-Satta, Orgiazzi J, Thivolet C. J Autoimmun. 1995;8:323–334. doi: 10.1006/jaut.1994.0025. [DOI] [PubMed] [Google Scholar]
- 33.Shizuru J A, Taylor-Edwards C, Banks B A, Gregory A K, Fathman C G. Science. 1988;240:659–661. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- 34.Hutchings P R E, Simpson E, O’Reilly L A, Lund T, Waldmann H, Cooke A. J Autoimmun. 1990;3:101–109. doi: 10.1016/s0896-8411(09)90018-x. [DOI] [PubMed] [Google Scholar]
- 35.Lombardi G, Sidhu S, Batchelor R, Lechler R. Science. 1994;264:1587–1589. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 36.Weiner H L, Friedman A, Miller A, Khoury S J, Al-Sabbagh A, Santos L, Sayegh M, Nussenblatt R B, Trentham D E, Hafler D A. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- 37.Fabien N, Bergerot I, Maguer-Satta, Orgiazzi J, Thivolet C. J Autoimmun. 1995;8:323–334. doi: 10.1006/jaut.1994.0025. [DOI] [PubMed] [Google Scholar]
- 38.Daniel D, Gill R G, Schloot N, Wegmann D. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 39.Tisch R X, Yang X, Singer S M, Liblau R S, Fugger L, McDevitt H O. Nature (London) 1993;366:72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 40.Healey D, Ozegbe P, Arden S, Chandler P, Hutton J, Cooke A. J Clin Invest. 1995;95:2979–2985. doi: 10.1172/JCI118006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel D, Wegmann D R. Proc Natl Acad Sci USA. 1996;93:956–960. doi: 10.1073/pnas.93.2.956. [DOI] [PMC free article] [PubMed] [Google Scholar]