Abstract
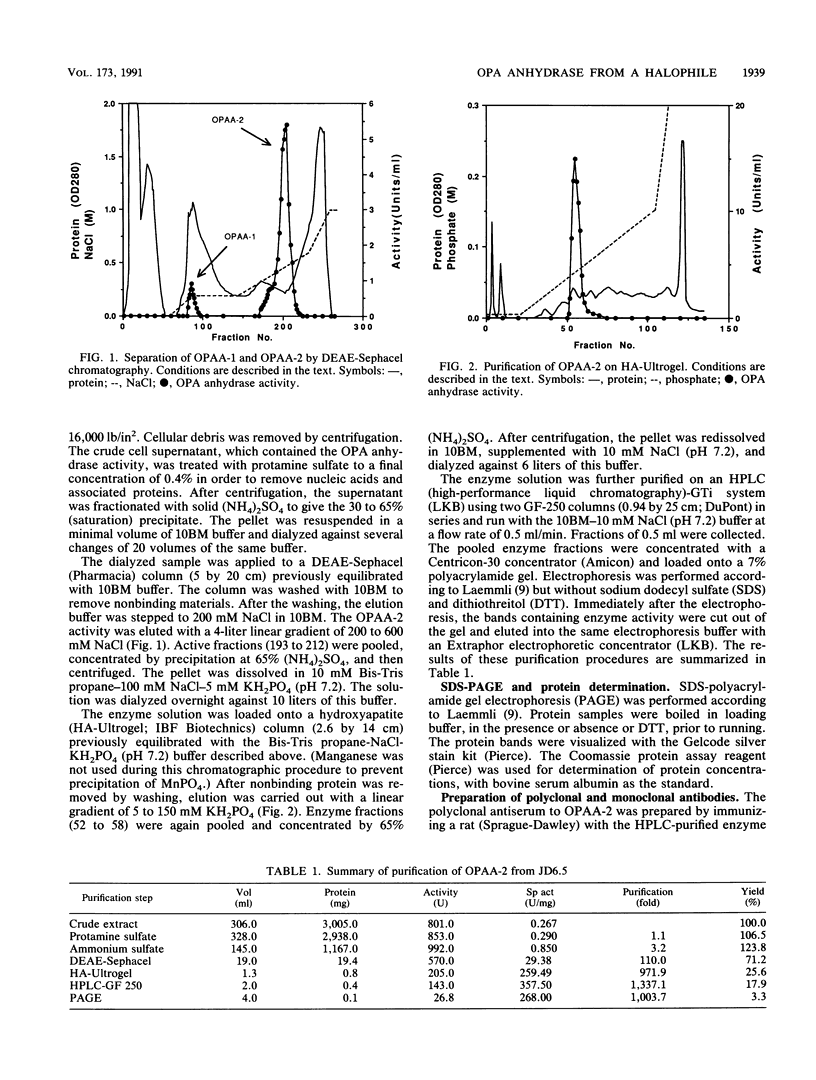
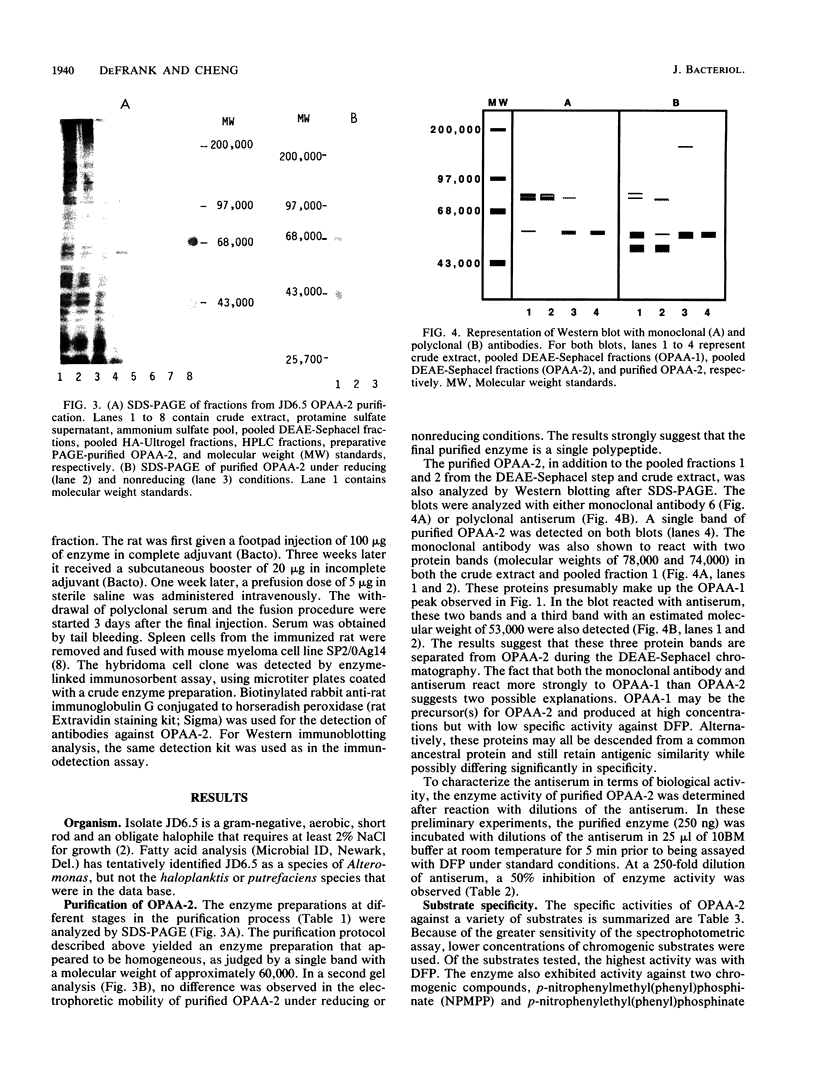
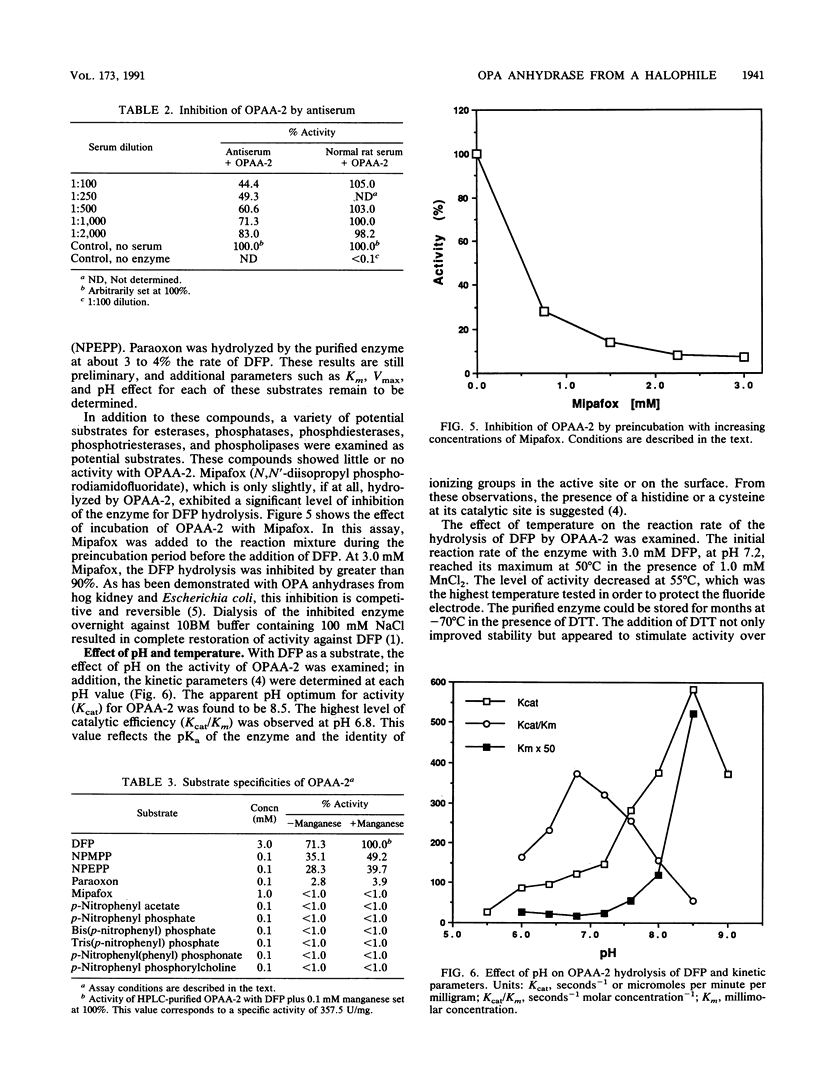
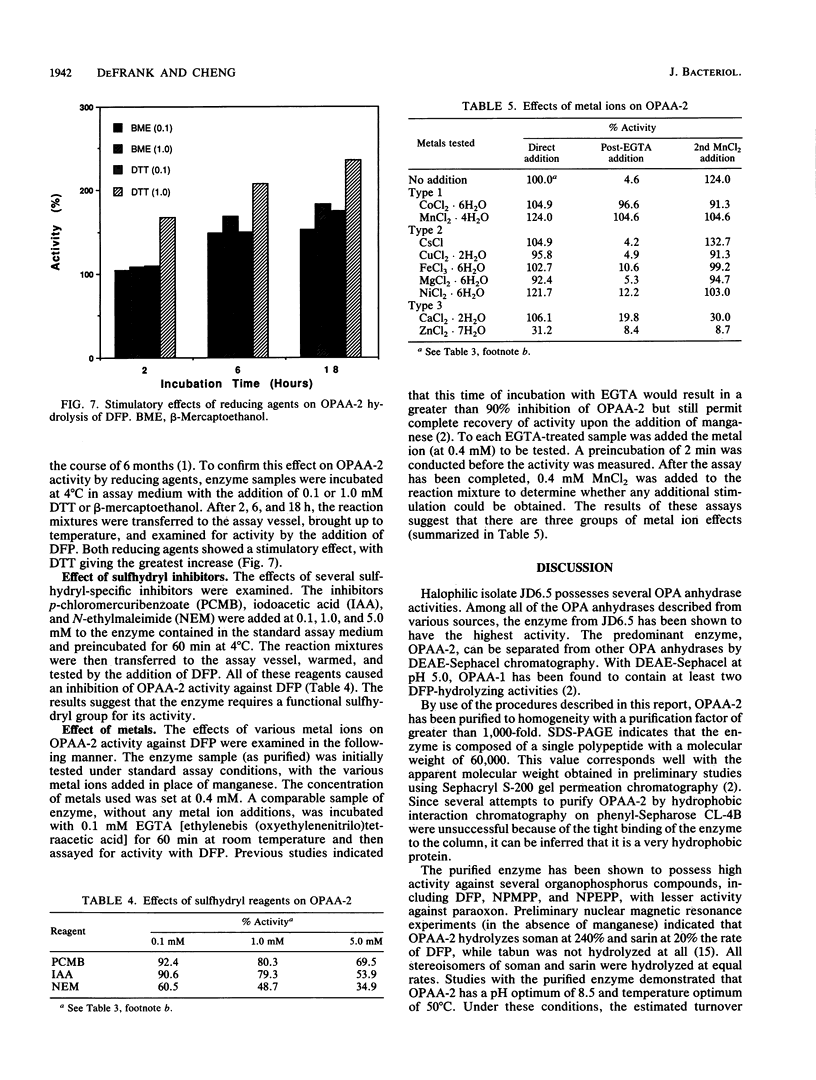
A moderately halophilic bacterial isolate has been found to possess high levels of enzymatic activity against several highly toxic organophosphorus compounds. The predominant enzyme, designated organophosphorus acid anhydrase 2, has been purified 1,000-fold to homogeneity and characterized. The enzyme is a single polypeptide with a molecular weight of 60,000. With diisopropylfluorophosphate as a substrate, the enzyme has optimum activity at pH 8.5 and 50 degrees C, and it is stimulated by manganese and cobalt.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hoskin F. C. Inhibition of a soman- and diisopropyl phosphorofluoridate (DFP)-detoxifying enzyme by Mipafox. Biochem Pharmacol. 1985 Jun 15;34(12):2069–2072. doi: 10.1016/0006-2952(85)90396-x. [DOI] [PubMed] [Google Scholar]
- Hoskin F. C., Roush A. H. Hydrolysis of nerve gas by squid-type diisopropyl phosphorofluoridate hydrolyzing enzyme on agarose resin. Science. 1982 Mar 5;215(4537):1255–1257. doi: 10.1126/science.7058344. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landis W. G., Haley M. V., Johnson D. W. Kinetics of the DFPase activity in Tetrahymena thermophila. J Protozool. 1986 May;33(2):216–218. doi: 10.1111/j.1550-7408.1986.tb05593.x. [DOI] [PubMed] [Google Scholar]



