Abstract
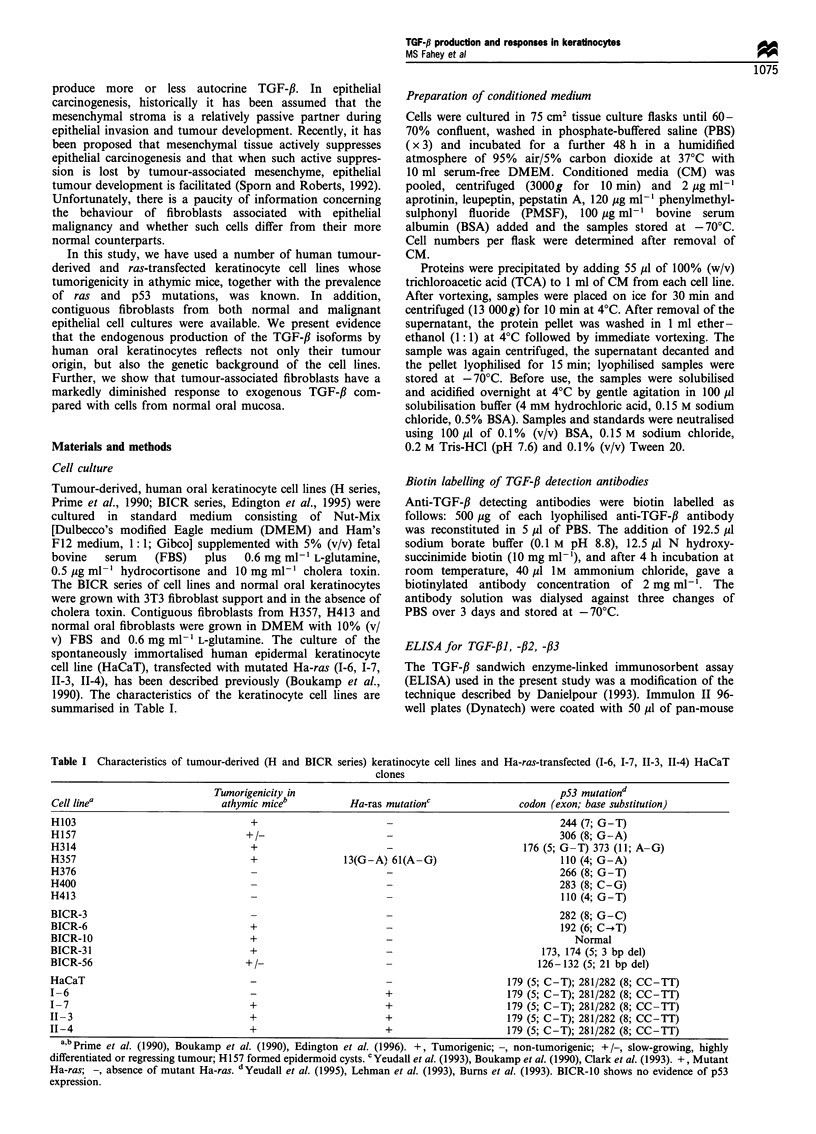
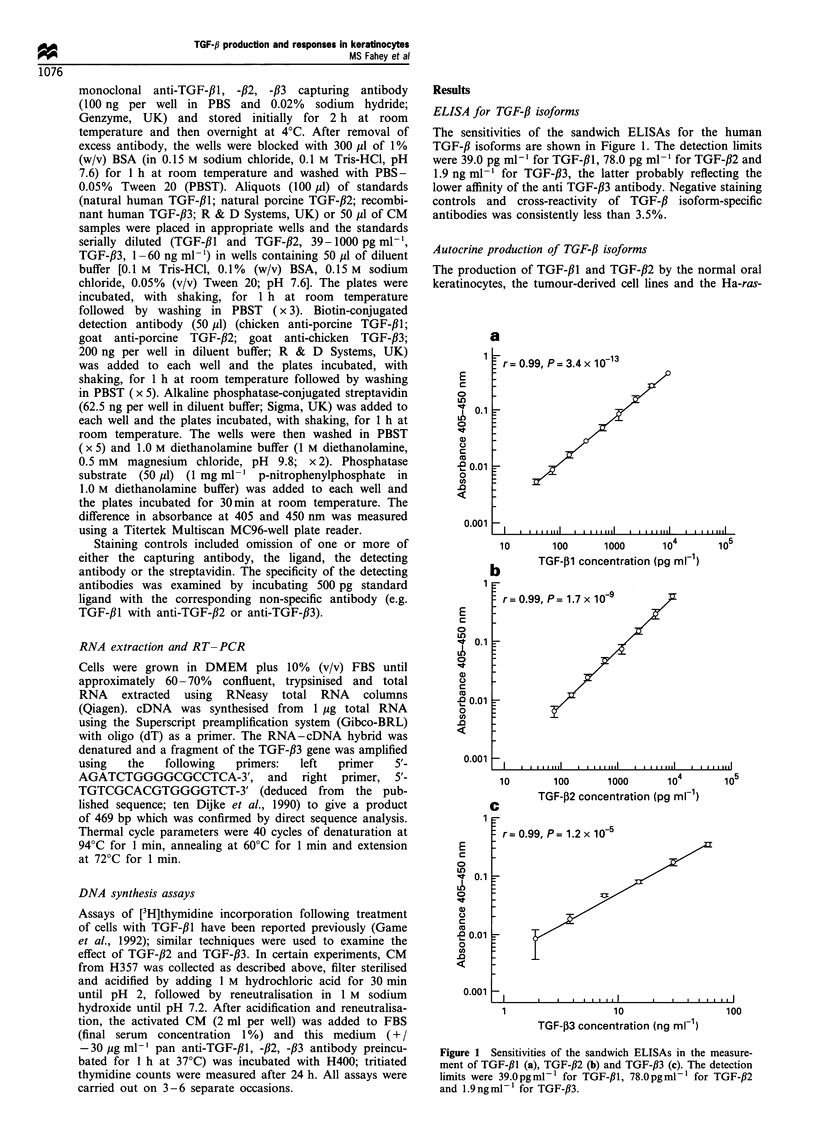
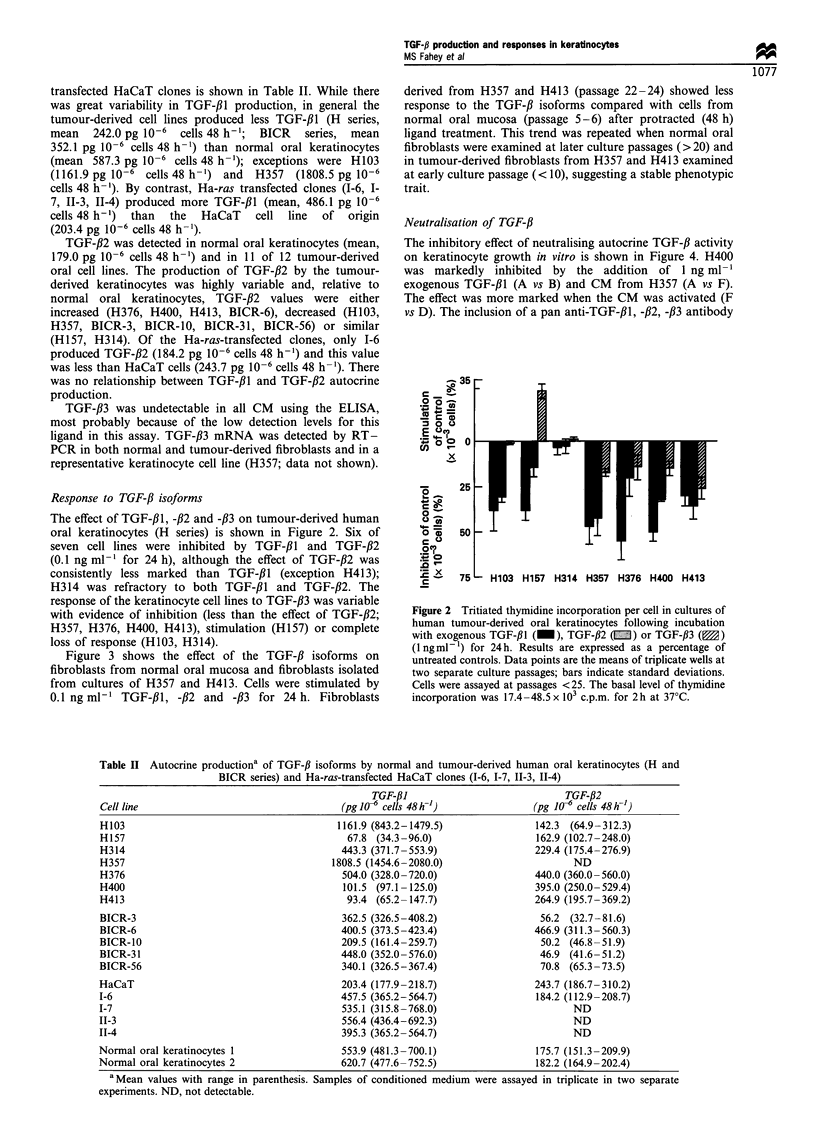
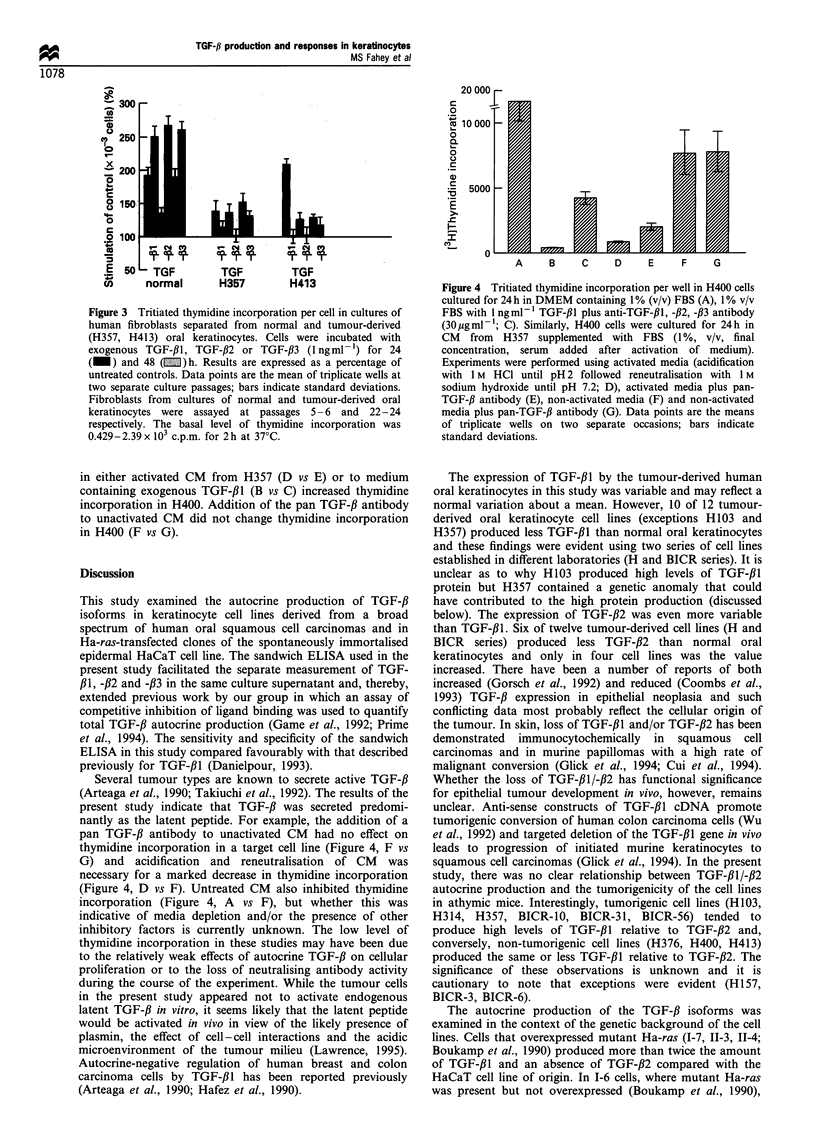
This study examined the autocrine production of TGF-beta 1, -beta 2 and -beta 3 in culture supernatants from tumour-derived (H series, n = 7; BICR series, n = 5), Ha-ras-transfected (n = 4) and normal (n = 2) human keratinocytes using a sandwich enzyme-linked immunosorbent assay (ELISA). Detection limits were 39.0 pg ml-1 for TGF-beta 1, 78.0 pg ml-1 for TGF-beta 2 and 1.9 ng ml-1 for TGF-beta 3. Tumour-derived oral keratinocytes predominantly produced less TGF-beta 1 than normal oral epithelial cells; the expression of endogenous TGF-beta 2 was variable. In keratinocytes containing mutant Ha-ras, TGF-beta 1 production was enhanced and TGF-beta 2 was undetectable. TGF-beta 3 mRNA was detected by reverse transcription-polymerase chain reaction (RT-PCR) but the protein was not detected in conditioned media, most probably because of the low detection limits of the ELISA for this isoform. Neutralisation experiments indicated that the latent TGF-beta peptide was secreted in keratinocyte conditioned medium. Seven tumour-derived keratinocyte cell lines (H series) and fibroblasts separated from normal (n = 1) and tumour-derived (n = 2) keratinocyte cultures were examined for their response to exogenous TGF-beta 1, -beta 2 and -beta 3. Six of seven tumour-derived keratinocyte cell lines were inhibited by TGF-beta 1 and TGF-beta 2 (-beta 1 > -beta 2); one cell line was refractory to both TGF-beta 1 and TGF-beta 2. Keratinocytes were inhibited (4 of 7), stimulated (1 of 7) or failed to respond (2 of 7) to TGF-beta 3, TGF-beta 1, -beta 2 and -beta 3 stimulated both normal and tumour-associated fibroblasts, but the tumour-associated fibroblasts showed less response to the ligands than their normal counterparts following prolonged treatment with each isoform. The results demonstrate variable autocrine production of TGF-beta isoforms by malignant keratinocytes, with loss of TGF-beta 1 generally associated with the tumour-derived phenotype and modification of endogenous isoform production dependent on the genetic background of the tumour cells. Further, the variable response of the tumour-derived keratinocytes and contiguous fibroblasts to the TGF-beta isoforms suggests that dysregulation of TGF-beta autocrine and paracrine networks are common characteristics of squamous epithelial malignancy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arteaga C. L., Coffey R. J., Jr, Dugger T. C., McCutchen C. M., Moses H. L., Lyons R. M. Growth stimulation of human breast cancer cells with anti-transforming growth factor beta antibodies: evidence for negative autocrine regulation by transforming growth factor beta. Cell Growth Differ. 1990 Aug;1(8):367–374. [PubMed] [Google Scholar]
- Arteaga C. L., Dugger T. C., Winnier A. R., Forbes J. T. Evidence for a positive role of transforming growth factor-beta in human breast cancer cell tumorigenesis. J Cell Biochem Suppl. 1993;17G:187–193. doi: 10.1002/jcb.240531134. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Stanbridge E. J., Foo D. Y., Cerutti P. A., Fusenig N. E. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990 May 1;50(9):2840–2847. [PubMed] [Google Scholar]
- Burns J. E., Baird M. C., Clark L. J., Burns P. A., Edington K., Chapman C., Mitchell R., Robertson G., Soutar D., Parkinson E. K. Gene mutations and increased levels of p53 protein in human squamous cell carcinomas and their cell lines. Br J Cancer. 1993 Jun;67(6):1274–1284. doi: 10.1038/bjc.1993.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheifetz S., Hernandez H., Laiho M., ten Dijke P., Iwata K. K., Massagué J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990 Nov 25;265(33):20533–20538. [PubMed] [Google Scholar]
- Clark L. J., Edington K., Swan I. R., McLay K. A., Newlands W. J., Wills L. C., Young H. A., Johnston P. W., Mitchell R., Robertson G. The absence of Harvey ras mutations during development and progression of squamous cell carcinomas of the head and neck. Br J Cancer. 1993 Sep;68(3):617–620. doi: 10.1038/bjc.1993.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Bascom C. C., Sipes N. J., Graves-Deal R., Weissman B. E., Moses H. L. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol. 1988 Aug;8(8):3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletta A. A., Wakefield L. M., Howell F. V., van Roozendaal K. E., Danielpour D., Ebbs S. R., Sporn M. B., Baum M. Anti-oestrogens induce the secretion of active transforming growth factor beta from human fetal fibroblasts. Br J Cancer. 1990 Sep;62(3):405–409. doi: 10.1038/bjc.1990.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs L. M., Pigott D. A., Eydmann M. E., Proctor A. J., Knowles M. A. Reduced expression of TGF beta is associated with advanced disease in transitional cell carcinoma. Br J Cancer. 1993 Mar;67(3):578–584. doi: 10.1038/bjc.1993.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Kemp C. J., Duffie E., Balmain A., Akhurst R. J. Lack of transforming growth factor-beta 1 expression in benign skin tumors of p53null mice is prognostic for a high risk of malignant conversion. Cancer Res. 1994 Nov 15;54(22):5831–5836. [PubMed] [Google Scholar]
- Danielpour D. Improved sandwich enzyme-linked immunosorbent assays for transforming growth factor beta 1. J Immunol Methods. 1993 Jan 14;158(1):17–25. doi: 10.1016/0022-1759(93)90254-5. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Kim K. Y., Winokur T. S., Sporn M. B. Differential regulation of the expression of transforming growth factor-beta s 1 and 2 by retinoic acid, epidermal growth factor, and dexamethasone in NRK-49F and A549 cells. J Cell Physiol. 1991 Aug;148(2):235–244. doi: 10.1002/jcp.1041480208. [DOI] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Dotto G. P., Weinberg R. A., Ariza A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6389–6393. doi: 10.1073/pnas.85.17.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington K. G., Loughran O. P., Berry I. J., Parkinson E. K. Cellular immortality: a late event in the progression of human squamous cell carcinoma of the head and neck associated with p53 alteration and a high frequency of allele loss. Mol Carcinog. 1995 Aug;13(4):254–265. doi: 10.1002/mc.2940130408. [DOI] [PubMed] [Google Scholar]
- Fynan T. M., Reiss M. Resistance to inhibition of cell growth by transforming growth factor-beta and its role in oncogenesis. Crit Rev Oncog. 1993;4(5):493–540. [PubMed] [Google Scholar]
- Game S. M., Huelsen A., Patel V., Donnelly M., Yeudall W. A., Stone A., Fusenig N. E., Prime S. S. Progressive abrogation of TGF-beta 1 and EGF growth control is associated with tumour progression in ras-transfected human keratinocytes. Int J Cancer. 1992 Sep 30;52(3):461–470. doi: 10.1002/ijc.2910520322. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Kim S. J., Roberts A. B., Sporn M. B. Characterization of the mouse transforming growth factor-beta 1 promoter and activation by the Ha-ras oncogene. Mol Cell Biol. 1991 Jan;11(1):84–92. doi: 10.1128/mcb.11.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. B., Kulkarni A. B., Tennenbaum T., Hennings H., Flanders K. C., O'Reilly M., Sporn M. B., Karlsson S., Yuspa S. H. Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6076–6080. doi: 10.1073/pnas.90.13.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. B., Lee M. M., Darwiche N., Kulkarni A. B., Karlsson S., Yuspa S. H. Targeted deletion of the TGF-beta 1 gene causes rapid progression to squamous cell carcinoma. Genes Dev. 1994 Oct 15;8(20):2429–2440. doi: 10.1101/gad.8.20.2429. [DOI] [PubMed] [Google Scholar]
- Gorsch S. M., Memoli V. A., Stukel T. A., Gold L. I., Arrick B. A. Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Cancer Res. 1992 Dec 15;52(24):6949–6952. [PubMed] [Google Scholar]
- Hafez M. M., Infante D., Winawer S., Friedman E. Transforming growth factor beta 1 acts as an autocrine-negative growth regulator in colon enterocytic differentiation but not in goblet cell maturation. Cell Growth Differ. 1990 Dec;1(12):617–626. [PubMed] [Google Scholar]
- Jennings J. C., Mohan S., Linkhart T. A., Widstrom R., Baylink D. J. Comparison of the biological actions of TGF beta-1 and TGF beta-2: differential activity in endothelial cells. J Cell Physiol. 1988 Oct;137(1):167–172. doi: 10.1002/jcp.1041370120. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Park K., Koeller D., Kim K. Y., Wakefield L. M., Sporn M. B., Roberts A. B. Post-transcriptional regulation of the human transforming growth factor-beta 1 gene. J Biol Chem. 1992 Jul 5;267(19):13702–13707. [PubMed] [Google Scholar]
- Lawrence D. A. Transforming growth factor-beta: an overview. Kidney Int Suppl. 1995 Jun;49:S19–S23. [PubMed] [Google Scholar]
- Lehman T. A., Modali R., Boukamp P., Stanek J., Bennett W. P., Welsh J. A., Metcalf R. A., Stampfer M. R., Fusenig N., Rogan E. M. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993 May;14(5):833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- Levine J. H., Moses H. L., Gold L. I., Nanney L. B. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993 Aug;143(2):368–380. [PMC free article] [PubMed] [Google Scholar]
- MacCallum J., Bartlett J. M., Thompson A. M., Keen J. C., Dixon J. M., Miller W. R. Expression of transforming growth factor beta mRNA isoforms in human breast cancer. Br J Cancer. 1994 Jun;69(6):1006–1009. doi: 10.1038/bjc.1994.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C., Ramon y Cajal S., Dotto G. P. Escape from transforming growth factor beta control and oncogene cooperation in skin tumor development. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9613–9617. doi: 10.1073/pnas.88.21.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Pietenpol J. A., Pittelkow M. R., Holt J. T., Moses H. L. Transforming growth factor beta 1 regulation of c-myc expression, pRB phosphorylation, and cell cycle progression in keratinocytes. Cell Growth Differ. 1992 May;3(5):291–298. [PubMed] [Google Scholar]
- Patamalai B., Burow D. L., Gimenez-Conti I., Zenklusen J. C., Conti C. J., Klein-Szanto A. J., Fischer S. M. Altered expression of transforming growth factor-beta 1 mRNA and protein in mouse skin carcinogenesis. Mol Carcinog. 1994 Apr;9(4):220–229. doi: 10.1002/mc.2940090406. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Holt J. T., Stein R. W., Moses H. L. Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci U S A. 1990 May;87(10):3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime S. S., Matthews J. B., Patel V., Game S. M., Donnelly M., Stone A., Paterson I. C., Sandy J. R., Yeudall W. A. TGF-beta receptor regulation mediates the response to exogenous ligand but is independent of the degree of cellular differentiation in human oral keratinocytes. Int J Cancer. 1994 Feb 1;56(3):406–412. doi: 10.1002/ijc.2910560320. [DOI] [PubMed] [Google Scholar]
- Prime S. S., Nixon S. V., Crane I. J., Stone A., Matthews J. B., Maitland N. J., Remnant L., Powell S. K., Game S. M., Scully C. The behaviour of human oral squamous cell carcinoma in cell culture. J Pathol. 1990 Mar;160(3):259–269. doi: 10.1002/path.1711600313. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Rushton G., Ferguson J. E., Howell A., Redford J., Schor S. L. Phenotypic heterogeneity in breast fibroblasts: functional anomaly in fibroblasts from histologically normal tissue adjacent to carcinoma. Int J Cancer. 1994 Oct 1;59(1):25–32. doi: 10.1002/ijc.2910590107. [DOI] [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995 Mar;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiuchi H., Tada T., Li X. F., Ogata M., Ikeda T., Fujimoto S., Fujiwara H., Hamaoka T. Particular types of tumor cells have the capacity to convert transforming growth factor beta from a latent to an active form. Cancer Res. 1992 Oct 15;52(20):5641–5646. [PubMed] [Google Scholar]
- Welch D. R., Fabra A., Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. P., Theodorescu D., Kerbel R. S., Willson J. K., Mulder K. M., Humphrey L. E., Brattain M. G. TGF-beta 1 is an autocrine-negative growth regulator of human colon carcinoma FET cells in vivo as revealed by transfection of an antisense expression vector. J Cell Biol. 1992 Jan;116(1):187–196. doi: 10.1083/jcb.116.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeudall W. A., Paterson I. C., Patel V., Prime S. S. Presence of human papillomavirus sequences in tumour-derived human oral keratinocytes expressing mutant p53. Eur J Cancer B Oral Oncol. 1995 Mar;31B(2):136–143. doi: 10.1016/0964-1955(94)00030-8. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Iwata K. K., Thorikay M., Schwedes J., Stewart A., Pieler C. Molecular characterization of transforming growth factor type beta 3. Ann N Y Acad Sci. 1990;593:26–42. doi: 10.1111/j.1749-6632.1990.tb16097.x. [DOI] [PubMed] [Google Scholar]


