Abstract
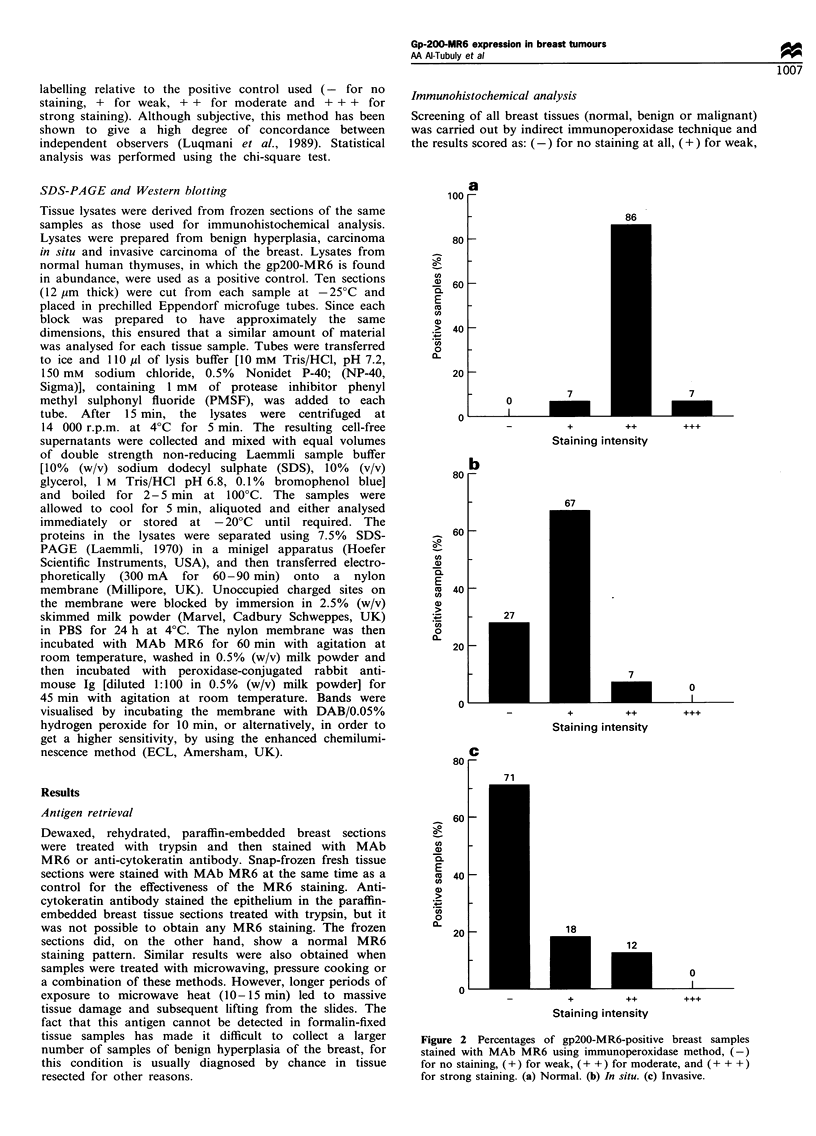
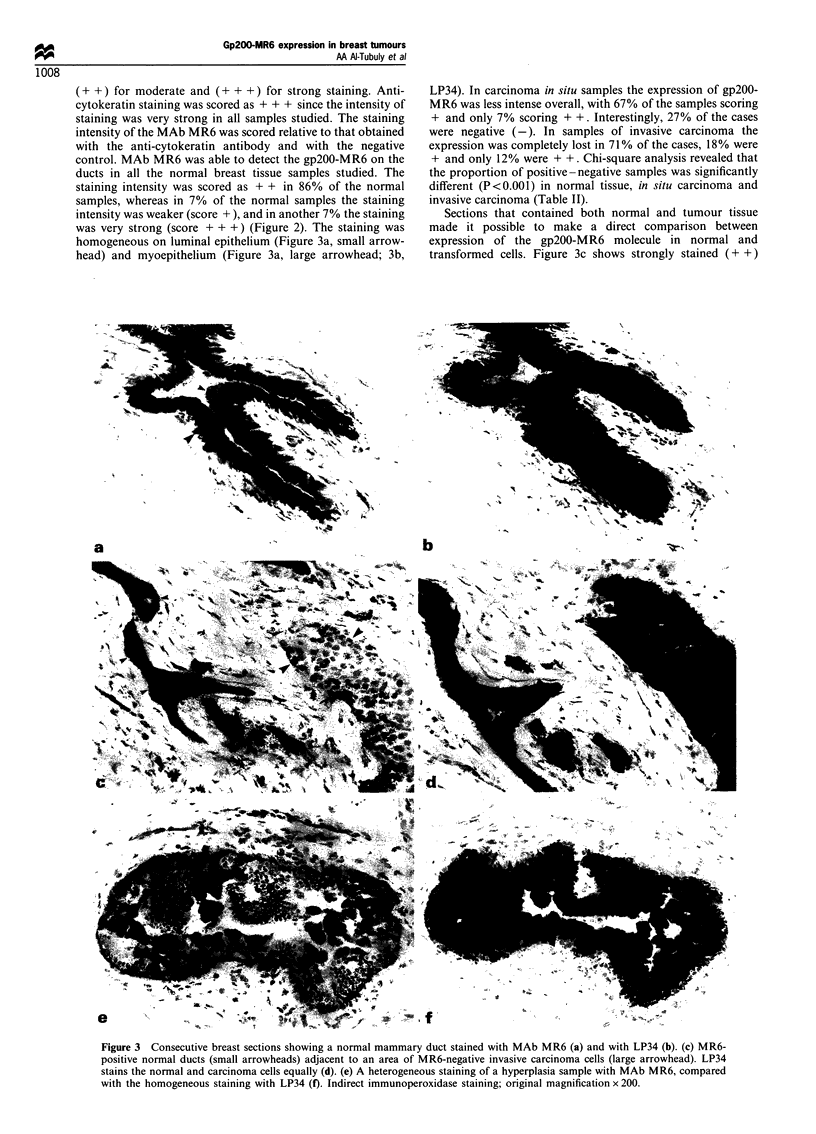
In this study, we used immunohistochemical and biochemical analysis to show that gp200-MR6, a 200 kDa molecule that is functionally associated with the human interleukin 4 (IL-4) receptor complex, is expressed at high levels on normal breast epithelial tissues, at lower levels on in situ carcinomas, and that the expression is lost in the invasive carcinoma of the breast. Furthermore, a preliminary study showed that benign epithelial hyperplasia of the breast expresses the gp200-MR6 heterogeneously. Two populations of cells have been observed: MR6 positive and MR6 negative. Interestingly, MR6-positive cells were observed to have different morphology from those that were MR6 negative; the nuclei of the former were larger and rounded in shape, whereas the nuclei of the latter were relatively small and oval in shape. In sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting, monoclonal antibody MR6 detects the same molecular weight molecule in both normal and transformed tissue, indicating that the molecule is not a product of a truncated gene. The intensity of the gp200-MR6 bands correlates with the immunohistochemical data, indicating that the molecule is expressed at high levels in normal tissue and at lower levels in malignant tissue. These results suggest that analysis of gp200-MR6 expression may be useful in tumour grading and prognostic evaluation in breast cancer. Moreover, the molecule may be involved early in the process of tumorigenesis of the breast, in which a loss or a down-regulation of gp200-MR6 could contribute towards tumour development and progression via an effect on cell growth and differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al Jabaari B., Ladyman H. M., Larché M., Sivolapenko G. B., Epenetos A. A., Ritter M. A. Elevated expression of the interleukin 4 receptor in carcinoma: a target for immunotherapy? Br J Cancer. 1989 Jun;59(6):910–914. doi: 10.1038/bjc.1989.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua G., Sobel M. E., Liotta L. A., Steeg P. S. Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas with lymph node involvement and other histopathological indicators of high metastatic potential. Cancer Res. 1989 Sep 15;49(18):5185–5190. [PubMed] [Google Scholar]
- Burchell J., Durbin H., Taylor-Papadimitriou J. Complexity of expression of antigenic determinants, recognized by monoclonal antibodies HMFG-1 and HMFG-2, in normal and malignant human mammary epithelial cells. J Immunol. 1983 Jul;131(1):508–513. [PubMed] [Google Scholar]
- Bussolati G., Botta G., Gugliotta P. Actin-rich (myoepithelial) cells in ductal carcinoma-in-situ of the breast. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(3):251–259. doi: 10.1007/BF02892422. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Guelstein V. I., Tchypysheva T. A., Ermilova V. D., Litvinova L. V., Troyanovsky S. M., Bannikov G. A. Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer. 1988 Aug 15;42(2):147–153. doi: 10.1002/ijc.2910420202. [DOI] [PubMed] [Google Scholar]
- Imami N., Larché M., Ritter M. A. Inhibition of alloreactivity by mAb MR6: differential effects on IL-2- and IL-4- producing human T cells. Int Immunol. 1994 Oct;6(10):1575–1584. doi: 10.1093/intimm/6.10.1575. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahm H., Schnyder B., Wyniger J., Borbenyi Z., Yilmaz A., Car B. D., Fischer J. R., Givel J. C., Ryffel B. Growth inhibition of human colorectal-carcinoma cells by interleukin-4 and expression of functional interleukin-4 receptors. Int J Cancer. 1994 Nov 1;59(3):440–447. doi: 10.1002/ijc.2910590325. [DOI] [PubMed] [Google Scholar]
- Larche M., Lamb J. R., O'Hehir R. E., Imami-Shita N., Zanders E. D., Quint D. E., Moqbel R., Ritter M. A. Functional evidence for a monoclonal antibody that binds to the human IL-4 receptor. Immunology. 1988 Dec;65(4):617–622. [PMC free article] [PubMed] [Google Scholar]
- Lennington W. J., Jensen R. A., Dalton L. W., Page D. L. Ductal carcinoma in situ of the breast. Heterogeneity of individual lesions. Cancer. 1994 Jan 1;73(1):118–124. doi: 10.1002/1097-0142(19940101)73:1<118::aid-cncr2820730121>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Luqmani Y., Bennett C., Paterson I., Corbishley C. M., Rio M. C., Chambon P., Ryall G. Expression of the pS2 gene in normal, benign and neoplastic human stomach. Int J Cancer. 1989 Nov 15;44(5):806–812. doi: 10.1002/ijc.2910440510. [DOI] [PubMed] [Google Scholar]
- Mahendran R., McIlhinney R., O'Hare M., Monaghan P., Gusterson B. Expression of the common acute lymphoblastic leukaemia antigen (CALLA) in the human breast. Mol Cell Probes. 1989 Mar;3(1):39–44. doi: 10.1016/0890-8508(89)90035-2. [DOI] [PubMed] [Google Scholar]
- Mat I., Larche M., Melcher D., Ritter M. A. Tumour-associated upregulation of the IL-4 receptor complex. Br J Cancer Suppl. 1990 Jul;10:96–98. [PMC free article] [PubMed] [Google Scholar]
- Norton A. J., Jordan S., Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994 Aug;173(4):371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Page D. L., Dupont W. D., Rogers L. W., Rados M. S. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985 Jun 1;55(11):2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Hughes C. M. Immunocytochemical identification of cell types in human mammary gland: variations in cellular markers are dependent on glandular topography and differentiation. J Histochem Cytochem. 1989 Jul;37(7):1087–1100. doi: 10.1177/37.7.2471725. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Pozzatti R., Liotta L. A., Sobel M. E. Altered expression of NM23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastasis. Cancer Res. 1988 Nov 15;48(22):6550–6554. [PubMed] [Google Scholar]
- Tavassoli F. A., Norris H. J. A comparison of the results of long-term follow-up for atypical intraductal hyperplasia and intraductal hyperplasia of the breast. Cancer. 1990 Feb 1;65(3):518–529. doi: 10.1002/1097-0142(19900201)65:3<518::aid-cncr2820650324>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Toi M., Bicknell R., Harris A. L. Inhibition of colon and breast carcinoma cell growth by interleukin-4. Cancer Res. 1992 Jan 15;52(2):275–279. [PubMed] [Google Scholar]
- Tungekar M. F., Gatter K. C., Ritter M. A. Bladder carcinomas and normal urothelium universally express gp200-MR6, a molecule functionally associated with the interleukin 4 receptor (CD 124). Br J Cancer. 1996 Feb;73(4):429–432. doi: 10.1038/bjc.1996.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungekar M. F., Turley H., Dunnill M. S., Gatter K. C., Ritter M. A., Harris A. L. Interleukin 4 receptor expression on human lung tumors and normal lung. Cancer Res. 1991 Jan 1;51(1):261–264. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., MacKenzie W. A., Schuurman H. J., Ritter M. A., Price K. M., Broekhuizen R., Kater L. The human thymus microenvironment: heterogeneity detected by monoclonal anti-epithelial cell antibodies. Immunology. 1985 Apr;54(4):745–754. [PMC free article] [PubMed] [Google Scholar]