Abstract
O6-benzylguanine (O6-BG) and 3-aminobenzamide (3-AB) inhibit the DNA repair proteins O6-alkylguanine-DNA alkyltransferase (AGT) and poly(ADP-ribose) polymerase (PARP) respectively. The effect of O6-BG and/or 3-AB on temozolomide and 1,3-bis(2-chloroethyl)-nitrosourea (BCNU) cytotoxicity, was assessed in seven human tumour cell lines: six with an AGT activity of > 80 fmol mg-1 protein (Mer+) and one with an AGT activity of < 3 fmol mg-1 protein (Mer-). Three of the Mer+ cell lines (LS174T, DLD1 and HCT116) were considered to exhibit resistance to methylation by a mismatch repair deficiency (MMR-), each being known to exhibit microsatellite instability, and DLD1 and HCT116 having well-characterised defects in DNA mismatch binding. Potentiation was defined as the ratio between an IC50 achieved without and with a particular inhibitor treatment. Temozolomide or BCNU cytotoxicity was not potentiated by either inhibitor in the Mer- cell line. Preincubation with O6-BG (100 microM for 1 h) was found to potentiate the cytotoxicity of temozolomide by 1.35- to 1.57-old in Mer+/MMR+ cells, but had no significant effect in Mer+/MMR- cells. In comparison, O6-BG pretreatment enhanced BCNU cytotoxicity by 1.94- to 2.57-fold in all Mer+ cell lines. Post-incubation with 3-AB (2 mM, 48 h) potentiated temozolomide by 1.35- to 1.59-fold in Mer+/MMR+ cells, and when combined with O6-BG pretreatment produced an effect which was at least additive, enhancing cytotoxicity by 1.97- to 2.16-fold. 3-AB treatment also produced marked potentiation (2.20- to 3.12-fold) of temozolomide cytotoxicity in Mer+/MMR- cells. In contrast, 3-AB produced marginal potentiation of BCNU cytotoxicity in only three cell lines (1.19- to 1.35-fold), and did not enhance the cytotoxicity of BCNU with O6-BG treatment in any cell line. These data suggest that the combination of an AGT and PARP inhibitor may have a therapeutic role in potentiating temozolomide activity, but that the inhibition of poly(ADP-ribosyl)ation has little effect on the cytotoxicity of BCNU.
Full text
PDF

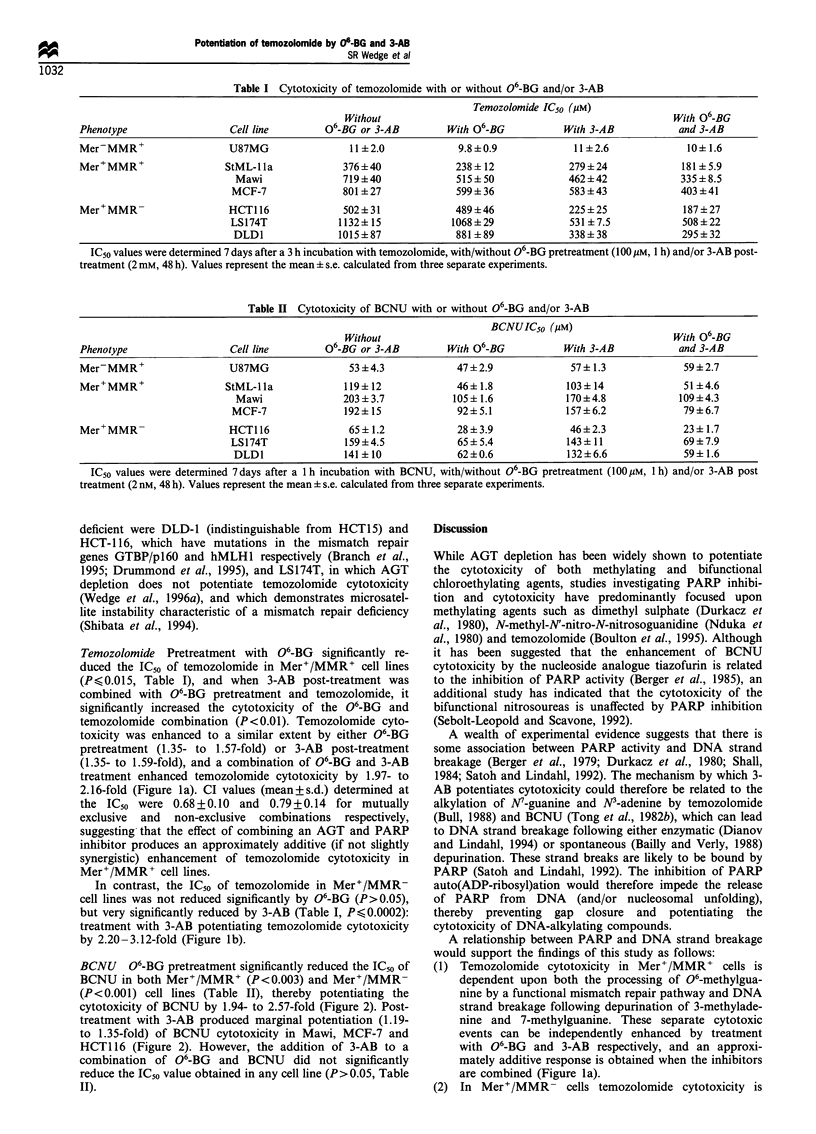
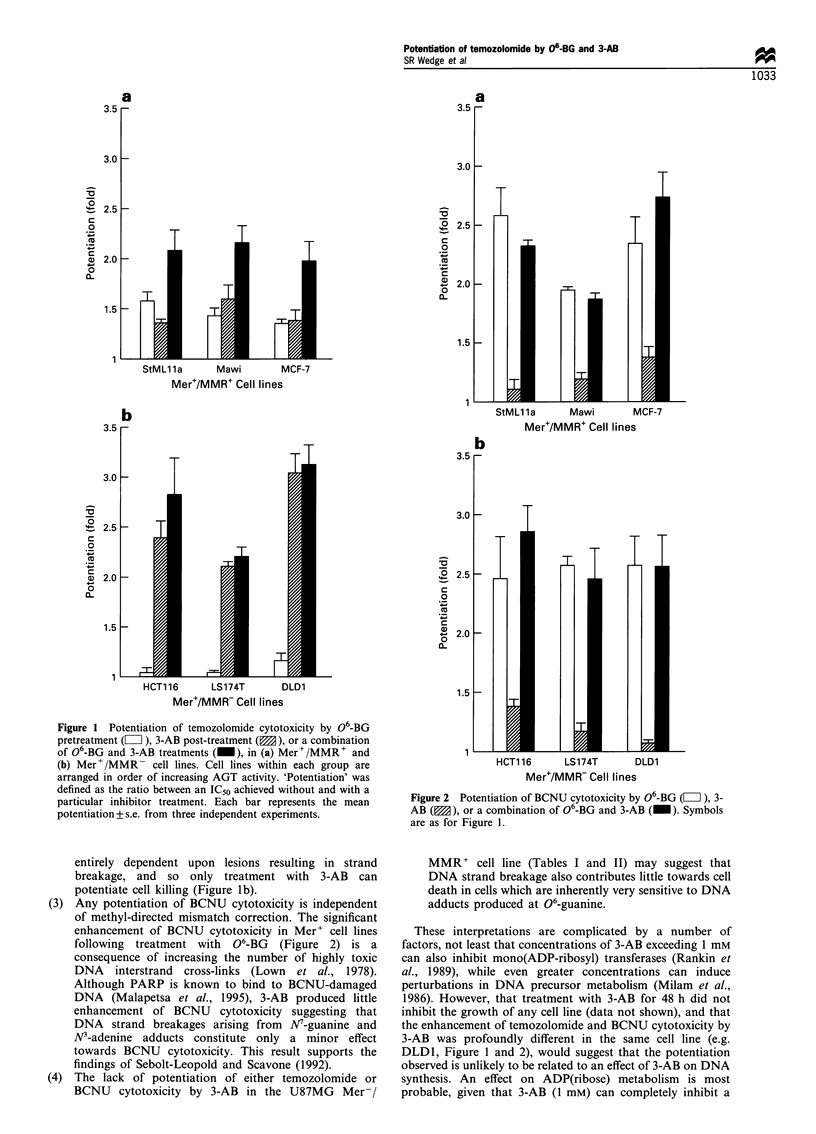



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Höfferer L., Kleczkowska H. E., Malanga M., Naegeli H., Panzeter P. L., Realini C. A. Histone shuttling by poly ADP-ribosylation. Mol Cell Biochem. 1994 Sep;138(1-2):53–59. doi: 10.1007/BF00928443. [DOI] [PubMed] [Google Scholar]
- Baer J. C., Freeman A. A., Newlands E. S., Watson A. J., Rafferty J. A., Margison G. P. Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer. 1993 Jun;67(6):1299–1302. doi: 10.1038/bjc.1993.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G. Possible roles of beta-elimination and delta-elimination reactions in the repair of DNA containing AP (apurinic/apyrimidinic) sites in mammalian cells. Biochem J. 1988 Jul 15;253(2):553–559. doi: 10.1042/bj2530553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A., Berger S. J., Catino D. M., Petzold S. J., Robins R. K. Modulation of nicotinamide adenine dinucleotide and poly(adenosine diphosphoribose) metabolism by the synthetic "C" nucleoside analogs, tiazofurin and selenazofurin. A new strategy for cancer chemotherapy. J Clin Invest. 1985 Feb;75(2):702–709. doi: 10.1172/JCI111750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W., Petzold S. J., Kurohara K. K. Association of poly(adenosine diphosphoribose) synthesis with DNA damage and repair in normal human lymphocytes. J Clin Invest. 1979 Jun;63(6):1164–1171. doi: 10.1172/JCI109410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell W. J., Gerosa M., Aida T., Berger M. S., Rosenblum M. L. Investigation of resistance to DNA cross-linking agents in 9L cell lines with different sensitivities to chloroethylnitrosoureas. Cancer Res. 1985 Aug;45(8):3460–3464. [PubMed] [Google Scholar]
- Boulton S., Pemberton L. C., Porteous J. K., Curtin N. J., Griffin R. J., Golding B. T., Durkacz B. W. Potentiation of temozolomide-induced cytotoxicity: a comparative study of the biological effects of poly(ADP-ribose) polymerase inhibitors. Br J Cancer. 1995 Oct;72(4):849–856. doi: 10.1038/bjc.1995.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branch P., Aquilina G., Bignami M., Karran P. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature. 1993 Apr 15;362(6421):652–654. doi: 10.1038/362652a0. [DOI] [PubMed] [Google Scholar]
- Branch P., Hampson R., Karran P. DNA mismatch binding defects, DNA damage tolerance, and mutator phenotypes in human colorectal carcinoma cell lines. Cancer Res. 1995 Jun 1;55(11):2304–2309. [PubMed] [Google Scholar]
- Buki K. G., Bauer P. I., Hakam A., Kun E. Identification of domains of poly(ADP-ribose) polymerase for protein binding and self-association. J Biol Chem. 1995 Feb 17;270(7):3370–3377. doi: 10.1074/jbc.270.7.3370. [DOI] [PubMed] [Google Scholar]
- Bürkle A., Meyer T., Hilz H., zur Hausen H. Enhancement of N-methyl-N'-nitro-N-nitrosoguanidine-induced DNA amplification in a Simian virus 40-transformed Chinese hamster cell line by 3-aminobenzamide. Cancer Res. 1987 Jul 15;47(14):3632–3636. [PubMed] [Google Scholar]
- Ceccotti S., Dogliotti E., Gannon J., Karran P., Bignami M. O6-methylguanine in DNA inhibits replication in vitro by human cell extracts. Biochemistry. 1993 Dec 14;32(49):13664–13672. doi: 10.1021/bi00212a035. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Berger N. A. Growth-phase-dependent response to DNA damage in poly(ADP-ribose) polymerase deficient cell lines: basis for a new hypothesis describing the role of poly(ADP-ribose) polymerase in DNA replication and repair. Mol Cell Biochem. 1994 Sep;138(1-2):61–69. doi: 10.1007/BF00928444. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Denny B. J., Wheelhouse R. T., Stevens M. F., Tsang L. L., Slack J. A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994 Aug 9;33(31):9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- Dianov G., Lindahl T. Reconstitution of the DNA base excision-repair pathway. Curr Biol. 1994 Dec 1;4(12):1069–1076. doi: 10.1016/s0960-9822(00)00245-1. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Mitchell R. B., Mummert C., Moschel R. C., Pegg A. E. Effect of O6-benzylguanine analogues on sensitivity of human tumor cells to the cytotoxic effects of alkylating agents. Cancer Res. 1991 Jul 1;51(13):3367–3372. [PubMed] [Google Scholar]
- Dolan M. E., Pegg A. E., Moschel R. C., Grindey G. B. Effect of O6-benzylguanine on the sensitivity of human colon tumor xenografts to 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Biochem Pharmacol. 1993 Jul 20;46(2):285–290. doi: 10.1016/0006-2952(93)90416-t. [DOI] [PubMed] [Google Scholar]
- Domoradzki J., Pegg A. E., Dolan M. E., Maher V. M., McCormick J. J. Correlation between O6-methylguanine-DNA-methyltransferase activity and resistance of human cells to the cytotoxic and mutagenic effect of N-methyl-N'-nitro-N-nitrosoguanidine. Carcinogenesis. 1984 Dec;5(12):1641–1647. doi: 10.1093/carcin/5.12.1641. [DOI] [PubMed] [Google Scholar]
- Drummond J. T., Li G. M., Longley M. J., Modrich P. Isolation of an hMSH2-p160 heterodimer that restores DNA mismatch repair to tumor cells. Science. 1995 Jun 30;268(5219):1909–1912. doi: 10.1126/science.7604264. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Levin V. A., Wilson C. B. Brain tumor chemotherapy: an evaluation of agents in current use for phase II and III trials. Cancer Treat Rep. 1980;64(12):1179–1205. [PubMed] [Google Scholar]
- Fairbairn L. J., Cowling G. J., Dexter T. M., Rafferty J. A., Margison G. P., Reipert B. bcl-2 delay of alkylating agent-induced apoptotic death in a murine hemopoietic stem cell line. Mol Carcinog. 1994 Sep;11(1):49–55. doi: 10.1002/mc.2940110109. [DOI] [PubMed] [Google Scholar]
- Fairbairn L. J., Watson A. J., Rafferty J. A., Elder R. H., Margison G. P. O6-benzylguanine increases the sensitivity of human primary bone marrow cells to the cytotoxic effects of temozolomide. Exp Hematol. 1995 Feb;23(2):112–116. [PubMed] [Google Scholar]
- Felker G. M., Friedman H. S., Dolan M. E., Moschel R. C., Schold C. Treatment of subcutaneous and intracranial brain tumor xenografts with O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Chemother Pharmacol. 1993;32(6):471–476. doi: 10.1007/BF00685892. [DOI] [PubMed] [Google Scholar]
- Friedman H. S., Dolan M. E., Pegg A. E., Marcelli S., Keir S., Catino J. J., Bigner D. D., Schold S. C., Jr Activity of temozolomide in the treatment of central nervous system tumor xenografts. Cancer Res. 1995 Jul 1;55(13):2853–2857. [PubMed] [Google Scholar]
- Gerson S. L., Zborowska E., Norton K., Gordon N. H., Willson J. K. Synergistic efficacy of O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) in a human colon cancer xenograft completely resistant to BCNU alone. Biochem Pharmacol. 1993 Jan 26;45(2):483–491. doi: 10.1016/0006-2952(93)90086-c. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Karran P., Lindahl T. O6-Methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983 Jul;43(7):3247–3252. [PubMed] [Google Scholar]
- Jiang B. Z., Bank B. B., Hsiang Y. H., Shen T., Potmesil M., Silber R. Lack of drug-induced DNA cross-links in chlorambucil-resistant Chinese hamster ovary cells. Cancer Res. 1989 Oct 15;49(20):5514–5517. [PubMed] [Google Scholar]
- Juarez-Salinas H., Sims J. L., Jacobson M. K. Poly(ADP-ribose) levels in carcinogen-treated cells. Nature. 1979 Dec 13;282(5740):740–741. doi: 10.1038/282740a0. [DOI] [PubMed] [Google Scholar]
- Karran P., Bignami M. DNA damage tolerance, mismatch repair and genome instability. Bioessays. 1994 Nov;16(11):833–839. doi: 10.1002/bies.950161110. [DOI] [PubMed] [Google Scholar]
- Karran P., Hjelmgren T., Lindahl T. Induction of a DNA glycosylase for N-methylated purines is part of the adaptive response to alkylating agents. Nature. 1982 Apr 22;296(5859):770–773. doi: 10.1038/296770a0. [DOI] [PubMed] [Google Scholar]
- Karran P., Macpherson P., Ceccotti S., Dogliotti E., Griffin S., Bignami M. O6-methylguanine residues elicit DNA repair synthesis by human cell extracts. J Biol Chem. 1993 Jul 25;268(21):15878–15886. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kat A., Thilly W. G., Fang W. H., Longley M. J., Li G. M., Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6424–6428. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley S. L., Basu A., Teicher B. A., Hacker M. P., Hamer D. H., Lazo J. S. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988 Sep 30;241(4874):1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Thatcher N., Margison G. P. O6-alkylguanine-DNA alkyltransferase depletion and regeneration in human peripheral lymphocytes following dacarbazine and fotemustine. Cancer Res. 1991 Jan 15;51(2):619–623. [PubMed] [Google Scholar]
- Ludlum D. B. DNA alkylation by the haloethylnitrosoureas: nature of modifications produced and their enzymatic repair or removal. Mutat Res. 1990 Nov-Dec;233(1-2):117–126. doi: 10.1016/0027-5107(90)90156-x. [DOI] [PubMed] [Google Scholar]
- Magull-Seltenreich A., Zeller W. J. Sensitization of human colon tumour cell lines to carmustine by depletion of O6-alkylguanine-DNA alkyltransferase. J Cancer Res Clin Oncol. 1995;121(4):225–229. doi: 10.1007/BF01366966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcomson R. D., Oren M., Wyllie A. H., Harrison D. J. p53-independent death and p53-induced protection against apoptosis in fibroblasts treated with chemotherapeutic drugs. Br J Cancer. 1995 Oct;72(4):952–957. doi: 10.1038/bjc.1995.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam K. M., Thomas G. H., Cleaver J. E. Disturbances in DNA precursor metabolism associated with exposure to an inhibitor of poly(ADP-ribose) synthetase. Exp Cell Res. 1986 Jul;165(1):260–268. doi: 10.1016/0014-4827(86)90550-1. [DOI] [PubMed] [Google Scholar]
- Mitchell R. B., Moschel R. C., Dolan M. E. Effect of O6-benzylguanine on the sensitivity of human tumor xenografts to 1,3-bis(2-chloroethyl)-1-nitrosourea and on DNA interstrand cross-link formation. Cancer Res. 1992 Mar 1;52(5):1171–1175. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992 Oct 1;52(19):5407–5411. [PubMed] [Google Scholar]
- Nduka N., Skidmore C. J., Shall S. The enhancement of cytotoxicity of N-methyl-N-nitrosourea and of gamma-radiation by inhibitors of poly(ADP-ribose) polymerase. Eur J Biochem. 1980 Apr;105(3):525–530. doi: 10.1111/j.1432-1033.1980.tb04528.x. [DOI] [PubMed] [Google Scholar]
- Newlands E. S., Blackledge G. R., Slack J. A., Rustin G. J., Smith D. B., Stuart N. S., Quarterman C. P., Hoffman R., Stevens M. F., Brampton M. H. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer. 1992 Feb;65(2):287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosseri C., Coppola S., Ghibelli L. Possible involvement of poly(ADP-ribosyl) polymerase in triggering stress-induced apoptosis. Exp Cell Res. 1994 Jun;212(2):367–373. doi: 10.1006/excr.1994.1156. [DOI] [PubMed] [Google Scholar]
- O'Reilly S. M., Newlands E. S., Glaser M. G., Brampton M., Rice-Edwards J. M., Illingworth R. D., Richards P. G., Kennard C., Colquhoun I. R., Lewis P. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993;29A(7):940–942. doi: 10.1016/s0959-8049(05)80198-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990 Oct 1;50(19):6119–6129. [PubMed] [Google Scholar]
- Rankin P. W., Jacobson E. L., Benjamin R. C., Moss J., Jacobson M. K. Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J Biol Chem. 1989 Mar 15;264(8):4312–4317. [PubMed] [Google Scholar]
- Satoh M. S., Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992 Mar 26;356(6367):356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- Satoh M. S., Poirier G. G., Lindahl T. NAD(+)-dependent repair of damaged DNA by human cell extracts. J Biol Chem. 1993 Mar 15;268(8):5480–5487. [PubMed] [Google Scholar]
- Sebolt-Leopold J. S., Scavone S. V. Enhancement of alkylating agent activity in vitro by PD 128763, a potent poly(ADP-ribose) synthetase inhibitor. Int J Radiat Oncol Biol Phys. 1992;22(3):619–621. doi: 10.1016/0360-3016(92)90889-p. [DOI] [PubMed] [Google Scholar]
- Shibata D., Peinado M. A., Ionov Y., Malkhosyan S., Perucho M. Genomic instability in repeated sequences is an early somatic event in colorectal tumorigenesis that persists after transformation. Nat Genet. 1994 Mar;6(3):273–281. doi: 10.1038/ng0394-273. [DOI] [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Evans C. G., Doane-Setzer P., Castro V. M., Tahir M. K., Mannervik B. Denitrosation of 1,3-bis(2-chloroethyl)-1-nitrosourea by class mu glutathione transferases and its role in cellular resistance in rat brain tumor cells. Cancer Res. 1989 May 15;49(10):2621–2625. [PubMed] [Google Scholar]
- Tano K., Shiota S., Collier J., Foote R. S., Mitra S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc Natl Acad Sci U S A. 1990 Jan;87(2):686–690. doi: 10.1073/pnas.87.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale M. J. Antitumour imidazotetrazines--XI: Effect of 8-carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one [CCRG 81045; M and B 39831 NSC 362856] on poly(ADP-ribose) metabolism. Br J Cancer. 1985 Nov;52(5):789–792. doi: 10.1038/bjc.1985.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W. P., Kirk M. C., Ludlum D. B. Formation of the cross-link 1-[N3-deoxycytidyl),2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N'-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. 1982 Aug;42(8):3102–3105. [PubMed] [Google Scholar]
- Tong W. P., Kohn K. W., Ludlum D. B. Modifications of DNA by different haloethylnitrosoureas. Cancer Res. 1982 Nov;42(11):4460–4464. [PubMed] [Google Scholar]
- Waxman D. J. Glutathione S-transferases: role in alkylating agent resistance and possible target for modulation chemotherapy--a review. Cancer Res. 1990 Oct 15;50(20):6449–6454. [PubMed] [Google Scholar]
- Wedge S. R., Newlands E. S. O6-benzylguanine enhances the sensitivity of a glioma xenograft with low O6-alkylguanine-DNA alkyltransferase activity to temozolomide and BCNU. Br J Cancer. 1996 May;73(9):1049–1052. doi: 10.1038/bjc.1996.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedge S. R., Porteus J. K., May B. L., Newlands E. S. Potentiation of temozolomide and BCNU cytotoxicity by O(6)-benzylguanine: a comparative study in vitro. Br J Cancer. 1996 Feb;73(4):482–490. doi: 10.1038/bjc.1996.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinkam R. J., Lin H. S. Reactions of 1,3-bis(2-chloroethyl)-1-nitrosourea and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea in aqueous solution. J Med Chem. 1979 Oct;22(10):1193–1198. doi: 10.1021/jm00196a009. [DOI] [PubMed] [Google Scholar]
- Whish W. J., Davies M. I., Shall S. Stimulation of poly(ADP-ribose) polymerase activity by the anti-tumour antibiotic, streptozotocin. Biochem Biophys Res Commun. 1975 Jul 22;65(2):722–730. doi: 10.1016/s0006-291x(75)80205-1. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Hsieh F. P., Lee P. C., Tseng H. J. Strand- and sequence-specific attenuation of N-methyl-N'-nitro-N-nitrosoguanidine-induced G.C to A.T transitions by expression of human 6-methylguanine-DNA methyltransferase in Chinese hamster ovary cells. Cancer Res. 1994 Jul 15;54(14):3857–3863. [PubMed] [Google Scholar]
- Young R. C., DeVita V. T., Jr, Serpick A. A., Canellos G. P. Treatment of advanced Hodgkin's disease with (1,3 bis (2-chloroethyl)-1-nitrosourea) BCNU. N Engl J Med. 1971 Aug 26;285(9):475–479. doi: 10.1056/NEJM197108262850902. [DOI] [PubMed] [Google Scholar]


