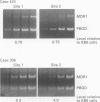
Abstract
We evaluated the MDR1 expression levels in 77 osteosarcomas and investigated whether MDR1 mRNA expression in osteosarcomas varies with location within the tumour, following chemotherapy, or after metastasis. We modified the semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) assay to determine accurately the levels of MDR1 mRNA expression in clinical specimens. We show that specimens collected from multiple locations in six tumours revealed very little variation in MDR1 expression suggesting that the levels of MDR1 in these tumours do not vary greatly with location within the tumour mass. In a comparison of pre and post-chemotherapy specimens it was found that MDR1 levels did not change appreciably following chemotherapy in 16 of 20 cases. In addition, in eight of ten specimens obtained before and after metastasis, the amount of MDR1 mRNA was found to remain relatively constant despite metastatic spread. Thus, many osteosarcomas exhibited intrinsic expression of MDR1 mRNA before multidrug regimens which invariably included doxorubicin and, in most cases, MDR1 expression was not induced following chemotherapeutic treatment. Our results suggest that some osteosarcoma patients may have primary tumours which are resistant to doxorubicin. These individuals may benefit from different chemotherapeutic regimens, e.g. the addition of MDR reversal agents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Bell R. S., O'Connor G., Bell D. F., Jacob J. Effect of doxorubicin on local recurrence following marginal resection in the MGH-OGS murine model. J Orthop Res. 1990 Jan;8(1):105–118. doi: 10.1002/jor.1100080114. [DOI] [PubMed] [Google Scholar]
- Borst P. Genetic mechanisms of drug resistance. A review. Acta Oncol. 1991;30(1):87–105. doi: 10.3109/02841869109091819. [DOI] [PubMed] [Google Scholar]
- Campos L., Guyotat D., Archimbaud E., Calmard-Oriol P., Tsuruo T., Troncy J., Treille D., Fiere D. Clinical significance of multidrug resistance P-glycoprotein expression on acute nonlymphoblastic leukemia cells at diagnosis. Blood. 1992 Jan 15;79(2):473–476. [PubMed] [Google Scholar]
- Chan H. S., Haddad G., Thorner P. S., DeBoer G., Lin Y. P., Ondrusek N., Yeger H., Ling V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N Engl J Med. 1991 Dec 5;325(23):1608–1614. doi: 10.1056/NEJM199112053252304. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Thorner P. S., Haddad G., Ling V. Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood. J Clin Oncol. 1990 Apr;8(4):689–704. doi: 10.1200/JCO.1990.8.4.689. [DOI] [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst. 1993 Apr 21;85(8):632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Finke J., Fritzen R., Ternes P., Lange W., Dölken G. An improved strategy and a useful housekeeping gene for RNA analysis from formalin-fixed, paraffin-embedded tissues by PCR. Biotechniques. 1993 Mar;14(3):448–453. [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Holzmayer T. A., Hilsenbeck S., Von Hoff D. D., Roninson I. B. Clinical correlates of MDR1 (P-glycoprotein) gene expression in ovarian and small-cell lung carcinomas. J Natl Cancer Inst. 1992 Oct 7;84(19):1486–1491. doi: 10.1093/jnci/84.19.1486. [DOI] [PubMed] [Google Scholar]
- Kandel R. A., Campbell S., Noble-Topham S., Bell R., Andrulis I. L. Correlation of p-glycoprotein detection by immunohistochemistry with mdr-1 mRNA levels in osteosarcomas. Pilot study. Diagn Mol Pathol. 1995 Mar;4(1):59–65. doi: 10.1097/00019606-199503000-00011. [DOI] [PubMed] [Google Scholar]
- Lin R. I., Schjeide O. A. Micro estimation of RNA by the cupric ion catalyzed orcinol reaction. Anal Biochem. 1969 Mar;27(3):473–483. doi: 10.1016/0003-2697(69)90061-x. [DOI] [PubMed] [Google Scholar]
- Marie J. P., Zittoun R., Sikic B. I. Multidrug resistance (mdr1) gene expression in adult acute leukemias: correlations with treatment outcome and in vitro drug sensitivity. Blood. 1991 Aug 1;78(3):586–592. [PubMed] [Google Scholar]
- Miller T. P., Grogan T. M., Dalton W. S., Spier C. M., Scheper R. J., Salmon S. E. P-glycoprotein expression in malignant lymphoma and reversal of clinical drug resistance with chemotherapy plus high-dose verapamil. J Clin Oncol. 1991 Jan;9(1):17–24. doi: 10.1200/JCO.1991.9.1.17. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Beck C., Holzmayer T. A., Chin J. E., Wunder J. S., Andrulis I. L., Gazdar A. F., Willman C. L., Griffith B., Von Hoff D. D. Quantitative analysis of MDR1 (multidrug resistance) gene expression in human tumors by polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7160–7164. doi: 10.1073/pnas.87.18.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. V., Anthony D. C., Piwnica-Worms D. MDR1 gene-specific monoclonal antibody C494 cross-reacts with pyruvate carboxylase. Cancer Res. 1994 Mar 15;54(6):1536–1541. [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Vergier B., Cany L., Bonnet F., Robert J., de Mascarel A., Coindre J. M. Expression of MDR1/P glycoprotein in human sarcomas. Br J Cancer. 1993 Dec;68(6):1221–1226. doi: 10.1038/bjc.1993.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R. S., Jakate S. M., Dominguez J. M., Lebovitz M. D., Koukoulis G. K., Kuszak J. R., Klusens L. F., Grogan T. M., Saclarides T. J., Roninson I. B. Relationship of the expression of the multidrug resistance gene product (P-glycoprotein) in human colon carcinoma to local tumor aggressiveness and lymph node metastasis. Cancer Res. 1991 May 15;51(10):2720–2726. [PubMed] [Google Scholar]
- Wunder J. S., Bell R. S., Wold L., Andrulis I. L. Expression of the multidrug resistance gene in osteosarcoma: a pilot study. J Orthop Res. 1993 May;11(3):396–403. doi: 10.1002/jor.1100110311. [DOI] [PubMed] [Google Scholar]