Abstract
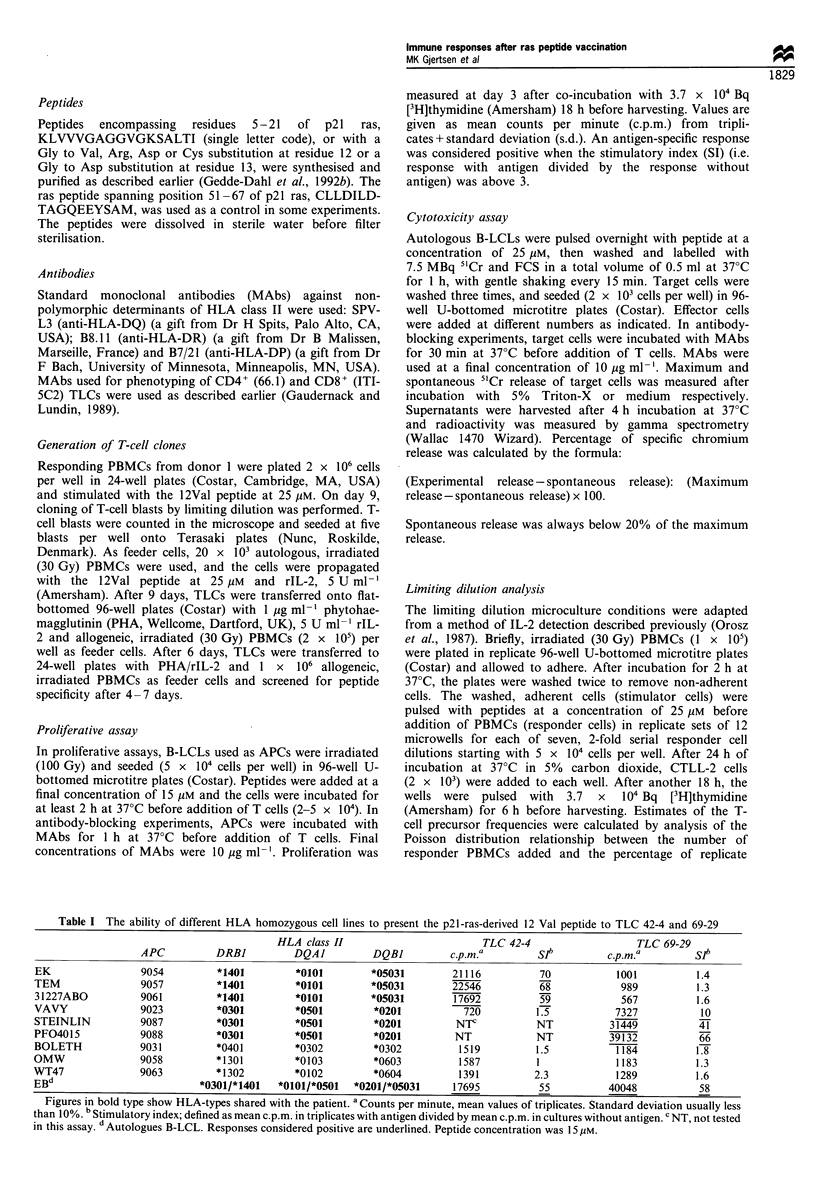
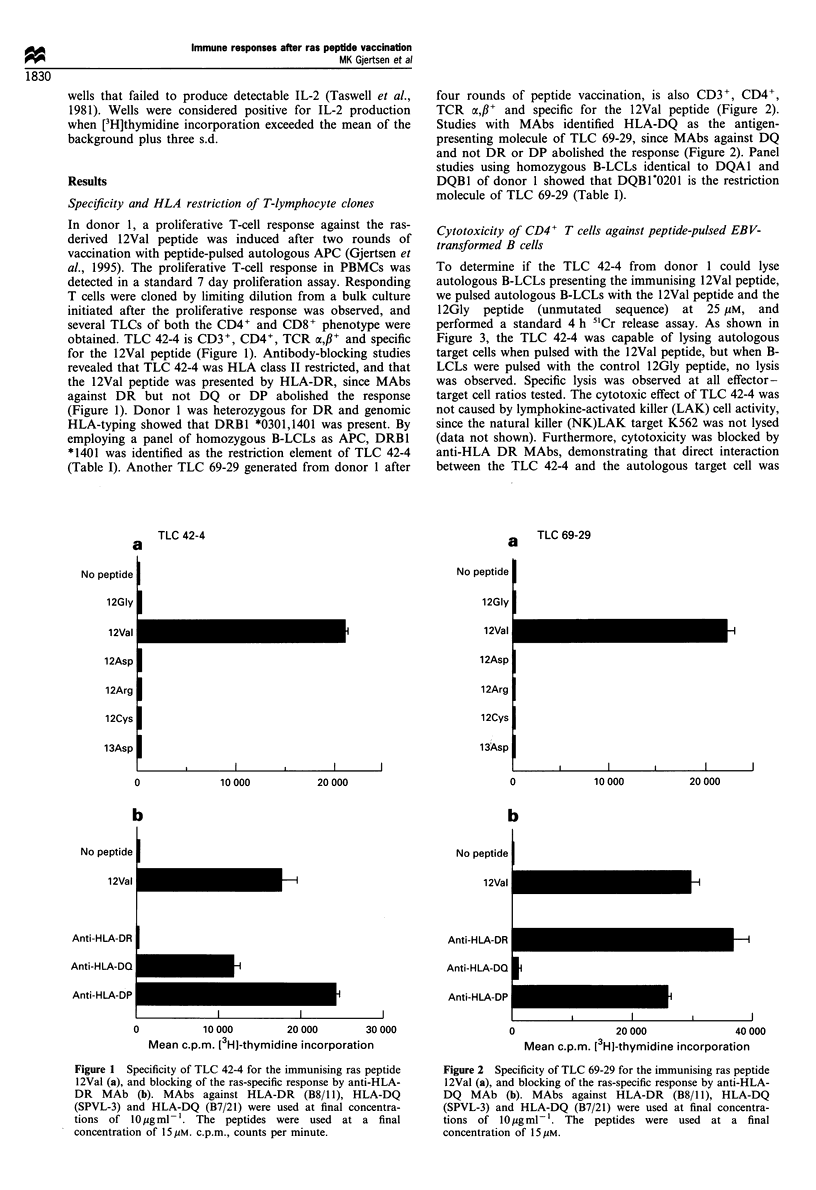
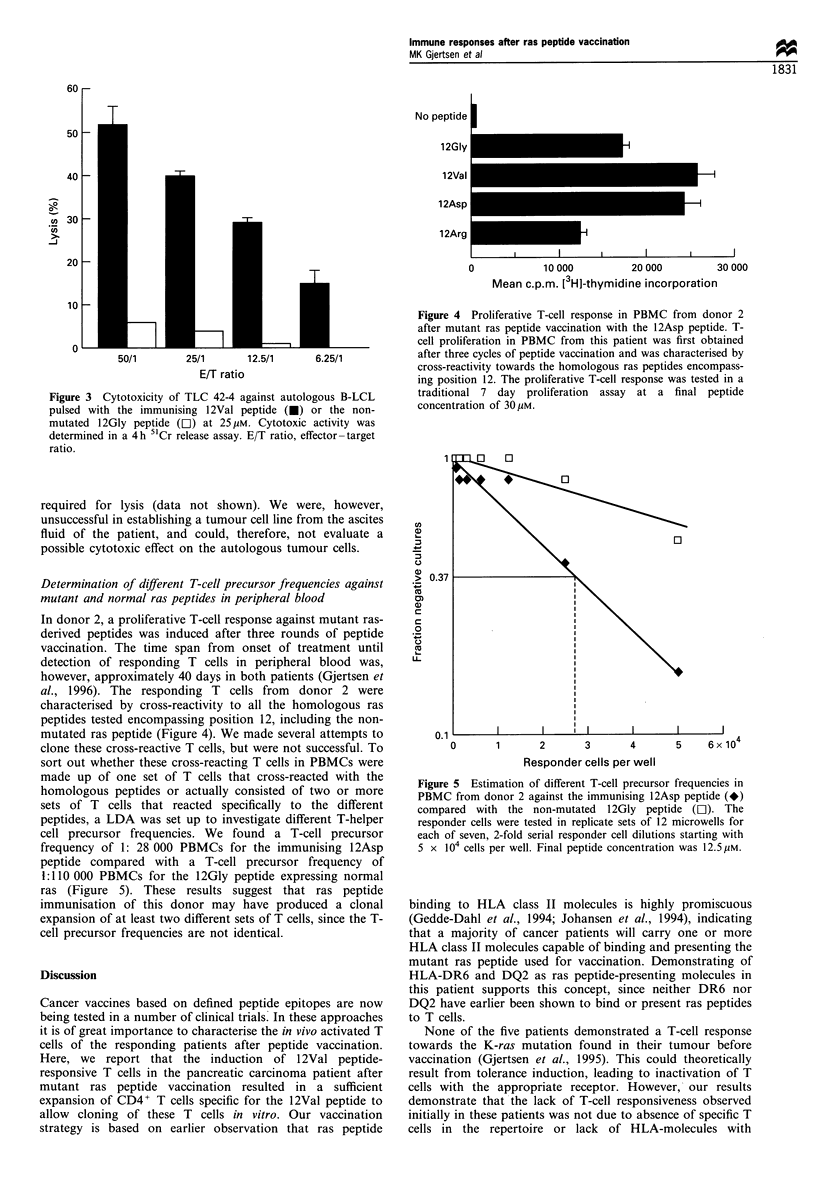
This is a study of immune responses generated by mutant ras peptide vaccination of patients with pancreatic adenocarcinoma. Responding T cells from one patient were cloned and two CD4+ T-lymphocyte clones (TLC) specific for the 12 Val peptide and restricted by HLA-DR6 or DQ2 were obtained. These class II molecules have not previously been found to bind or present mutant ras peptides to T cells. The DR6-restricted TLC showed marked cytotoxicity against autologous target cells pulsed with the 12 Val peptide. Target cells pulsed with the control peptide were not killed. Responding T cells from another patient showed cross-reactivity towards the homologous ras peptides. Investigation by limiting dilution analysis (LDA) revealed different T-cell precursor frequencies for the immunising, mutant ras peptide (1:28000), compared with the normal ras peptide (1:110000).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Boon T., Cerottini J. C., Van den Eynde B., van der Bruggen P., Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- Capella G., Cronauer-Mitra S., Pienado M. A., Perucho M. Frequency and spectrum of mutations at codons 12 and 13 of the c-K-ras gene in human tumors. Environ Health Perspect. 1991 Jun;93:125–131. doi: 10.1289/ehp.9193125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Fossum B., Gedde-Dahl T., 3rd, Breivik J., Eriksen J. A., Spurkland A., Thorsby E., Gaudernack G. p21-ras-peptide-specific T-cell responses in a patient with colorectal cancer. CD4+ and CD8+ T cells recognize a peptide corresponding to a common mutation (13Gly-->Asp). Int J Cancer. 1994 Jan 2;56(1):40–45. doi: 10.1002/ijc.2910560108. [DOI] [PubMed] [Google Scholar]
- Fossum B., Gedde-Dahl T., 3rd, Hansen T., Eriksen J. A., Thorsby E., Gaudernack G. Overlapping epitopes encompassing a point mutation (12 Gly-->Arg) in p21 ras can be recognized by HLA-DR, -DP and -DQ restricted T cells. Eur J Immunol. 1993 Oct;23(10):2687–2691. doi: 10.1002/eji.1830231045. [DOI] [PubMed] [Google Scholar]
- Fossum B., Olsen A. C., Thorsby E., Gaudernack G. CD8+ T cells from a patient with colon carcinoma, specific for a mutant p21-Ras-derived peptide (Gly13-->Asp), are cytotoxic towards a carcinoma cell line harbouring the same mutation. Cancer Immunol Immunother. 1995 Mar;40(3):165–172. doi: 10.1007/BF01517348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudernack G., Lundin K. E. Rapid immunomagnetic phenotyping of cells. J Immunogenet. 1989 Apr;16(2):169–175. doi: 10.1111/j.1744-313x.1989.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Eriksen J. A., Thorsby E., Gaudernack G. T-cell responses against products of oncogenes: generation and characterization of human T-cell clones specific for p21 ras-derived synthetic peptides. Hum Immunol. 1992 Apr;33(4):266–274. doi: 10.1016/0198-8859(92)90334-j. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Fossum B., Eriksen J. A., Thorsby E., Gaudernack G. T cell clones specific for p21 ras-derived peptides: characterization of their fine specificity and HLA restriction. Eur J Immunol. 1993 Mar;23(3):754–760. doi: 10.1002/eji.1830230328. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Spurkland A., Eriksen J. A., Thorsby E., Gaudernack G. Memory T cells of a patient with follicular thyroid carcinoma recognize peptides derived from mutated p21 ras (Gln-->Leu61). Int Immunol. 1992 Nov;4(11):1331–1337. doi: 10.1093/intimm/4.11.1331. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Spurkland A., Fossum B., Wittinghofer A., Thorsby E., Gaudernack G. T cell epitopes encompassing the mutational hot spot position 61 of p21 ras. Promiscuity in ras peptide binding to HLA. Eur J Immunol. 1994 Feb;24(2):410–414. doi: 10.1002/eji.1830240221. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gjertsen M. K., Bakka A., Breivik J., Saeterdal I., Gedde-Dahl T., 3rd, Stokke K. T., Sølheim B. G., Egge T. S., Søreide O., Thorsby E. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer. 1996 Feb 8;65(4):450–453. doi: 10.1002/(SICI)1097-0215(19960208)65:4<450::AID-IJC10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gjertsen M. K., Bakka A., Breivik J., Saeterdal I., Solheim B. G., Søreide O., Thorsby E., Gaudernack G. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet. 1995 Nov 25;346(8987):1399–1400. doi: 10.1016/s0140-6736(95)92408-6. [DOI] [PubMed] [Google Scholar]
- Johansen B. H., Gedde-Dahl T., 3rd, Sollid L. M., Vartdal F., Thorsby E., Gaudernack G. Binding of ras oncogene peptides to purified HLA-DQ(alpha 1*0102,beta 1*0602) and -DR(alpha,beta 1*0101) molecules. Scand J Immunol. 1994 Jun;39(6):607–612. doi: 10.1111/j.1365-3083.1994.tb03420.x. [DOI] [PubMed] [Google Scholar]
- Jung S., Schluesener H. J. Human T lymphocytes recognize a peptide of single point-mutated, oncogenic ras proteins. J Exp Med. 1991 Jan 1;173(1):273–276. doi: 10.1084/jem.173.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S. M., Jiang W., Culbertson T. A., Weinstein I. B., Williams G. M., Tomita N., Ronai Z. Rapid and sensitive nonradioactive detection of mutant K-ras genes via 'enriched' PCR amplification. Oncogene. 1991 Jun;6(6):1079–1083. [PubMed] [Google Scholar]
- Mukherji B., Chakraborty N. G., Yamasaki S., Okino T., Yamase H., Sporn J. R., Kurtzman S. K., Ergin M. T., Ozols J., Meehan J. Induction of antigen-specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide-pulsed autologous antigen presenting cells. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):8078–8082. doi: 10.1073/pnas.92.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz C. G., Adams P. W., Ferguson R. M. Frequency of human alloantigen-reactive T lymphocytes. II. Method for limiting dilution analysis of alloantigen-reactive helper T cells in human peripheral blood. Transplantation. 1987 May;43(5):718–724. [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Skipper J., Stauss H. J. Identification of two cytotoxic T lymphocyte-recognized epitopes in the Ras protein. J Exp Med. 1993 May 1;177(5):1493–1498. doi: 10.1084/jem.177.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Kerr I. B. Elevated expression of the human ras oncogene family in premalignant and malignant tumours of the colorectum. Br J Cancer. 1984 Jun;49(6):681–688. doi: 10.1038/bjc.1984.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]


