Abstract
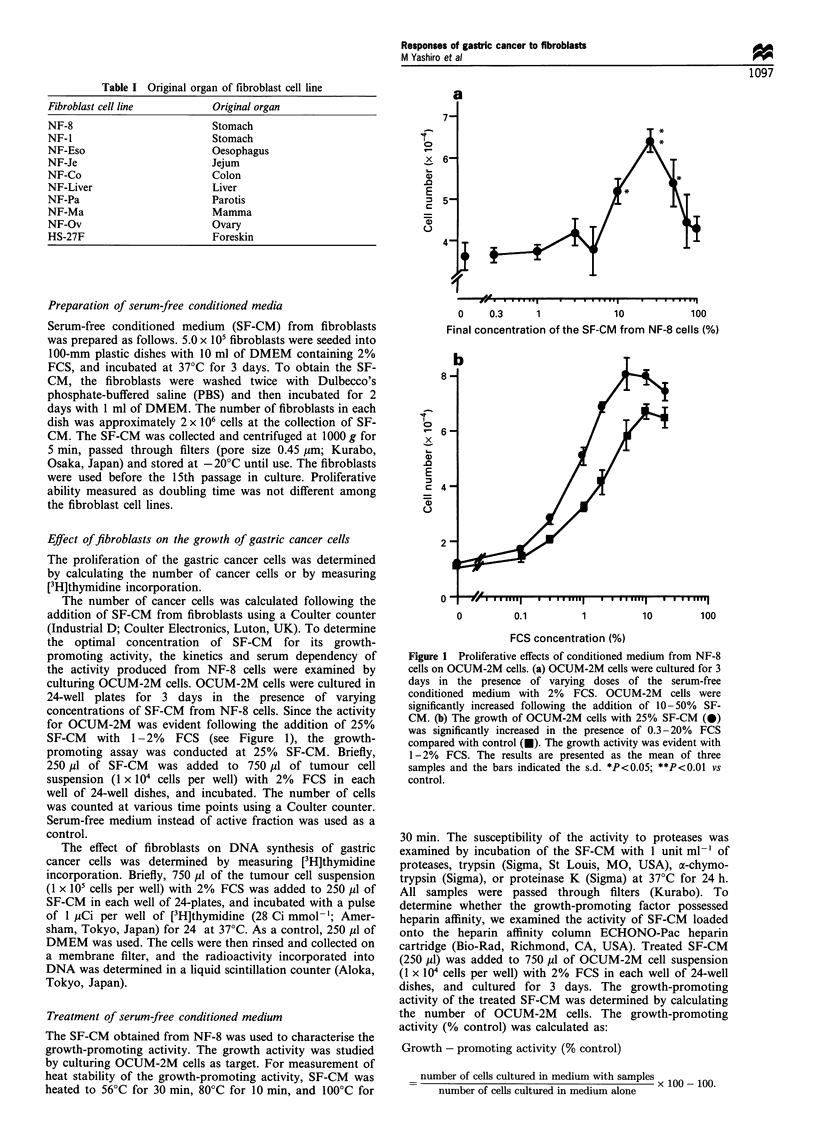
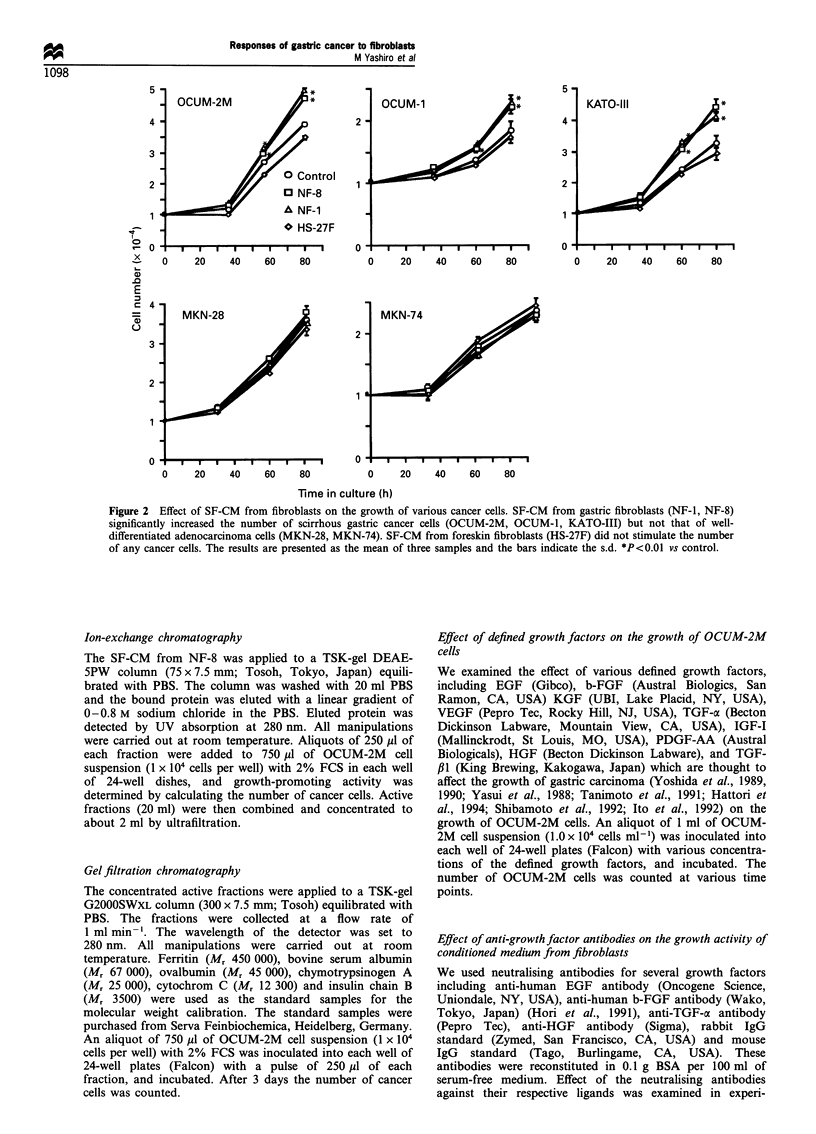
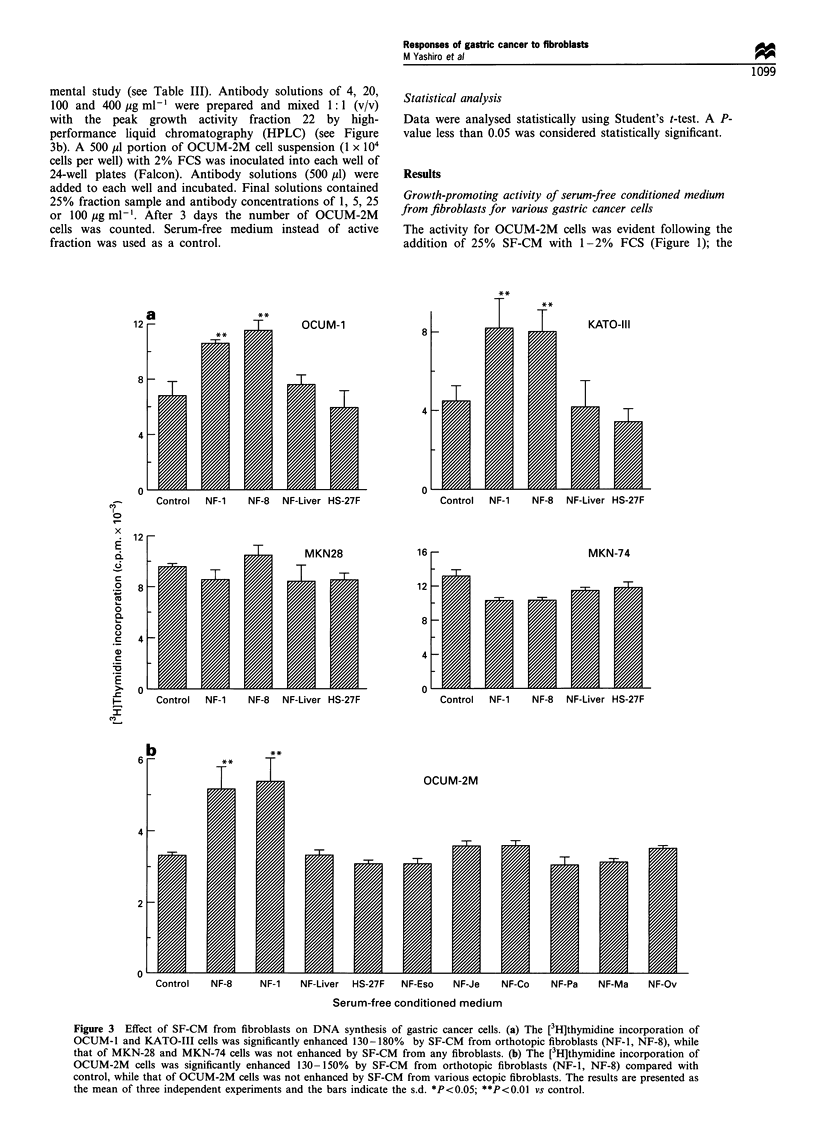
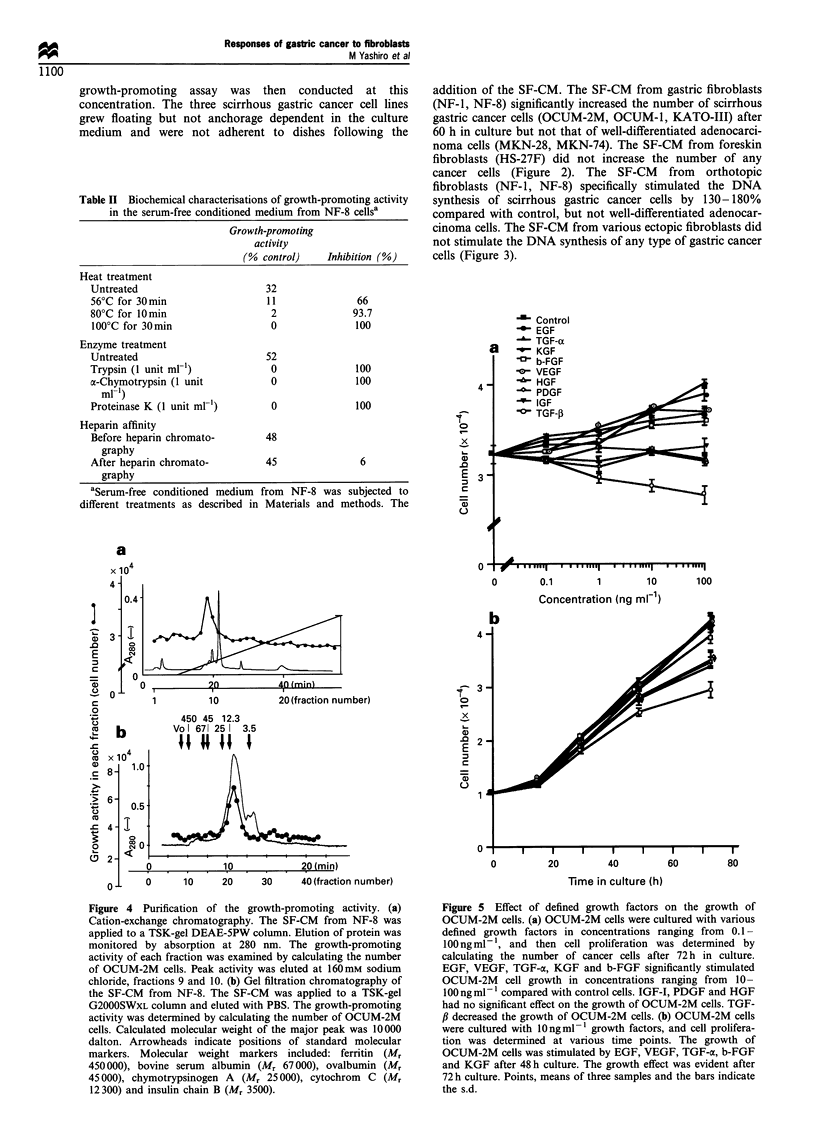
Scirrhous gastric cancer cells proliferate rapidly with fibrosis, when the cancer cells invade into the submucosa of the stomach. To investigate the mechanisms responsible for the rapid proliferation, the growth interaction between gastric cancer cells and fibroblasts was examined. Human gastric cancer cell lines established from scirrhous carcinoma or well-differentiated adenocarcinoma were used. Human fibroblast cell lines were obtained from various organs. The growth interaction between gastric cancer cells and fibroblasts was examined by calculating the number of cancer cells or by measuring [3H]thymidine incorporation of cancer cells. Gastric fibroblasts specifically stimulated the growth of scirrhous gastric cancer cells, but not that of well-differentiated adenocarcinoma cells. The growth factor(s) produced from gastric fibroblasts were then partially purified and characterised. The growth-promoting factor(s) had apparent molecular weights of 10000 dalton and was sensitive both to heat and proteinase treatment. No inhibition for the factor(s) was achieved with defined anti-growth factor antibodies. In this study, differential responses of scirrhous and well-differentiated gastric cancer cells to orthotopic fibroblasts were shown. Rapid proliferation of scirrhous gastric carcinoma should be partly controlled by orthotopic fibroblasts. The growth factor(s) from gastric fibroblasts, which was distinct from various defined growth factors such as epidermal growth factor (EGF), basic fibroblast growth factor (b-FGF), transforming growth factor-alpha (TGF-alpha), keratinocyte growth factor (KGF), vascular endothelial growth factor (VEGF), insulin-like growth factor I (IGF-I), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF) and transforming growth factor beta 1 (TGF-beta 1) may play an important role in the progression of scirrhous gastric cancer cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankrapp D. P., Bevan D. R. Insulin-like growth factor-I and human lung fibroblast-derived insulin-like growth factor-I stimulate the proliferation of human lung carcinoma cells in vitro. Cancer Res. 1993 Jul 15;53(14):3399–3404. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A. Participation of tenascin and transforming growth factor-beta in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 1989 Aug 1;49(15):4322–4325. [PubMed] [Google Scholar]
- Cornil I., Theodorescu D., Man S., Herlyn M., Jambrosic J., Kerbel R. S. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami J., Enami S., Koga M. Growth of normal and neoplastic mouse mammary epithelial cells in primary culture: stimulation by conditioned medium from mouse mammary fibroblasts. Gan. 1983 Dec;74(6):845–853. [PubMed] [Google Scholar]
- Gleave M., Hsieh J. T., Gao C. A., von Eschenbach A. C., Chung L. W. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991 Jul 15;51(14):3753–3761. [PubMed] [Google Scholar]
- Hattori Y., Odagiri H., Nakatani H., Miyagawa K., Naito K., Sakamoto H., Katoh O., Yoshida T., Sugimura T., Terada M. K-sam, an amplified gene in stomach cancer, is a member of the heparin-binding growth factor receptor genes. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5983–5987. doi: 10.1073/pnas.87.15.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlatky L., Tsionou C., Hahnfeldt P., Coleman C. N. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994 Dec 1;54(23):6083–6086. [PubMed] [Google Scholar]
- Horgan K., Jones D. L., Mansel R. E. Mitogenicity of human fibroblasts in vivo for human breast cancer cells. Br J Surg. 1987 Mar;74(3):227–229. doi: 10.1002/bjs.1800740326. [DOI] [PubMed] [Google Scholar]
- Hori A., Sasada R., Matsutani E., Naito K., Sakura Y., Fujita T., Kozai Y. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res. 1991 Nov 15;51(22):6180–6184. [PubMed] [Google Scholar]
- Ito M., Yasui W., Kyo E., Yokozaki H., Nakayama H., Ito H., Tahara E. Growth inhibition of transforming growth factor beta on human gastric carcinoma cells: receptor and postreceptor signaling. Cancer Res. 1992 Jan 15;52(2):295–300. [PubMed] [Google Scholar]
- Kiyasu Y., Kaneshima S., Koga S. Morphogenesis of peritoneal metastasis in human gastric cancer. Cancer Res. 1981 Mar;41(3):1236–1239. [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Mooradian D. L., McCarthy J. B., Komanduri K. V., Furcht L. T. Effects of transforming growth factor-beta 1 on human pulmonary adenocarcinoma cell adhesion, motility, and invasion in vitro. J Natl Cancer Inst. 1992 Apr 1;84(7):523–527. doi: 10.1093/jnci/84.7.523. [DOI] [PubMed] [Google Scholar]
- Mukai M., Shinkai K., Komatsu K., Akedo H. Potentiation of invasive capacity of rat ascites hepatoma cells by transforming growth factor-beta. Jpn J Cancer Res. 1989 Feb;80(2):107–110. doi: 10.1111/j.1349-7006.1989.tb02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Kino I., Horiuchi K., Fujimoto D. Promotion of collagen production by human fibroblasts with gastric cancer cells in vitro. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46(1-2):145–154. doi: 10.1007/BF02890304. [DOI] [PubMed] [Google Scholar]
- Shibamoto S., Hayakawa M., Hori T., Oku N., Miyazawa K., Kitamura N., Ito F. Hepatocyte growth factor and transforming growth factor-beta stimulate both cell growth and migration of human gastric adenocarcinoma cells. Cell Struct Funct. 1992 Jun;17(3):185–190. doi: 10.1247/csf.17.185. [DOI] [PubMed] [Google Scholar]
- Shirasuna K., Morioka S., Watatani K., Hayashido Y., Furusawa H., Sugiyama M., Okura M., Matsuya T. Growth inhibition and differentiation of human salivary adenocarcinoma cells by medium conditioned with normal human fibroblasts. Cancer Res. 1988 May 15;48(10):2819–2824. [PubMed] [Google Scholar]
- Tahara E. Growth factors and oncogenes in human gastrointestinal carcinomas. J Cancer Res Clin Oncol. 1990;116(2):121–131. doi: 10.1007/BF01612665. [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Yoshida K., Yokozaki H., Yasui W., Nakayama H., Ito H., Ohama K., Tahara E. Expression of basic fibroblast growth factor in human gastric carcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;61(4):263–267. doi: 10.1007/BF02890427. [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Iishi H., Tatsuta M., Nakamura H., Terada N., Komatsu K., Matsusaka T. Enhancement of mucus accumulation in a human gastric scirrhous carcinoma cell line (KATO-III) by fibroblast-tumor cell interaction. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(1):26–31. doi: 10.1007/BF02899383. [DOI] [PubMed] [Google Scholar]
- Yashiro M., Chung Y. S., Nishimura S., Inoue T., Sowa M. Establishment of two new scirrhous gastric cancer cell lines: analysis of factors associated with disseminated metastasis. Br J Cancer. 1995 Nov;72(5):1200–1210. doi: 10.1038/bjc.1995.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro M., Chung Y. S., Sowa M. Role of orthotopic fibroblasts in the development of scirrhous gastric carcinoma. Jpn J Cancer Res. 1994 Sep;85(9):883–886. doi: 10.1111/j.1349-7006.1994.tb02963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui W., Sumiyoshi H., Hata J., Kameda T., Ochiai A., Ito H., Tahara E. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 1988 Jan 1;48(1):137–141. [PubMed] [Google Scholar]
- Yoshida K., Kyo E., Tsujino T., Sano T., Niimoto M., Tahara E. Expression of epidermal growth factor, transforming growth factor-alpha and their receptor genes in human gastric carcinomas; implication for autocrine growth. Jpn J Cancer Res. 1990 Jan;81(1):43–51. doi: 10.1111/j.1349-7006.1990.tb02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Yokozaki H., Niimoto M., Ito H., Ito M., Tahara E. Expression of TGF-beta and procollagen type I and type III in human gastric carcinomas. Int J Cancer. 1989 Sep 15;44(3):394–398. doi: 10.1002/ijc.2910440303. [DOI] [PubMed] [Google Scholar]


