Abstract
Gonadotropin-releasing hormone (GnRH) deficiency in the human presents either as normosmic idiopathic hypogonadotropic hypogonadism (nIHH) or with anosmia [Kallmann syndrome (KS)]. To date, several loci have been identified to cause these disorders, but only 30% of cases exhibit mutations in known genes. Recently, murine studies have demonstrated a critical role of the prokineticin pathway in olfactory bulb morphogenesis and GnRH secretion. Therefore, we hypothesize that mutations in prokineticin 2 (PROK2) underlie some cases of KS in humans and that animals deficient in Prok2 would be hypogonadotropic. One hundred IHH probands (50 nIHH and 50 KS) with no known mutations were examined for mutations in the PROK2 gene. Mutant PROK2s were examined in functional studies, and the reproductive phenotype of the Prok2−/− mice was also investigated. Two brothers with KS and their sister with nIHH harbored a homozygous deletion in the PROK2 gene (p.[I55fsX1]+[I55fsX1]). Another asymptomatic brother was heterozygous for the deletion, whereas both parents (deceased) had normal reproductive histories. The identified deletion results in a truncated PROK2 protein of 27 amino acids (rather than 81 in its mature form) that lacks bioactivity. In addition, Prok2−/− mice with olfactory bulb defects exhibited disrupted GnRH neuron migration, resulting in a dramatic decrease in GnRH neuron population in the hypothalamus as well as hypogonadotropic hypogonadism. Homozygous loss-of-function PROK2 mutations cause both KS and nIHH.
Keywords: gonadotropin-releasing hormone deficiency
Idiopathic hypogonadotropic hypogonadism (IHH) caused by gonadotropin-releasing hormone (GnRH) deficiency in the human occurs either with anosmia [Kallmann syndrome (KS)] (1) or with a normal sense of smell [normosmic IHH (nIHH)] (2). Genetic defects on several loci have been identified in <30% of this population either alone or in combination (3). KAL1 mutations cause X-linked KS (4–6), whereas gonadotropin-releasing hormone receptor (GNRHR) and G protein couple receptor 54 (GPR54) mutations underlie autosomal recessive nIHH (7–9). KS and nIHH were previously believed to be distinct entities caused by different genes; however, the finding that FGFR1 mutations can cause both syndromes has provided insight into this genetic heterogeneity (10, 11).
PROK2 is a secreted protein containing 10 conserved cysteine residues within the 81 aa in its mature form (12). PROK2 signals through two cognate G protein-coupled receptors, prokineticin receptor 1 and 2 (PROKR1, PROKR2) (12). The PROK2 signaling system regulates diverse biological processes, including olfactory bulb (OB) morphogenesis and reproduction, through multiple intracellular signaling pathways including calcium mobilization (13–15). Genetic studies in the mouse demonstrated a key role for PROK2 signaling in OB morphogenesis (16). Furthermore, a recent study found heterozygous mutations in the PROK2 gene associated with KS, although no functional data on the mutant proteins were provided (17). Herein, we demonstrate that homozygous loss-of-function mutations in the PROK2 gene cause IHH in mice and humans.
Results
Molecular Analysis of PROK2 Gene.
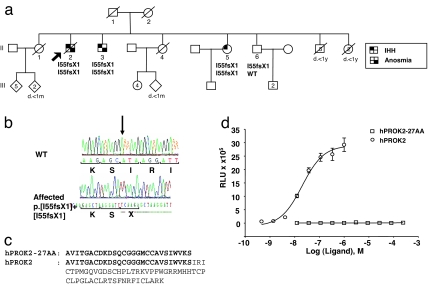
A homozygous single base pair deletion in exon 2 of the PROK2 gene (c.[163delA]+ [163delA]) was identified in the proband, in his brother with KS, and in his sister with nIHH (Fig. 1 a and b). The asymptomatic brother was hesterozygous for the mutation. This base pair deletion results in a frameshift (p.I55fsX1) and premature stop codon at amino acid 55. This deletion should result in a truncated protein of 27 aa in its mature form (rather than the 81 aa) (Fig. 1c). Alternatively, this premature stop codon may result in mRNA decay (18).
Fig. 1.
IHH pedigree with a homozygous loss-of-function PROK2 mutation. (a) The pedigree reveals a family with two affected KS brothers (II-02 and II-03) and a normosmic IHH sister (II-05) carrying a homozygous deletion in PROK2 (p.[I55fsX1]+ [I55fsX1]). The unaffected brother (II-06) is heterozygous for the mutation. The proband is identified by the arrow. ○, females; □, males. Phenotypes are as described in the text. (b) Sequence electropherograms of WT and affected individuals for the homozygous PROK2 deletion. (c) Alignment of the truncated form of PROK2 (hRPOK2–27AA) with mature PROK2 (hPROK2). (d) In vitro analysis of the activity of the truncated hPROK2-27AA (open squares) and the mature PROK2 on a CHO cell line that stably expressed PROKR2 (open circles). No activation activity of the truncated hPROK2-27AA was observed at any of the tested concentrations.
Truncated PROK2 Is Nonfunctional in Vitro.
To test whether the truncated PROK2 was functional in vitro, we synthesized the 27-aa peptide and tested its activity by using a CHO cell line that stably expresses the receptor PROKR2 (19). As shown in Fig. 1d, full-length human PROK2 (hPROK2) potently activated PROKR2 at a low concentration (EC50 = 19 nM). However, the truncated PROK2 (hPROK2-27AA) could not activate PROKR2 even at very high concentrations (>200 μM). Thus, the truncated form of PROK2 lacks functionality in vitro.
Genotype–Phenotype Correlation.
II-2.
The proband, a Caucasian male of Portugese descent, was homozygous for the p.I55fsX1 mutation. He failed to go through puberty, eventually married, but was unable to father a child. At age 29, he had a convulsive episode without predisposing factors. At age 52, he developed type II diabetes mellitus, followed by a cerebrovascular event at age 62. At age 68, he was admitted to the hospital for colon cancer. Upon presentation, he was anosmic, unvirilized, overweight (body mass index of 29 kg/m2), with bilateral gynecomastia, microphallus, and prepubertal testes (2 ml bilaterally). His hormonal profile revealed hypogonadal testosterone and undetectable gonadotropins. In addition, he had a blunted gonadotropin releasing hormone (GnRH) test: luteninizing hormone (LH) rose from 0.5 to 0.7 units/liter, and follicule-stimulating hormone (FSH) rose from 0.7 to 2.1 units/liter). Upon imaging, he had normal kidneys, hypoplastic olfactory sulci, and no OB leading to a diagnosis of KS. He died 1 year later from colon cancer. Thus, the proband had a severe form of KS, characterized by microphallus, congenital anosmia, apparent absent OB, and absent puberty.
II-3.
The proband's younger brother, homozygous for p.I55fsX1, was a 67-year-old male (Fig. 1a). He also had congenital anosmia and microphallus at birth, and he failed to go through puberty. He married but did not father any children. The diagnosis of KS was not made until age 60 years. At that time, he had no secondary sexual characteristics, and he had prepubertal testes, hypogonadal serum testosterone (<35 ng/dl; adult range from 300 to 1,000 ng/dl), and undetectable gonadotropins. He exhibited otherwise normal pituitary function. Formal smell testing confirmed anosmia (he had a score of 6/40), ultrasound showed normal kidneys, and dual x-ray absorptometry indicated lumbar osteoporosis. He was started on testosterone replacement, and he noted improved sexual function. In summary, the proband's brother also presented with a severe form of KS with osteoporosis.
II-5.
One of the proband's younger sisters also harbors the homozygous p.I55fsX1 mutation. She presented at age 18 years with primary amenorrhea and normal sense of smell. She had minimal pubic and axillary hair, and she exhibited no spontaneous breast development. She was treated with hormone replacement therapy and acquired secondary sexual characteristics. She later presented with infertility and underwent three cycles of ovulation induction resulting in a successful pregnancy. Her only son is now 27 years old with normal facial hair and a normal sense of smell.
II-6.
The proband's younger brother is heterozygous for the p.I55fsX1 mutation and had normal puberty and fertility. He now has two sons. Like the oldest brother, he presented with type II diabetes mellitus at age 54.
II-1.
The proband had an older sister (1930–2003) with seven descendents. Two died in the neonatal period; the remaining five are married and have children.
II-4.
The proband had another younger sister (1939–2003) with five descendents. One died in the neonatal period; the remaining four daughters are alive and well with children.
Interestingly, the proband also had five brothers and two sisters who died in their first years of life. Because the family was raised in a remote village in rural Portugal, there were no clinical or pathological studies in these cases. Oral history indicates they died of infectious disease related to the poor living conditions. However, it should be noted that both the Prok2−/− and Prokr2−/− mice also have a high frequency of neonatal death (20). The father (I-1) and mother (I-2) apparently went through normal puberty and had normal reproductive function, and both died at old ages (Fig. 1a).
Reproductive Phenotype in Prok2−/− Mice.
Abnormalities of the OB in adult Prok2−/− mice are described in ref. 16. We also noted that the OB of Prok2−/− embryos were overtly smaller than those of WT controls (21).
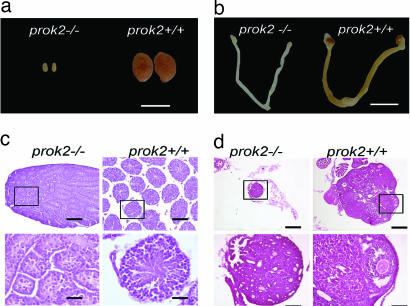
Anatomical examination of the reproductive tissues of 12-week-old Prok2−/− mice revealed hypoplasia of reproductive organs in both male and female mice. The testes of the male Prok2−/− mice were significantly smaller than those of age-matched controls (109 ± 6.6 mg for WT vs. 3.1 ± 0.8 mg for mutants; Fig. 2a).
Fig. 2.
Hypoplasia of the reproductive organs in Prok2−/− mice. (a and b) Macroscopic view of the testis, ovary, and uterus of 12-week-old Prok2−/− and WT mice. The reproductive organs of both male and female prok2−/− mice were small. (Scale bars: 5 mm.) (c) H&E-stained sections of testis from 12-week-old Prok2−/− and WT mice. Low magnification showed smaller seminiferous tubule in Prok2−/− mice. Higher magnification (rectangle in Upper Left) revealed no sperm in Prok2−/− mice. (Scale bars: 100 μm for low magnification and 25 μm for high magnification.) (d) H&E-stained sections of ovary from 12-week-old Prok2−/− and WT mice. Higher magnification (rectangle in the lower magnification) showed undeveloped follicles in Prok2−/− mice. (Scale bars: 200 μm for low magnification and 50 μm for high magnification.)
Male Prok2−/− mice had immature testes with absent lumen and greatly reduced seminiferous tubule diameters (Fig. 2c). There were no haploid spermatids. Seminal vesicles were commonly absent (data not shown), and female Prok2−/− mice also were characterized by defective sexual development and an absence of vaginal opening. Their ovaries and uteri were significantly smaller than those in WT animals (Fig. 2b). The ovaries contained primary and secondary follicles and occasional early antral follicles but lacked Graafian follicles and corpora lutea (Fig. 2d). Consistent with these immature gonads, we observed that Prok2−/− mice were infertile. No abnormalities were observed in Prok2+/− mice (data not shown).
The lack of estrus cyclicity and the inability to produce sperm were due to low serum gonadotropins. Both serum LH and FSH were significantly lower in male Prok2−/− mice than in WT mice (n = 4; Fig. 3 d and e). There was a trend for lower serum LH and FSH levels in female Prok2−/− mice vs. those in WT, although the difference was not significant (n = 3; Fig. 3 d and e).
Fig. 3.
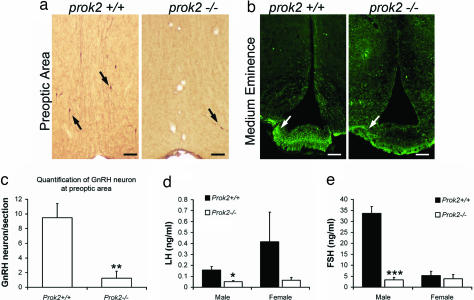
Abnormalities of the GnRH neurons in Prok2−/− mice. (a and b) Immunohistochemical staining of the preoptic region (a) and immunofluorescence staining of the median eminence (b) of 12-week-old WT and Prok2−/− mice. Fewer GnRH neurons were observed in Prok2−/− mice. The arrows in a indicate the cell bodies of GnRH-positive neurons; the arrows in b indicate the GnRH-immunoreactive axons. (Scale bars, 100 μm.) (c) Quantification of GnRH-positive neurons in the preoptic area of WT and Prok2−/− mice (**, P < 0.001; Student's t test; n = 4). (d and e) Measurement of LH and FSH hormones in age-matched WT and Prok2−/− mice. Both LH and FSH in male Prok2−/− were significantly lower than in WT control (*, P < 0.05; ***, P < 0.0001; Student's t test; n = 4).
To investigate potential additional gonadal defects, we performed ovulation induction in the female Prok2−/− mice. These mice could be induced to ovulate after sequential injection of gonadotropins and human chorionic gonadotropin (data not shown), consistent with hypogonadotropic hypogonadism.
Defects in GnRH Neuron Migration in Prok2−/− Mice.
We performed GnRH immunohistochemistry in 12-week-old Prok2−/− mice and WT mice. In the WT mouse brain, the cell bodies of most GnRH-immunoreactive neurons were scattered in the basal forebrain areas (+1.3 mm to +0.4 mm to bregma) with their axons converging onto the medium eminence (Fig. 3 a and b). In contrast, few GnRH-immunoreactive neurons were observed in the basal forebrain of Prok2−/− mice (Fig. 3a). We counted the number of GnRH+ neurons in the preoptic area (≈0.6 mm to bregma, three adjacent sections of each animal and four animals in each genotype) of the WT and Prok2−/− mice, which revealed a dramatic reduction of GnRH-positive cells in the Prok2−/− mice (Fig. 3c). Consistent with the severely decreased GnRH neuron numbers, only a few GnRH positive axons were observed in the medium eminence in Prok2−/− mice (Fig. 3b).
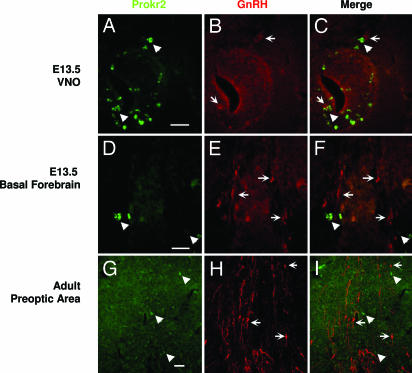
To test whether the PROK2–PROKR2 signaling pathway acts directly on GnRH neurons, we performed in situ hybridization analysis for Prokr2 and immunostaining for GnRH in WT mice. Surprisingly, GnRH and Prokr2, although expressed in the same area of the brain (Fig. 4), did not colocalize during embryogenesis nor in the adult stages in WT mice (Fig. 5).
Fig. 4.
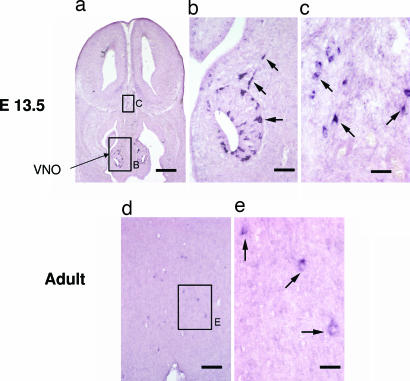
prokr2 expression in the brain. Digoxigenin-labeled in situ hybridization of prokr2 in E13.5 (a–c) and adult (d and e) mouse brain. (a–c) In E13.5, prokr2-expressing cells were observed in the vomeronasal organ (high magnification in b) and in the basal forebrain (high magnification in c). (Scale bars: a, 250 μm; b, 50 μm; c, 25 μm.) In the adult (d and e), several prokr2-expressing cells were observed in the preoptic area. (e) High magnification of the rectangle in d. (Scale bars: d, 100 μm; e, 25 μm.)
Fig. 5.
prokr2 does not colocalize with GnRH. prokr2 expression was revealed by in situ hybridization (green), and GnRH was revealed by immunostaining (red) in the vomeronasal organ (A–C), in the basal forebrain (D–F) of E13.5, and in the preoptic area of adult mouse brain (G–I). (Scale bar: 50 μm.)
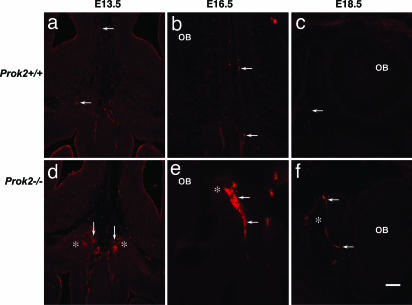
We then examined the migration of GnRH neurons at embryonic stages in Prok2−/− mice (Fig. 6). As expected, most of the GnRH neurons did not reach their destination at the preoptic area in the Prok2−/− mice. Many GnRH neurons were still in the nasal septum at embryonic day (E) 16.5, but fewer were seen in the WT littermates. After crossing the cribriform plate, GnRH neurons were trapped in an exogenous, spherical-shaped structure, which appeared as early as E13.5 between those two olfactory bulbs in the Prok2−/− mice (Fig. 6e). Because the development of the OB was greatly compromised (16), the spherical structure probably was formed by olfactory/vomeronasal axons that did not reach their targets.
Fig. 6.
GnRH neurons were stalled in an exogenous spherical structure in the Prok2−/− mice. Migrating GnRH neurons passed the OB through their migration route at E13.5, E16.5, and E18.5 (a–c). In Prok2−/− mice, an exogenous spherical structure (asterisks in d–f) appeared between the two olfactory bulbs. GnRH neurons were stalled in the structure. (Scale bar: 100 μm.)
Discussion
We report a homozygous loss-of-function mutation in PROK2 in a kindred with both KS and nIHH. These findings are consistent with the phenotype of the Prok2−/− mice that exhibit variable olfactory bulb defects, disrupted GnRH neuron migration, and consequent hypogonadotropic hypogonadism.
Herein, we identify a homozygous PROK2 deletion leading to a frameshift (p.[I55fsX1]+ [I55fsX1]) and premature stop codon in three affected KS/nIHH siblings. This termination codon could either mediate nonsense mRNA decay (18) or lead to a truncated mature protein of 27 aa instead of the full-length mature protein (81 aa). It should be noted that an alternatively spliced form encoding an additional 21 basic aa has also been isolated in the brain, although with low expression (22). PROK2 contains two highly conserved structural features: 10 cysteines and an N-terminal hexapeptide sequence (AVITGA) (19). Previous biochemical studies have shown that both the AVITGA and the cysteine-rich C-terminal domains are critical for the bioactivity of prokineticins (19). Because the putative truncated protein of 27 aa identified in our sibship lacks a large part of the C-terminal, cysteine-rich domain, it was predicted to lack functionality (19). Indeed, this was confirmed in vitro.
The three affected siblings carrying the homozygous PROK2 deletion presented with absent puberty indicating severe GnRH deficiency. The normosmic sister conceived with gonadotropin therapy consistent with a hypothalamic defect and functional gonads. Interestingly, the Prok2−/− mice revealed striking phenotypic similarities with the human hypogonadotropic hypogonadism; they lack sexual maturation, have low serum gonadotropins, and respond to exogenous gonadotropins consistent with hypogonadotropic hypogonadism. Furthermore, they exhibit a marked decrease in the number of GnRH neurons in the hypothalamus.
Interestingly, the three siblings with IHH carrying the identical PROK2 deletion differed in terms of olfactory phenotype. Both brothers had congenital anosmia, and the sister was normosmic. These data suggest a role for other gene(s) and/or environmental cues acting as modifiers of the olfactory phenotype.
It is well known that the olfactory system and GnRH neuron migration are intimately linked during development. GnRH neurons originate in the nasal placode and migrate along their olfactory guiding fibers into the forebrain toward the OB and ultimately dissociate from their guiding fibers to reach the preoptic area (23). Herein, we demonstrate that GnRH neuronal migration in the Prok2−/− mice was arrested in the forebrain after crossing the cribiform plate. The GnRH neurons in the Prok2−/− mice were identified in a spherical structure, likely formed by the olfactory/vomeronasal axons, between two defective OB (16, 21). This is consistent with the phenotypes of the two KS brothers with absent PROK2 activity who exhibited absent puberty, congenital anosmia, and absent OB. Previous studies have shown that Prok2 is expressed in the developing OB, serves as a chemo-attractant for neuronal progenitors of the subventricular zone, and is critical for the establishment of a normal OB architecture (16). In addition, we demonstrate that both Prokr2 and GnRH are expressed in the same vicinity within the vomeronasal organ and along the GnRH migratory route during embryology. Therefore, PROK2, expressed in the developing OB, may also participate as a chemo-attractant for the olfactory guiding fibers. Furthermore, Prokr2−/− mice exhibit an even more severe disruption of the OB and hypogonadotropic hypogonadism (20), suggesting that a fully functioning prokineticin pathway is critical for OB ontogenesis and GnRH neuron migration.
Interestingly, only 50% of the Prok2−/− mice displayed asymmetric OBs (16), yet all have sexual immaturity and infertility. This finding suggests that additional defects, apart from OB dysgenesis, may occur in the Prok2−/− mice. Indeed, these mice have severely decreased numbers of GnRH neurons in the preoptic area. PROK2 deficiency may lead to an isolated defect in GnRH neuron migration. This has been demonstrated in the knock-out mouse model for another gene, Ebf2, showing abnormal GnRH neuron migration yet normal olfactory bulbs and sulci (24). Furthermore, the few GnRH neurons in the preoptic area of the Prok2−/− mice may not be functional, consistent with their sexual immaturity. Indeed, GnRH implants in the hpg mouse model showed that few neurons are needed to rescue function of the reproductive axis (25). Both Prok2 and Prokr2 are expressed in the preoptic area (21) and therefore may be involved in the maturation/survival of the GnRH neurons. Prok2 also acts as an output protein from the suprachiasmatic nucleus (26) and therefore could play a role in GnRH pacemaking. These hypotheses on the impact of PROK2 mutations on GnRH ontogeny are supported by the normosmic phenotype of the IHH sister in our kindred. Interestingly, Prokr2 and GnRH do not colocalize in GnRH neurons, suggesting that the prokineticin pathway acts indirectly to support GnRH neuron development, migration, or function.
The unaffected brother carried a heterozygous mutation, and both parents (likely heterozygote for the mutation) were asymptomatic. A recent report of a pedigree with a proposed PROK2 heterozygous mutation (p.79fsX100) is informative for its variable olfactory and reproductive phenotypes and hence incomplete penetrance (17). Combined with our data, it appears that two mutated PROK2 alleles are needed to cause nIHH/KS. Alternatively, additional gene defect(s) critical to the integrity of the GnRH neuronal system could synergize with one mutated PROK2 allele to cause nIHH/KS. This hypothesis would be consistent with our recent demonstration that oligogenicity underlies some cases of IHH (3).
In conclusion, this report establishes a gene (PROK2) causing IHH in both mice and humans with variable olfactory phenotypes. Defining the roles of the prokineticin system in GnRH neuron ontogeny will provide invaluable information on the control of human sexual maturation.
Methods
Subjects.
IHH Population.
One hundred unrelated probands with IHH (50 with hyposmia/anosmia and 50 with normosmia) with no mutations in loci previously shown to cause IHH (KAL1, GNRHR, GPR54, and FGFR1) were studied. IHH was characterized by (i) absent/incomplete puberty by age 18 years; (ii) serum testosterone ≤ 100 ng/dl in men or serum estradiol (E2) ≤ 20 pg/ml in women in association with low or normal levels of serum gonadotropins; (iii) otherwise normal pituitary function; (iv) normal serum ferritin concentrations; and (v) normal MRI of the hypothalamic pituitary region.
Controls.
The Caucasian control population consisted of 170 healthy Caucasian subjects with a normal sexual maturation from Massachusetts General Hospital. The study was approved by the Human Research Committee of Massachusetts General Hospital, and all subjects provided written informed consent before participation.
Mutation Analysis of PROK2 Gene.
Genomic DNA was obtained from peripheral blood samples by using a standard extraction procedure. The PROK2 gene is located on 3p13 (GenBank accession no. NM_021935) and consists of four exons. Primers were designed to amplify across all intron–exon boundaries and exons of the PROK2 gene. The following primers were used: PROK2-e1F: 5′-CTTT A T AACGGCCCGGAGG-3′; PROK2-e1R: 5′-CCTC T A G C C TGCCCTTCAG-3′; PROK2-e2F: 5′-GATTTTCATAATCCAGGGGC-3′; PROK2-e2R: 5′-TGTTTGTCGAGCACGTTACC-3′; PROK2-e3F: 5′-GTATCTTGCTCCGC C A GTTC-3′; PROK2-e3R: 5′-AGGACAGACCCAACTCTATGG-3′; PROK2-e4F: 5′-TGAGCATATTGCCTAATGGG-3′; and PROK2-e4R: 5′-TTGAGGAAGCAAGAGCATTTC-3′. Amplification was performed for 35 cycles. The annealing temperatures were 60, 60, 63, and 56°C for exons 1, 2, 3, and 4, respectively. Amplified products were sequenced using the AmpliTaq Dye Terminator Cycle Sequencing kit and an ABI PRISM 377 DNA sequencer (Perkin-Elmer, Foster City, CA). All sequence variations were found on both strands and were confirmed in a separate PCR. All genes and proteins are described using standard nomenclature (27).
Generation of Mutant PROK2.
The mutant PROK2 peptide of 27 aa was chemically synthesized and purified via reverse phase HPLC as described in ref. 12.
Functional Studies of PROK2 Mutant.
An aequorin-based luminescent assay was used to measure the activity of mutant PROK2 peptides (19). Briefly, CHO cells stably expressing the photoprotein aequorin and PROKR2 were charged in Opti-MEM (Invitrogen, Carlsbad, CA) containing 8 μM coelenterazine cp at 37°C for 2 h and were treated with increasing doses of PROK2. Cells were harvested by trypsinization and maintained in Hanks' balanced salt solution plus 10 mM Hepes, pH 7.5, and 0.1% BSA at 5 × 105 cells/ml. Luminescence was measured with a Sirius luminometer (Berthold Detection Systems, Nashua, NH).
Reproductive Characterization of Prok2−/− Mice.
In parallel to human genetic studies to investigate the roles of PROK2 in the neuroendocrine system, we studied the Prok2−/− mice as described in ref. 16. All procedures regarding the care and use of animals were in accordance with institutional guidelines.
Histologic Studies.
Adult mice were anesthetized with ketamine and xylazine and perfused with saline and 4% paraformaldehyde. After perfusion, brains and reproductive organs of the adult mice were removed and postfixed in 4% paraformaldehyde at 4°C overnight, cryoprotected in 30% sucrose, snap-frozen in cold isopentane, and stored frozen at −70°C.
For the embryos, at 1200 hours on the day on which the vaginal plug was found counted as E0.5. E13.5–E18.5 embryos were collected from pregnant mice by cesarean section. Embryos older than E15.5 were perfused transcardiacally. Embryos were fixed in 4% paraformaldehyde at 4°C overnight, cryoprotected in 30% sucrose, snap-frozen in cold isopentane, and stored frozen at −70°C. Frozen sections were cut on a cryostat and mounted onto superfrost slides (Fisher Scientific, Hanover Park, IL). Routine H&E staining was carried out for histological examination.
Immunostaining of GnRH Neurons.
Immunohistochemistry was carried out as described in ref. 16. Briefly, 40-μm coronal sections were first blocked in PBS containing 0.2% Triton X-100 (PBST) plus 10% horse serum, then incubated with rabbit anti-luteinizing hormone-releasing hormone (1:5,000; Chemicon, Temecula, CA) in PBST with 3% horse serum at 4°C overnight. Diaminobenzidine staining was done with the Vector ABC Elite kit (Vector Laboratories, Burlingame, CA) as suggested by the vendor.
For immunofluorescence staining, FITC-labeled donkey anti-rabbit IgG (1:200; Jackson ImmunoResearch, West Grove, PA) was added after primary antibody incubation. Sections were counterstained with DAPI (Vector Laboratories) and viewed with a Zeiss (Oberkochen, Germany) fluorescence microscope.
Response to Gonadotropins.
To assess the gonadal response to gonadotropin stimulations of female Prok2−/− mice, we gave pregnant mare serum gonadotropin (G4527; Sigma, St. Louis, MO) 5 units i.p. at 1400 hours followed 48 h later by hCG (C8554; Sigma) to induce superovulation to 4 week-old Prok2−/− female mice. Twelve hours later, the ova were collected from the oviduct.
Hormone Assays.
All murine hormone assays were performed by the Ligand Assay Core of the Specialized Cooperative Center for Research in Reproduction at the University of Virginia (Charlottesville, VA). All samples were analyzed in one assay. The reportable range of the LH assay was 0.04–37.4 ng/ml, and the intraassay coefficient of variation was 7%. The reportable range of the FSH assay was 3.4–35.9 ng/ml, and the intraassay coefficient of variation was 8%. The reportable range of the testosterone assay was 7–801 ng/dl, and the intraassay coefficient of variation was 7%.
In Situ Hybridization Using Nonradiolabeled Probes.
Digoxigenin-labeled Prokr2 and Prok2 riboprobes were made by using digoxigenin RNA labeling mix from Roche (Indianapolis, IN). In situ hybridization using digoxigenin-labeled riboprobes was performed as described in ref. 28.
Statistical Analysis.
Results are expressed as the mean ± SD. Differences between groups were examined for statistical significance by using Student's two-tailed unpaired t test.
Acknowledgments
We thank two clinical colleagues who first studied the proband: Dr. Jorge Herbert and Dr. Maria João Oliveira, from Porto, Portugal. This work was supported by National Institutes of Health Grants U54 HD028138 and 5R01 HD015788 to W.F.C.; MH067753 to Q.-Y.Z.; and DK044838, U54 HD012303, and HD020377 to P.L.M.
Abbreviations
- IHH
idiopathic hypogonadotropic hypogonadism
- GnRH
gonadotropin-releasing hormone
- KS
Kallmann syndrome
- nIHH
normosmic idiopathic hypogonadotropic hypogonadism
- PROKR
prokineticin receptor
- OB
olfactory bulb
- LH
luteinizing hormone
- FSH
follicule-stimulating hormone
- En
embryonic day n.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 17247.
References
- 1.Kallmann FJ, Schoenfeld WA. Am J Mental Defic. 1944;158:203–236. [Google Scholar]
- 2.Boyar RM, Wu RH, Kapen S, Hellman L, Weitzman ED, Finkelstein JW. J Clin Endocrinol Metab. 1976;43:1268–1275. doi: 10.1210/jcem-43-6-1268. [DOI] [PubMed] [Google Scholar]
- 3.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, et al. J Clin Invest. 2007;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, et al. Nature. 1991;353:529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- 5.Hardelin JP, Levilliers J, Blanchard S, Carel J-C, Leutenegger M, Pinard-Bertelletto J-P, Bouloux P, Petit C. Hum Mol Genet. 1993;2:373–377. doi: 10.1093/hmg/2.4.373. [DOI] [PubMed] [Google Scholar]
- 6.Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millasseau P, Le Paslier D, Cohen D, Caterina D, et al. Cell. 1991;67:423–435. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- 7.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. N Engl J Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- 9.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 10.Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, et al. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- 11.Pitteloud N, Acierno JS, Jr, Meysing A, Eliseenkova AV, Ma J, Ibrahimi OA, Metzger DL, Hayes FJ, Dwyer AA, Hughes VA, et al. Proc Natl Acad Sci USA. 2006;103:6281–6286. doi: 10.1073/pnas.0600962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 13.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 14.Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, et al. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 15.Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 16.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 17.Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, et al. PLoS Genet. 2006;2:e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maquat LE, Carmichael GG. Cell. 2001;104:173–176. doi: 10.1016/s0092-8674(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 19.Bullock CM, Li JD, Zhou QY. Mol Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. Proc Natl Acad Sci USA. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Ng KL, Li JD, He F, Anderson DJ, Sun YE, Zhou QY. J Biol Chem. 2007;282:6917–6921. doi: 10.1074/jbc.C600290200. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg T, Liu C. Mol Pharmacol. 2005;67:2070–2076. doi: 10.1124/mol.105.011619. [DOI] [PubMed] [Google Scholar]
- 23.Tobet SA, Schwarting GA. Endocrinology. 2006;147:1159–1165. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- 24.Corradi A, Croci L, Broccoli V, Zecchini S, Previtali S, Wurst W, Amadio S, Maggi R, Quattrini A, Consalez GG. Development (Cambridge, UK) 2003;130:401–410. doi: 10.1242/dev.00215. [DOI] [PubMed] [Google Scholar]
- 25.Krieger DT, Perlow MJ, Gibson MJ, Davies TF, Zimmerman EA, Ferin M, Charlton HM. Nature. 1982;298:468–471. doi: 10.1038/298468a0. [DOI] [PubMed] [Google Scholar]
- 26.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 27.Antonarakis SE. Hum Mutat. 1998;11:1–3. doi: 10.1002/(SICI)1098-1004(1998)11:1<1::AID-HUMU1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Schaeren-Wiemers N, Gerfin-Moser A. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]