Abstract
The extracellular matrix protein tenascin (TN) is overexpressed in a number of solid tumours. Thi however, is the first study to examine TN expression in ovarian tumours. TN protein was examined in froze sections of 50 human ovarian tumours by immunohistochemistry. Malignant and borderline tumours showed significantly greater incidence and intensity of stromal staining than benign tumours (P < 0.0001 and P = 0.038 respectively). Seven omental metastases were also examined and showed a strikingly similar protein distribution to their primary tumour counterparts. The expression pattern of different RNA isoforms, created by alternative splicing of the primary transcript, was identified using reverse transcription-polymerase chain reactions (RT-PCR). The smallest TN RNA splice variant (284 bp) was found in all tumours examined, while the appearance of larger molecular weight transcripts (approximately 490 and 556 bp), as major forms, was predominantly limited to malignant tumours, with 9/12 malignant tumours showing this pattern compared with 1/6 benign tumours. These data suggest that malignant ovarian tumours have increased expression of TN compared with benign tumours and this may be associated with induction of specific isoforms.
Full text
PDF


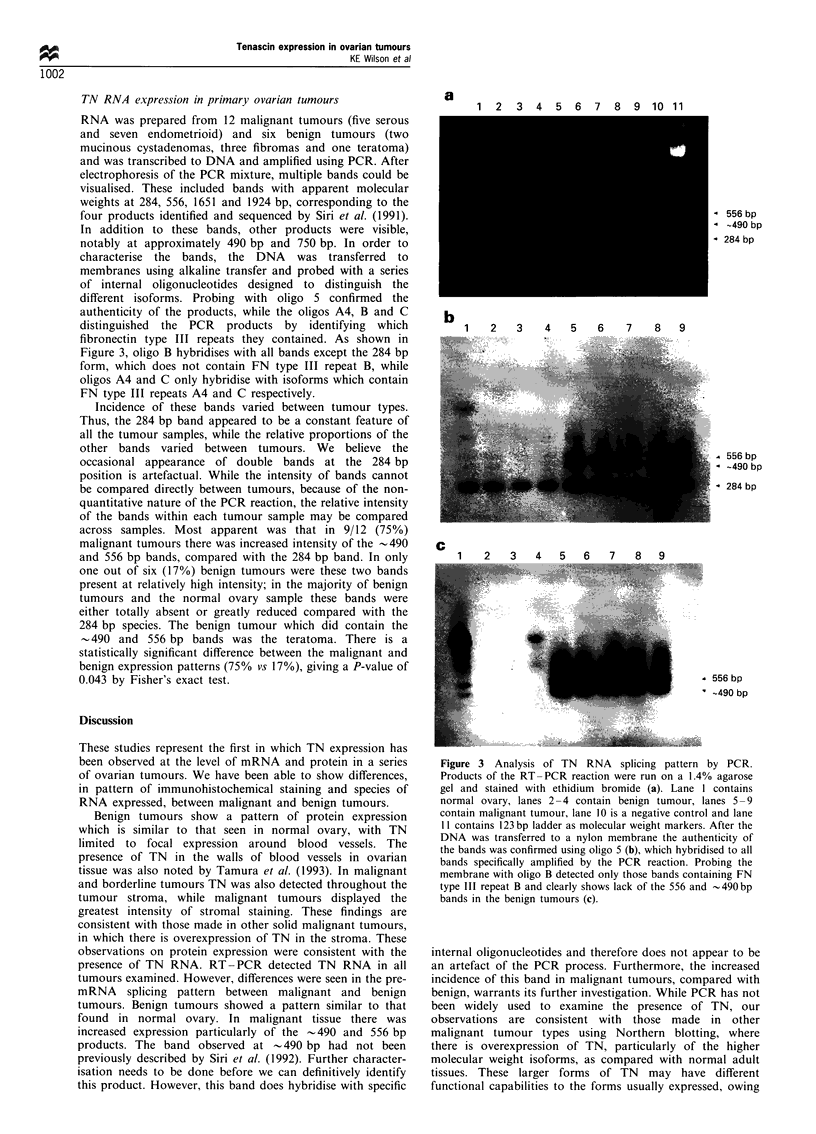


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borsi L., Balza E., Castellani P., Carnemolla B., Ponassi M., Querzé G., Zardi L. Cell-cycle dependent alternative splicing of the tenascin primary transcript. Cell Adhes Commun. 1994 Jan;1(4):307–317. doi: 10.3109/15419069409097262. [DOI] [PubMed] [Google Scholar]
- Borsi L., Balza E., Gaggero B., Allemanni G., Zardi L. The alternative splicing pattern of the tenascin-C pre-mRNA is controlled by the extracellular pH. J Biol Chem. 1995 Mar 17;270(11):6243–6245. doi: 10.1074/jbc.270.11.6243. [DOI] [PubMed] [Google Scholar]
- Borsi L., Carnemolla B., Nicolò G., Spina B., Tanara G., Zardi L. Expression of different tenascin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int J Cancer. 1992 Nov 11;52(5):688–692. doi: 10.1002/ijc.2910520504. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A., Beck K., Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988 May 6;53(3):383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A. Participation of tenascin and transforming growth factor-beta in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 1989 Aug 1;49(15):4322–4325. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Ferguson J. E., Schor A. M., Howell A., Ferguson M. W. Tenascin distribution in the normal human breast is altered during the menstrual cycle and in carcinoma. Differentiation. 1990 Feb;42(3):199–207. doi: 10.1111/j.1432-0436.1990.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Gulcher J. R., Nies D. E., Marton L. S., Stefansson K. An alternatively spliced region of the human hexabrachion contains a repeat of potential N-glycosylation sites. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1588–1592. doi: 10.1073/pnas.86.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howeedy A. A., Virtanen I., Laitinen L., Gould N. S., Koukoulis G. K., Gould V. E. Differential distribution of tenascin in the normal, hyperplastic, and neoplastic breast. Lab Invest. 1990 Dec;63(6):798–806. [PubMed] [Google Scholar]
- Ibrahim S. N., Lightner V. A., Ventimiglia J. B., Ibrahim G. K., Walther P. J., Bigner D. D., Humphrey P. A. Tenascin expression in prostatic hyperplasia, intraepithelial neoplasia, and carcinoma. Hum Pathol. 1993 Sep;24(9):982–989. doi: 10.1016/0046-8177(93)90112-t. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Yoshida T., Tamaki H., Sakakura T. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res. 1995 Sep;1(9):1035–1041. [PubMed] [Google Scholar]
- Jones F. S., Hoffman S., Cunningham B. A., Edelman G. M. A detailed structural model of cytotactin: protein homologies, alternative RNA splicing, and binding regions. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1905–1909. doi: 10.1073/pnas.86.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. K., Gould V. E., Bhattacharyya A., Gould J. E., Howeedy A. A., Virtanen I. Tenascin in normal, reactive, hyperplastic, and neoplastic tissues: biologic and pathologic implications. Hum Pathol. 1991 Jul;22(7):636–643. doi: 10.1016/0046-8177(91)90285-w. [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Chiquet-Ehrismann R., Pearson C. A., Inaguma Y., Taya K., Kawarada Y., Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Bigotti A., Botti C., Castellani P., Risso A. M., Zardi L. Comparative analysis of the expression of the extracellular matrix protein tenascin in normal human fetal, adult and tumor tissues. Int J Cancer. 1991 Apr 1;47(6):811–816. doi: 10.1002/ijc.2910470603. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Erickson H. P., Albino A. P., Garin-Chesa P. Induction of human tenascin (neuronectin) by growth factors and cytokines: cell type-specific signals and signalling pathways. J Cell Sci. 1994 Feb;107(Pt 2):487–497. [PubMed] [Google Scholar]
- Riedl S., Bodenmüller H., Hinz U., Holle R., Möller P., Schlag P., Herfarth C., Faissner A. Significance of tenascin serum level as tumor marker in primary colorectal carcinoma. Int J Cancer. 1995 Feb 20;64(1):65–69. doi: 10.1002/ijc.2910640113. [DOI] [PubMed] [Google Scholar]
- Siri A., Carnemolla B., Saginati M., Leprini A., Casari G., Baralle F., Zardi L. Human tenascin: primary structure, pre-mRNA splicing patterns and localization of the epitopes recognized by two monoclonal antibodies. Nucleic Acids Res. 1991 Feb 11;19(3):525–531. doi: 10.1093/nar/19.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Sriramarao P., Bourdon M. A. A novel tenascin type III repeat is part of a complex of tenascin mRNA alternative splices. Nucleic Acids Res. 1993 Jan 11;21(1):163–168. doi: 10.1093/nar/21.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Sasano H., Suzuki T., Fukaya T., Watanabe T., Kusakabe M., Sakakura T., Yajima A. Distribution of tenascin in normal cycling human ovary. Hum Reprod. 1993 Dec;8(12):2015–2018. doi: 10.1093/oxfordjournals.humrep.a137974. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Hammarback J. A., Jenrath D. A., Mackie E. J., Xu Y. Tenascin expression in the mouse: in situ localization and induction in vitro by bFGF. J Cell Sci. 1993 Jan;104(Pt 1):69–76. doi: 10.1242/jcs.104.1.69. [DOI] [PubMed] [Google Scholar]
- Umbhauer M., Riou J. F., Spring J., Smith J. C., Boucaut J. C. Expression of tenascin mRNA in mesoderm during Xenopus laevis embryogenesis: the potential role of mesoderm patterning in tenascin regionalization. Development. 1992 Sep;116(1):147–157. doi: 10.1242/dev.116.1.147. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Siegal G. P., Chiquet-Ehrismann R., Lightner V. A., Arnholdt H., Knuppen R. Tenascin expression in the human endometrium and in endometrial adenocarcinomas. Lab Invest. 1990 Jun;62(6):725–730. [PubMed] [Google Scholar]
- Yamada S., Ichida T., Matsuda Y., Miyazaki Y., Hatano T., Hata K., Asakura H., Hirota N., Geerts A., Wisse E. Tenascin expression in human chronic liver disease and in hepatocellular carcinoma. Liver. 1992 Feb;12(1):10–16. doi: 10.1111/j.1600-0676.1992.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Zagzag D., Friedlander D. R., Miller D. C., Dosik J., Cangiarella J., Kostianovsky M., Cohen H., Grumet M., Greco M. A. Tenascin expression in astrocytomas correlates with angiogenesis. Cancer Res. 1995 Feb 15;55(4):907–914. [PubMed] [Google Scholar]