Abstract
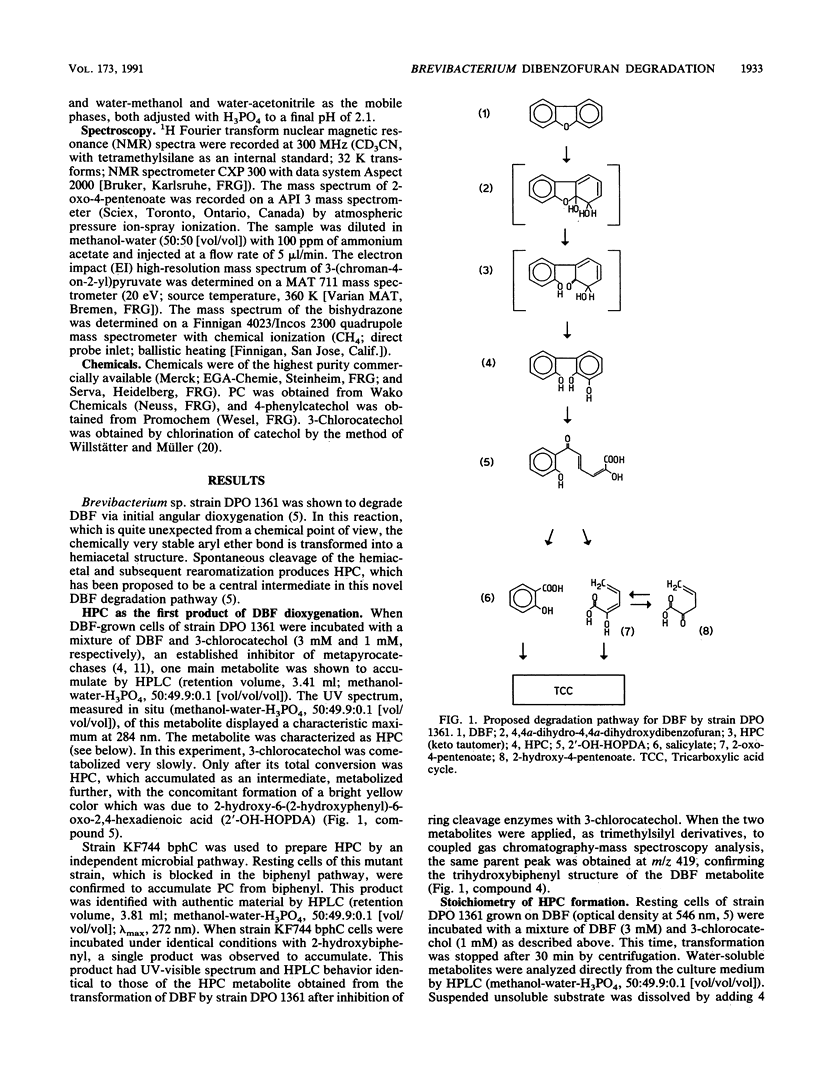
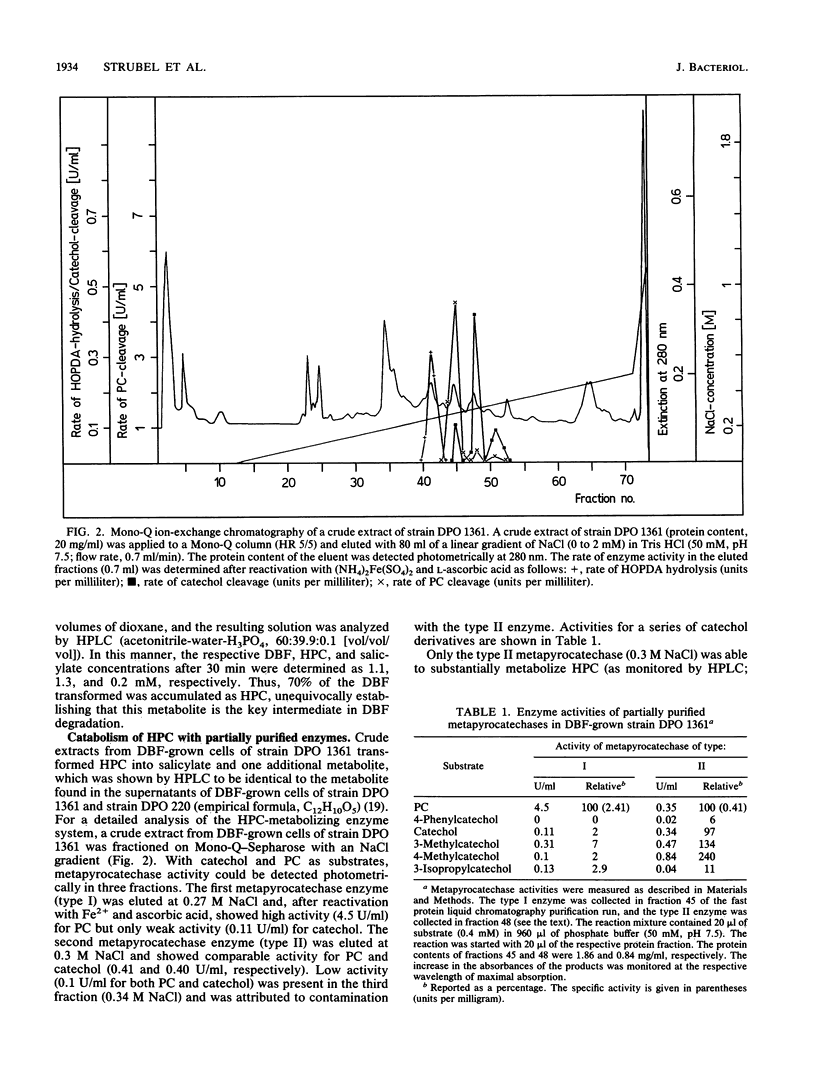
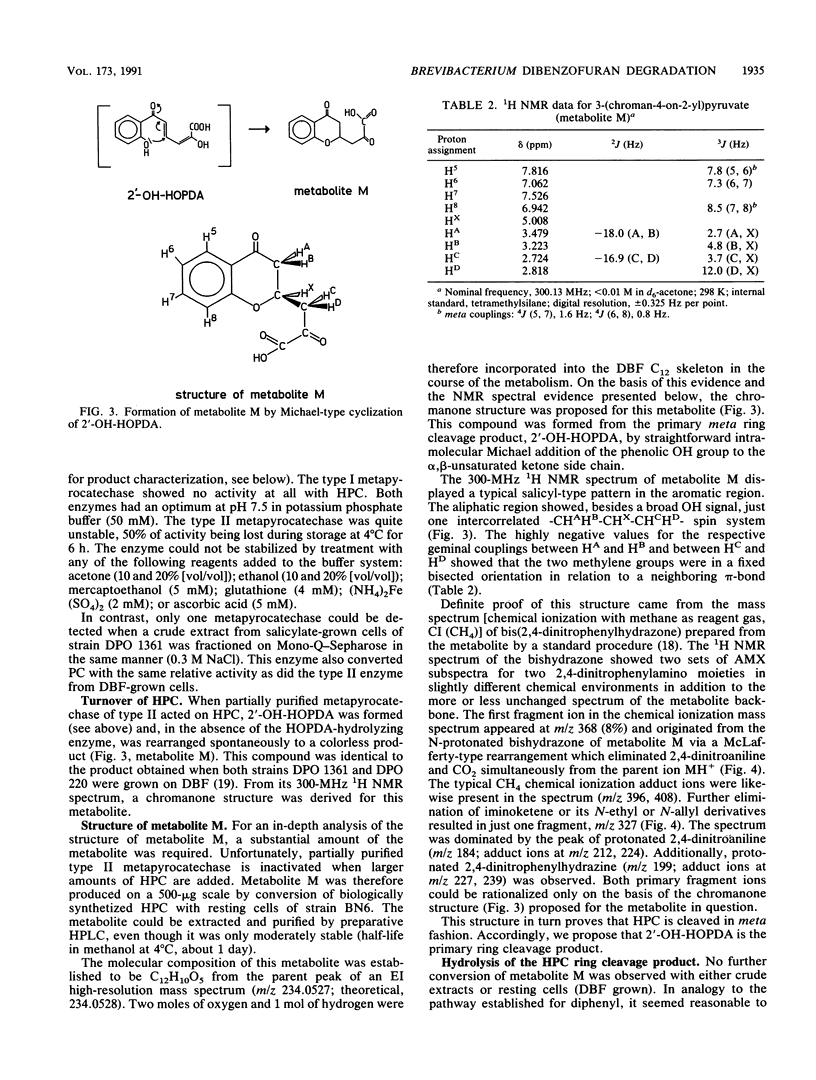
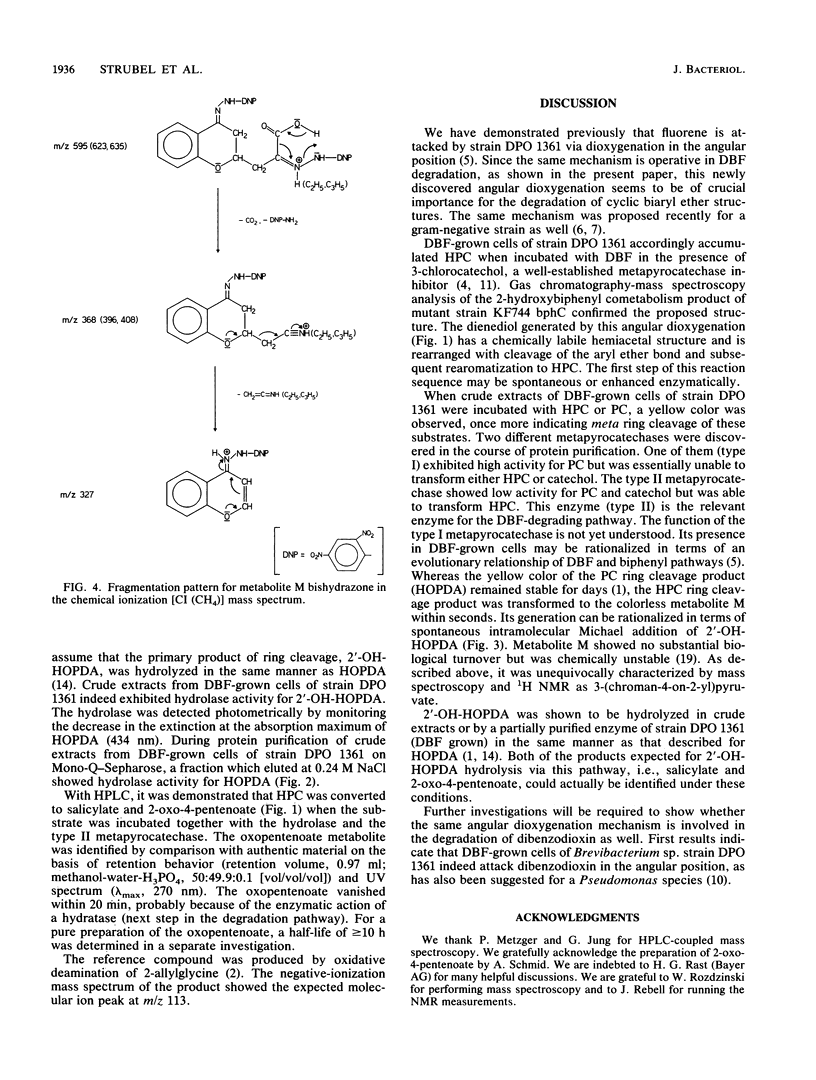
Brevibacterium sp. strain DPO 1361 oxygenates dibenzofuran in the unusual angular position. The 3-(2-hydroxyphenyl)catechol thus generated is subject to meta ring cleavage in the proximal position, yielding 2-hydroxy-6-(2-hydroxyphenyl)-6-oxo-2,4-hexadienoic acid, which is hydrolyzed to 2-oxo-4-pentenoate and salicylate by 2-hydroxy-6-oxo-6-phenyl-2,4-hexadienoic acid hydrolase. The proximal mode of ring cleavage is definitely established by isolation and unequivocal structural characterization of a cyclization product of 2-hydroxy-6-(2-hydroxyphenyl)-6-oxo-2,4-hexadienoic acid, i.e., 3-(chroman-4-on-2-yl)pyruvate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catelani D., Colombi A. Metabolism of biphenyl. Structure and physicochemical properties of 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid, the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1974 Nov;143(2):431–434. doi: 10.1042/bj1430431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinsworth W. L., Chapman P. J., Dagley S. Stereospecific enzymes in the degradation of aromatic compounds by pseudomonas putida. J Bacteriol. 1973 Feb;113(2):922–931. doi: 10.1128/jb.113.2.922-931.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engesser K. H., Schulte P. Degradation of 2-bromo-, 2-chloro- and 2-fluorobenzoate by Pseudomonas putida CLB 250. FEMS Microbiol Lett. 1989 Jul 15;51(1):143–147. doi: 10.1016/0378-1097(89)90497-7. [DOI] [PubMed] [Google Scholar]
- Engesser K. H., Strubel V., Christoglou K., Fischer P., Rast H. G. Dioxygenolytic cleavage of aryl ether bonds: 1,10-dihydro-1,10-dihydroxyfluoren-9-one, a novel arene dihydrodiol as evidence for angular dioxygenation of dibenzofuran. FEMS Microbiol Lett. 1989 Nov;53(1-2):205–209. doi: 10.1016/0378-1097(89)90392-3. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Francke W., Krohn S., Meyer H. Cleavage of dibenzofuran and dibenzodioxin ring systems by a Pseudomonas bacterium. Naturwissenschaften. 1989 May;76(5):222–223. doi: 10.1007/BF00627694. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Krohn S., Meyer H., Sinnwell V., Wilkes H., Francke W. Metabolism of Dibenzofuran by Pseudomonas sp. Strain HH69 and the Mixed Culture HH27. Appl Environ Microbiol. 1990 Apr;56(4):1148–1156. doi: 10.1128/aem.56.4.1148-1156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Simon J. R., Chakrabarty A. M. Common induction and regulation of biphenyl, xylene/toluene, and salicylate catabolism in Pseudomonas paucimobilis. J Bacteriol. 1983 Jun;154(3):1356–1362. doi: 10.1128/jb.154.3.1356-1362.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms H., Wittich R. M., Sinnwell V., Meyer H., Fortnagel P., Francke W. Transformation of Dibenzo-p-Dioxin by Pseudomonas sp. Strain HH69. Appl Environ Microbiol. 1990 Apr;56(4):1157–1159. doi: 10.1128/aem.56.4.1157-1159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Inhibition of catechol 2,3-dioxygenase from Pseudomonas putida by 3-chlorocatechol. Appl Environ Microbiol. 1981 May;41(5):1159–1165. doi: 10.1128/aem.41.5.1159-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nörtemann B., Baumgarten J., Rast H. G., Knackmuss H. J. Bacterial communities degrading amino- and hydroxynaphthalene-2-sulfonates. Appl Environ Microbiol. 1986 Nov;52(5):1195–1202. doi: 10.1128/aem.52.5.1195-1202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]


