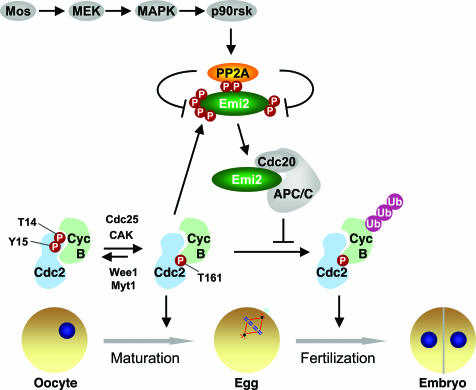
Immature oocytes of most vertebrates, including humans, arrest at prophase of meiosis I. Upon hormonal stimulation, the oocytes undergo maturation and complete meiosis I. The mature eggs are then arrested at metaphase of meiosis II until fertilization. In the early 1970s, Yoshio Masui and coworkers (1) identified two biochemical activities in the mature frog egg: the maturation-promoting factor (MPF), which when injected into oocytes promoted their maturation, and the cytostatic factor (CSF), which when injected into dividing embryos reestablished metaphase arrest (1). Subsequent molecular characterizations of MPF and CSF in the frog eggs and egg extracts have highlighted the central roles of dynamic, site-specific protein phosphorylation and dephosphorylation in cell cycle transitions. For example, MPF is the active heterodimeric complex of cyclin B and Cdc2 (also known as cyclin-dependent kinase 1 or Cdk1) (Fig. 1) (2). Phosphorylation of Cdc2 at T14 and Y15 by Wee1 and other kinases inhibits Cdc2 and can be removed by the Cdc25 phosphatase (2). In contrast, phosphorylation of T161 in the activation loop of Cdc2 by Cdk-activating kinase (CAK) activates Cdc2 (3). In a recent issue of PNAS, Wu et al. (4) present another stunning example of how site-specific dephosphorylation of Emi2, a key component of CSF, by protein phosphatase 2A (PP2A) contributes to the metaphase block of the egg (Fig. 1).
Fig. 1.
Model for the regulation of Emi2 by the p90rsk-dependent recruitment of PP2A in CSF-mediated metaphase arrest of vertebrate eggs. Entry into mitosis and activation of cyclin B/Cdc2 in the egg also are shown to highlight the importance of dynamic, site-specific phosphorylation in controlling cell cycle transitions.
Earlier studies of CSF in frog and mouse focused on the Mos–MEK–MAPK–p90rsk kinase cascade (5). This pathway is active in the mature egg and is inactivated upon fertilization or by an elevation in the cytoplasmic Ca2+ concentration. Introduction of either wild-type Mos or constitutively active mutants of other kinases in this pathway to dividing embryos or cycling egg extracts is sufficient to induce metaphase arrest. Conversely, inactivation of enzymes in this cascade through various means abrogates CSF. Therefore, the Mos–MAPK pathway is crucial for the establishment and maintenance of CSF.
A recent line of research focused on the direct mechanism by which CSF maintains the activity of cyclin B/Cdc2 or MPF in the egg (6). Active cyclin B/Cdc2 is necessary and sufficient for mitotic arrest. Cyclin B degradation is an important mechanism for Cdc2 inactivation and exit from mitosis. In association with its substrate-recruiting activator Cdc20, the anaphase-promoting complex or cyclosome (APC/C), a multisubunit ubiquitin ligase, mediates the ubiquitination and degradation of cyclin B (7). An inhibitor of APC/C called early mitotic inhibitor 2 (Emi2; also known as Erp1) was recently shown to be a key component of CSF in both frog and mouse eggs (8–10). Binding of Emi2 to APC/C blocks substrate binding and inhibits its ligase activity (11). Addition of Emi2 to cycling extracts results in a mitotic arrest with the stabilization of cyclin B (8, 9). Depletion of Emi2 from CSF extracts causes cyclin B degradation and mitotic exit (8, 9). Emi2 is stable in CSF extracts. Upon fertilization or Ca2+ addition, calmodulin-dependent kinase II (CaMKII) and the polo-like kinase Plk1 (Plx1 in Xenopus) collaborate to phosphorylate Emi2 to generate a phospho-degron that is recognized by βTrCP, an adaptor for the Skp1–Cul1–F-box (SCF) ubiquitin ligase complex (12, 13). Ubiquitination of phosphorylated Emi2 by SCFβTrCP leads to its rapid degradation, activation of APC/C, degradation of cyclin B, and exit from mitosis. Thus, Emi2 is a key downstream component of CSF.
Two recent studies further showed that p90rsk phosphorylates Emi2 at multiple residues in its central region and that these phosphorylation events are critical for the establishment of CSF (14, 15). These studies placed Emi2 downstream of the Mos–MAPK pathway and effectively unified the two main lines of research on CSF. A key unresolved issue, however, was how phosphorylation of Emi2 by p90rsk promoted the function of Emi2. The study by Wu et al. (4) has now filled this significant gap (Fig. 1).
Wu et al. (4) began their study by confirming a requirement for Emi2 in Mos-induced CSF arrest in Xenopus egg extracts. By blocking the translation of Emi2 with antisense morpholino oligonucleotides, Wu et al. effectively reduced the level of Emi2 in cycling extracts. Addition of purified Mos to these Emi2-deficient cycling extracts failed to induce metaphase arrest, suggesting that ectopic Mos likely acted through Emi2 to induce CSF. To explore the mechanism by which the Mos–MAPK pathway regulated Emi2, they examined the behavior of Emi2 in extracts treated with U0126, a chemical inhibitor of MEK. As expected, MEK inactivation abrogated CSF and caused degradation of Emi2. Unexpectedly, inhibition of MEK by U0126 caused hyperphosphorylation of Emi2, as evidenced by the appearance of slower migrating Emi2 species on gels. Furthermore, addition of Mos to interphase extracts enhanced dephosphorylation of Emi2 fragments that had been phosphorylated by cyclin B/Cdc2 in vitro at either a cluster of N-terminal sites or two C-terminal sites, T545 and T551. These results suggested the counterintuitive possibility that the Mos–MAPK pathway promoted the dephosphorylation of Emi2 at multiple sites.
It had been shown previously by the same groups that phosphorylation of T545 and T551 of Emi2 by Cdc2 dissociated the Emi2–APC/C interaction (11). They show in the current study (4) that chemical inhibition of p90rsk in extracts reduced APC/C-binding of a stable, exogenously added Emi2 fragment but not that of the Emi2 T545A/T551A mutant. Therefore, Mos-induced dephosphorylation of Emi2 could enhance its APC/C-inhibitory activity. On the other hand, an Emi2 mutant with its two p90rsk sites eliminated was degraded in CSF extracts with slow kinetics, suggesting that phosphorylation of Emi2 by p90rsk prevented the chronic degradation of Emi2. This chronic degradation required the N-terminal cluster of Cdc2 sites in Emi2 and its Plx1 phosphorylation site. Together, these experiments revealed two independent functions of Mos/p90rsk-dependent dephosphorylation of Emi2 in CSF. Dephosphorylation at the C-terminal Cdc2 sites of Emi2 enhances its ability to inhibit APC/C, whereas dephosphorylation of N-terminal Cdc2 sites of Emi2 preserves its stability.
Wu et al. (4) next investigated the mechanism by which p90rsk phosphorylation of Emi2 promoted its dephosphorylation at Cdc2 sites. They showed that a small central fragment of Emi2 containing the two p90rsk sites bound to PP2A in CSF extracts. Addition of this Emi2 fragment to CSF extracts caused hyperphosphorylation and degradation of the endogenous Emi2 and inactivation of CSF, presumably
The phosphatase thus acts as a mercenary in this war between kinases.
by blocking the Emi2–PP2A interaction in a dominant-negative manner. Mutations of the p90rsk sites within this Emi2 fragment abolished PP2A-binding, suggesting that the Emi2–PP2A interaction required p90rsk phosphorylation. Mutations of residues neighboring the p90rsk sites in Emi2 abolished its binding to PP2A without affecting its phosphorylation by p90rsk. The same mutations also disrupted the binding of Emi2 to APC/C in extracts. These results support a model in which phosphorylation of Emi2 by p90rsk creates a docking site for PP2A (as opposed to a conformational change that unmasks a hidden PP2A-binding motif) and recruits PP2A to Emi2 to counteract Cdc2-dependent phos-phorylation of Emi2 at other sites (Fig. 1). Residues surrounding the p90rsk-phosphorylation sites of Emi2 are conserved, suggesting that phosphorylation-dependent PP2A-binding to Emi2 might also be important for CSF in other vertebrates.
PP2A is a multisubunit enzyme and consists of the scaffolding subunit (A), the catalytic subunit (C), and a variable B subunit that confers substrate specificity (16). It is unclear, at present, which PP2A holoenzyme binds to Emi2 and how phosphorylation enhances its binding to Emi2. Regardless, the study by Wu et al. (4) establishes an elegant mechanism by which the Mos–MAPK pathway regulates Emi2, a key component of CSF. In addition, this study also reveals a new scheme in the regulation of protein phosphorylation. In this scheme, phosphorylation of a given protein (Emi2) at one site by one kinase (p90rsk) can recruit a protein phosphatase (PP2A) to remove phosphates on the same protein that are added by another kinase (Cdc2) (Fig. 1). The two kinases are expected to play opposite roles in a signaling pathway and antagonize each other's function through the phosphatase. The phosphatase thus acts as a mercenary in this war between kinases.
Intriguingly, Plk1 and the spindle checkpoint kinase Bub1 play antagonistic roles in the regulation of Shugoshin (Sgo1), a protector of centromeric sister-chromatid cohesion (17). Bub1 is required for binding of PP2A to Sgo1 and for the centromeric localization of Sgo1 (18, 19). In contrast, phosphorylation of Sgo1 by Plk1 is required for the dissociation of Sgo1 from centromeres (18, 20). In light of the new principle revealed by Wu et al. (4), it will be interesting to examine whether phosphorylation of Sgo1 by Bub1 directly recruits PP2A and whether this recruitment enhances dephosphorylation of Sgo1 at Plk1 sites. Thus, PP2A and the army of phosphatase mercenaries may fight in other cellular kinase wars that exist in various signal transduction processes.
Footnotes
The author declares no conflict of interest.
See the companion article on page 16564 in issue 42 of volume 104.
References
- 1.Masui Y, Markert CL. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 2.Norbury C, Nurse P. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RP, Morgan DO. Cell. 1994;78:713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 4.Wu JQ, Hansen DV, Guo Y, Wang MZ, Tang W, Freel CD, Tung JJ, Jackson PK, Kornbluth S. Proc Natl Acad Sci USA. 2007;104:16564–16569. doi: 10.1073/pnas.0707537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tunquist BJ, Maller JL. Genes Dev. 2003;17:683–710. doi: 10.1101/gad.1071303. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt A, Rauh NR, Nigg EA, Mayer TU. J Cell Sci. 2006;119:1213–1218. doi: 10.1242/jcs.02919. [DOI] [PubMed] [Google Scholar]
- 7.Yu H. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU. Genes Dev. 2005;19:502–513. doi: 10.1101/gad.320705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR, III, Jackson PK. Proc Natl Acad Sci USA. 2005;102:4318–4323. doi: 10.1073/pnas.0501108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC. EMBO J. 2006;25:834–845. doi: 10.1038/sj.emboj.7600953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, Freel CD, Tung JJ, Tang W, Margolis SS, et al. Curr Biol. 2007;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU. Nature. 2005;437:1048–1052. doi: 10.1038/nature04093. [DOI] [PubMed] [Google Scholar]
- 13.Hansen DV, Tung JJ, Jackson PK. Proc Natl Acad Sci USA. 2006;103:608–613. doi: 10.1073/pnas.0509549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue D, Ohe M, Kanemori Y, Nobui T, Sagata N. Nature. 2007;446:1100–1104. doi: 10.1038/nature05688. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T, Ohsumi K, Kishimoto T. Nature. 2007;446:1096–1099. doi: 10.1038/nature05696. [DOI] [PubMed] [Google Scholar]
- 16.Janssens V, Goris J. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe Y. Curr Opin Cell Biol. 2005;17:590–595. doi: 10.1016/j.ceb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H. Dev Cell. 2006;10:575–585. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Tang Z, Sun Y, Harley SE, Zou H, Yu H. Proc Natl Acad Sci USA. 2004;101:18012–18017. doi: 10.1073/pnas.0408600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke AS, Tang TT, Ooi DL, Orr-Weaver TL. Dev Cell. 2005;8:53–64. doi: 10.1016/j.devcel.2004.12.003. [DOI] [PubMed] [Google Scholar]