Abstract
Myasthenia gravis (MG) and experimental autoimmune MG are T cell-dependent antibody-mediated autoimmune diseases. A dual altered peptide ligand (APL), composed of the tandemly arranged two single amino acid analogs of two myasthenogenic peptides, p195-212 and p259-271, down-regulated in vitro and in vivo MG-associated T cell responses. In the present study, we investigated the role of CD8+CD28− regulatory cells in the mechanism of action of the dual APL. We demonstrated that treatment of mice with the dual APL concomitant with immunization with a myasthenogenic peptide resulted in an increased population of CD8+CD28− cells that express forkhead box P3 (Foxp3). The dual APL inhibited the proliferation of lymph node (LN) cells of the Torpedo acetylcholine receptor-immunized WT C57BL/6 mice, whereas the inhibition was abrogated in CD8−/− knockout mice. Moreover, the dual APL did not inhibit the secretion of IFN-γ by LN cells from CD8−/− mice immunized with Torpedo acetylcholine receptor. However, the mRNA expression of IL-10 and TGF-β by LN cells from CD8−/− mice was up-regulated similarly to that of the WT mice. Furthermore, the dual APL elevated the proapoptotic markers caspases 3 and caspase 8, whereas it down-regulated the antiapoptotic marker Bcl-xL in both CD8−/− and WT mice. Finally, the dual APL-induced CD4+CD25+Foxp3+ cells were up-regulated in CD8−/− mice to a similar extent to that observed in the WT mice. Thus, we suggest that CD8+CD28− regulatory cells play a partial role in the mechanism of action by which the dual APL suppresses experimental autoimmune MG-associated T cell responses.
Keywords: apoptotic markers, cytokines, regulatory T cells, experimental autoimmune myasthenia gravis
Myasthenia gravis (MG) is a T cell-regulated, antibody-mediated autoimmune disease characterized by muscular weakness and excessive fatigue owing to antibodies directed against acetylcholine receptors (AChRs) of skeletal muscles (1, 2). Previous work performed in our laboratory demonstrated that two peptides representing sequences of the human AChR α-subunit, namely p195-212 and p259-271, were able to stimulate peripheral blood lymphocytes of patients with MG and were shown to be immunodominant T cell epitopes of SJL and BALB/c mice, respectively (3, 4). A dual altered peptide ligand (APL), composed of the tandemly arranged two single amino acid analogs of the two above peptides, was shown to inhibit efficiently the in vivo priming of lymph node (LN) cells to either myasthenogenic peptides and to down-regulate the clinical manifestations of an ongoing experimental autoimmune MG (EAMG) (5–9). The dual APL up-regulated the secretion of TGF-β and down-regulated IFN-γ and IL-2 secretion (10). Moreover, the inhibitory effect of the dual APL could be adoptively transferred to p195–212 or Torpedo AChR (TAChR)-immunized mice by cells of treated mice (10). CD4+CD25+ regulatory cells were shown to play an important role in the suppressive action of the dual APL (11, 12).
Immunological tolerance functions as a fundamental concept in the control of a broad spectrum of immune responses to auto and foreign antigens. CD4+CD25+ regulatory T cells (≈ 10% of peripheral CD4+ T cells) have emerged as key players in the development of immunological tolerance (13). In healthy individuals, CD4+CD25+ cells have several characteristic markers such as CD45RBlow in mice and CD45RO+ in humans (14), the cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) (15), glucocorticoid-induced transforming neuronal factor receptor (16), and the forkhead box P3 (Foxp3) transcription factor (17). Foxp3 expression was shown to correlate with the acquisition of regulatory function (18), and two factors appear to be involved in its induction: TGF-β and the mode of antigen presentation. The dual APL was previously shown to induce CD4+CD25+ regulatory cells that expressed CTLA-4, CD45RBlow, TGF-β, and Foxp3 (11, 19). Moreover, depletion of these cells was reported to abrogate the inhibitory effects of the dual APL in mice immunized with either of the myasthenogenic peptides or the TAChR (20).
In addition to CD4+CD25+ regulatory cells, subsets of CD8+ T cells were found to play a regulatory role. One class of CD8+ Treg cells recognizes peptides derived from cell-surface antigens in association with MHC class I antigens. The underlying mechanism of suppression by these CD8+ T cells is unknown, although it may involve the differentiation of CD8+ T cells to cytotoxic T cells or the secretion of suppressor cytokines (21). A second class of CD8+ Treg cells resembles the CD4+ Tr1 cells, because their suppressive effects are mediated primarily by IL-10. A subpopulation of CD8+, IL-10-producing cells, expresses CD122, the IL-2 receptor β-chain, and was found to inhibit activated CD8 and CD4 T cells in vitro, in the absence of antigen-presenting cells (APCs) (21). The CD8+CD28− subset of cells develops after repeated stimulation with antigen-pulsed APCs and expresses Foxp3. CD8+CD28− cells target APCs and render them tolerogenic because exposure of dendritic cells to CD8+CD28− cells results in increased expression of genes encoding Ig-like transcript (ILT) 3 and ILT4. Expression of ILT3 and ILT4 receptors is associated with a reduced capacity of the APCs to transcribe NF-κB-dependent costimulatory molecules (21–23).
Both CD4+ and CD8+ cells were shown to play a role in the induction of EAMG (24). In the present study, we investigated the role of CD8+CD28− regulatory cells in suppressing myasthenogenic T cell responses by the dual APL. We demonstrated that treatment with the dual APL concomitant with the immunization with a myasthenogenic peptide or TAChR, resulted in increases in CD8+CD28−Foxp3+ cells. The latter cells are of importance because the dual APL inhibited neither the proliferation nor the secretion of IFN-γ by LN cells of TAChR-immunized CD8−/− mice. However, the expression of IL-10, TGF-β, caspase 3, caspase 8, and Bcl-xL by LN cells from TAChR immunized CD8−/− mice was similar to that observed in the WT mice. Finally, treatment of TAChR-immunized CD8−/− mice with the dual APL up-regulated CD4+CD25+Foxp3+ cells to levels similar to those observed in the WT mice. Thus, the CD8+CD28− regulatory cells play a partial role in the suppression of EAMG-associated autoreactive T cell responses by the dual APL.
Results
The Dual APL Up-Regulates Foxp3-Expressing CD8+CD28− Cells.
To determine whether CD8+CD28− regulatory cells play a role in the down-regulating effects of the dual APL, we monitored the kinetics of their development and compared it with that of CD4+CD25+ regulatory cells. To this end, BALB/c mice were immunized with the myasthenogenic peptide p259-271 alone or concomitant with treatment with the dual APL. LN cells obtained from these mice were stained for CD8, CD28, CD4, and CD25 markers and analyzed by FACS on different days after immunization and treatment. Fig. 1 presents the kinetics of CD8+CD28− and CD4+CD25+ cell development (one representative experiment of three performed). As shown, the CD8+CD28− cell population was up-regulated in the dual APL-treated cells 10 days after immunization and treatment (Fig. 1A). A similar pattern of up-regulation is shown for CD4+CD25+ cells in the dual APL-treated mice (Fig. 1B). Fig. 1C shows the proportion of CD8+CD28− cells relative to CD8+CD28+ cells 10 days after immunization with the myasthenogenic peptide and treatment with the dual APL. As shown, treatment with the dual APL resulted in an increased CD8+CD28−/CD8+CD28+ ratio. To assess the potential regulatory function of the CD8+CD28− cells, we immunized C57BL/6 mice with the whole macromolecule of TAChR, either with or without concomitant treatment with the dual APL, and 10 days later stained their LN cells for CD8+CD28− cells (Fig. 1D) and for Foxp3 out of CD8+CD28− cells (Fig. 1E). Fig. 1D demonstrates the elevation in the CD8+CD28− cell population in mice that were immunized with TAChR and treated with the dual APL. Fig. 1E shows that treatment with the dual APL increased Foxp3 expression in the gated CD8+CD28− cells, as compared with untreated cells (the mean increase in Foxp3 expression in the gated CD8+CD28− cells was 29.5% ± 2.1 for three experiments performed). Thus, regulatory CD8+CD28−Foxp3+ cells were elevated after treatment with the dual APL concomitant with immunization with either the myasthenogenic peptide or TAChR.
Fig. 1.
The dual APL up-regulates CD8+CD28− regulatory cells. (A and B) BALB/c mice were immunized with the myasthenogenic peptide p259-271 alone (▴) or concomitant with treatment with the dual APL (■). LN cells obtained from these mice were stained for CD8 and CD28 (A) or CD4 and CD25 (B) markers and analyzed by FACS at the indicated days after immunization and treatment. (C) The ratio between CD8+CD28+ and CD8+CD28− was determined 10 days after immunization with p259-271 and treatment with the dual APL. C57BL/6 mice were immunized with TAChR and either treated or not with the dual APL. (D and E) LN cells were taken 10 days later and stained for CD8+CD28− cells (D) or for Foxp3 out of the gated CD8+CD28− cells (E). Results are from one representative experiment of three performed.
The Inhibitory Effect of the Dual APL Is Abrogated in CD8−/− Knockout Mice Immunized with TAChR.
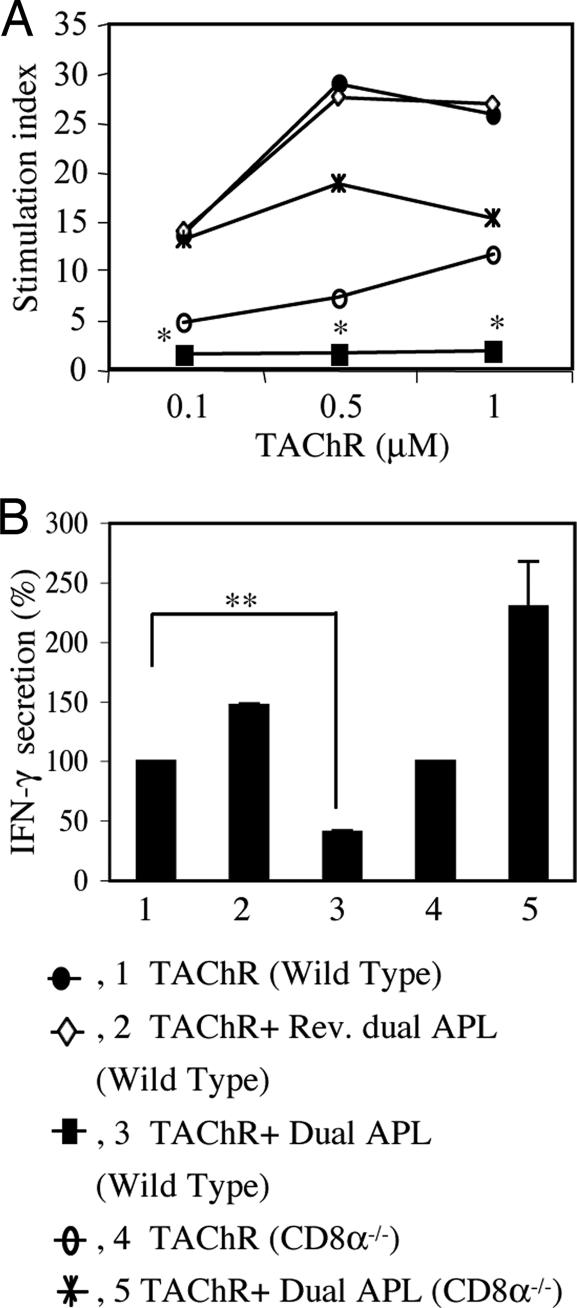
To assess the role of the CD8+CD28− cells in the mechanism of action of the dual APL, we compared the effect of treatment with the dual APL on CD8−/− (knock out for CD8α) and C57BL/6 WT mice. To this end, CD8−/− and C57BL/6 WT mice were immunized with TAChR alone or concomitantly treated with the dual APL. A control peptide, the reversed dual APL, was used for specificity control. The proliferation responses and the secretion of IFN-γ, which is considered to be the pathogenic cytokine in MG and EAMG, were tested in LN cells obtained from these mice 10 days later. Fig. 2A shows the proliferation responses of one experiment of two performed. As can be seen, treatment of the WT mice with the dual APL led to effective inhibition of proliferation (P ≤ 0.05 at all concentrations of TAChR), unlike the reversed dual APL, which did not suppress the proliferation. However, the dual APL did not inhibit the proliferation of LN cells of the CD8−/− mice, but instead elevated this response relative to the TAChR-immunized, untreated CD8−/− mice. Fig. 2B demonstrates the secretion of IFN-γ by LN cells, expressed as the mean percentage of IFN-γ secretion of three experiments. It can be seen that whereas the secretion of IFN-γ by LN cells from the WT mice was significantly inhibited after treatment with the dual APL, as compared with untreated mice (P = 0.002), secretion of IFN-γ by LN cells from CD8−/− mice was not inhibited, but rather, it was elevated after treatment with the dual APL. Because, as seen in Fig. 1C, most (≈ 90%) of the CD8 cells were CD28−, after immunization with TAChR and treatment with the dual APL, it is suggested that the latter cells play an important role in inhibiting proliferation and IFN-γ secretion by the dual APL.
Fig. 2.
The inhibition of proliferation and IFN-γ secretion by the dual APL is abrogated in CD8−/− mice immunized with TAChR. WT and CD8−/− mice were immunized with TAChR (10 μg per mouse in complete Freund's adjuvant) alone or concomitant with treatment with the dual APL (200 μg per mouse). Treatment of the WT mice with the reversed dual APL (200 μg per mouse) served as a control. (A) Proliferation of LN cells was performed as described in Materials and Methods. Results are expressed as the mean stimulation index of triplicate cultures (mean cpm for background of cells incubated with medium only was 1,808 ± 787). *, P ≤ 0.05 for dual APL-treated mice as compared with untreated mice. (B) LN cells (5 × 106 cells/ml) were incubated in the presence of TAChR (1 μg/ml), and their supernatants were collected after 48 h and assayed by ELISA for IFN-γ secretion. Results are expressed as the mean percent (100% = 1.3 ng/ml ± 0.168) secretion of three experiments. **, P = 0.002.
The Dual APL Up-Regulates the Secretion of TGF-β and IL-10 Regardless of the Presence of CD8 Cells.
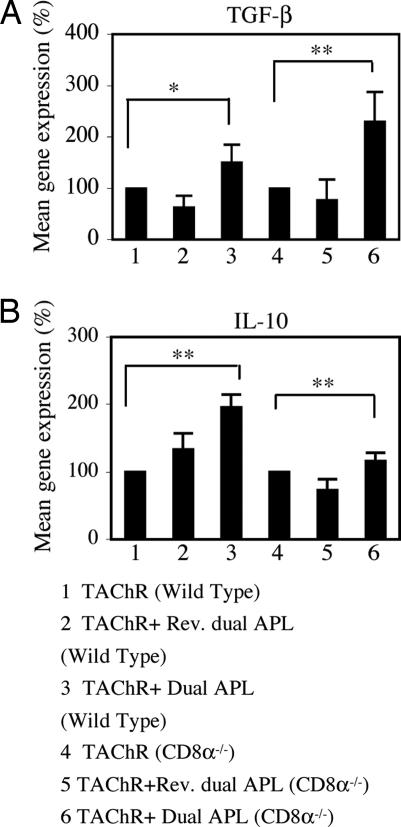
Treatment with the dual APL was previously shown in our laboratory to up-regulate the secretion of IL-10 and TGF-β, by LN cells from TAChR-immunized mice and EAMG-afflicted mice (10). To determine whether CD8 cells play a role in the up-regulation of these cytokines, we immunized WT and CD8−/− mice with TAChR alone or concomitant with treatment with the dual APL or the reversed dual APL. Ten days later, LN cells of these mice were tested for the gene expression levels of TGF-β and IL-10. Results are of a representative experiment of two performed. As can be seen in Fig. 3, immunization of WT and CD8−/− mice with TAChR and treatment with the dual APL resulted in similar elevated expression levels of TGF-β and IL-10 genes. Thus, the dual APL up-regulated the expression of TGF-β and IL-10 independently of the presence of CD8+CD28− cells.
Fig. 3.
TGF-β and IL-10 expression is up-regulated in WT and CD8−/− mice. WT mice and CD8−/− mice were immunized with TAChR alone, concomitant with treatment with the dual APL, or with the reversed (control) dual APL. Ten days later, total RNA of LN cells obtained from these mice was isolated, reverse-transcribed, as described in Materials and Methods, and subjected to real-time RT-PCR to determine the expression of TGF-β (A) and IL-10 (B) genes. Results were normalized to the gene expression levels of β-actin and compared with the TAChR-immunized WT group (defined as 100%). Statistical analysis was based on two individual experiments (*, P < 0.05; **, P ≤ 0.0001).
The Dual APL Up-Regulates the Expression of Caspase 3 and Caspase 8 and Down-Regulates the Expression of Bcl-xL in TAChR-Immunized CD8−/− Mice.
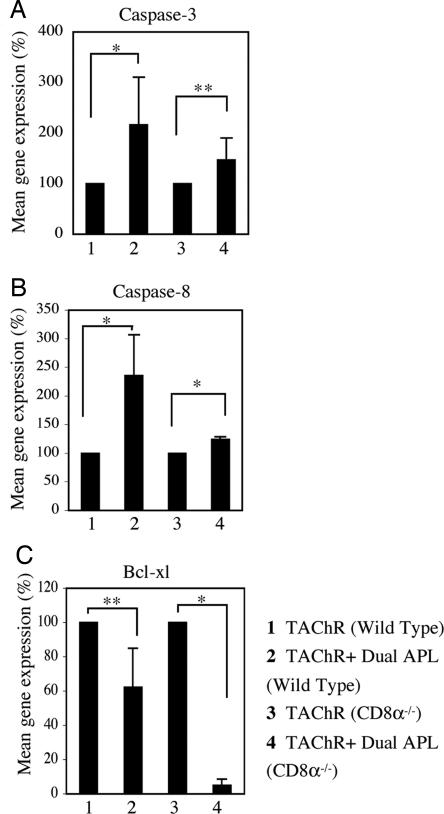
We have previously reported that the dual APL up-regulated several apoptotic markers in LN cells from mice that were immunized with either the myasthenogenic peptides or TAChR (11, 12). It was of interest to find out whether CD8+CD28− cells are involved in the dual APL induced up-regulation of proapoptotic markers, while down-regulating the antiapoptotic markers. To this end, we immunized WT and CD8−/− mice with TAChR alone or concomitant with treatment with the dual APL. Ten days later, LN cells obtained from these mice were tested for the mRNA expression levels of caspase 3, caspase 8, and Bcl-xL (Fig. 4). Results are of a representative experiment of two performed. As shown in Fig. 4 A and B, the mRNA expression levels of caspase 3 and 8 were elevated significantly after treatment with the dual APL in WT mice, as compared with nontreated mice. This trend was similar in CD8−/− mice that were immunized with TAChR and treated with the dual APL. Furthermore, the mRNA expression levels of the antiapoptotic Bcl-xL factor were significantly down-regulated after treatment with the dual APL, and the diminished expression was even more prominent in the CD8−/− mice (Fig. 4C). Thus, the dual APL up-regulated apoptotic markers and down-regulated antiapoptotic Bcl-xL in both WT and CD8−/− mice.
Fig. 4.
The dual APL up-regulates the mRNA expression levels of caspase 3 and caspase 8 and down-regulates the mRNA expression levels of Bcl-xL in CD8−/− mice. WT mice and CD8−/− mice were immunized with TAChR alone or concomitant with treatment with the dual APL. Ten days later, RNA isolated from these LN cells was reverse-transcribed, as described in Materials and Methods, and subjected to real-time RT-PCR of the caspase 3 (A), caspase 8 (B), and Bcl-xL (C) genes. Results were normalized to the gene expression levels of β-actin and compared with the TAChR-immunized WT group (defined as 100%). Statistical analysis was based on two individual experiments (*, P ≤ 0.05; **, P < 0.0001).
The Dual APL Induces CD4+CD25+ Regulatory T Cells in Both TAChR-Immunized CD8−/− and WT Mice.
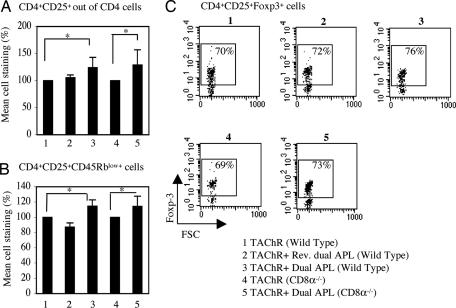
As previously reported, the dual APL up-regulated the CD4+CD25+ regulatory cells in mice that were immunized with either the myasthenogenic peptides or TAChR (11, 19–20). It was of interest to find out whether the absence of CD8+CD28− cells would affect the development of CD4+CD25+ regulatory cells. To this end, we immunized WT mice and CD8−/− mice with TAChR alone or concomitant with treatment with the dual APL or the reversed dual APL. Ten days later, LN cells obtained from these mice were stained for CD4, CD25, CD45RB, and Foxp3 markers and analyzed by FACS. Fig. 5 presents the results of staining of CD4+CD25+ regulatory cells in the tested groups. Results are presented as the mean percent of staining (Fig. 5 A and B) or as a representative staining experiment of three performed (Fig. 5C). As can be seen in Fig. 5A, the staining of CD4+CD25+ cells out of CD4+ cells was significantly elevated in WT mice that were immunized with TAChR and treated with the dual APL, whereas treatment with the reversed dual APL had no effect on the percentage of CD4+CD25+ cells. This up-regulation in CD4+CD25+ cells was also observed in CD8−/− mice that were immunized with TAChR and treated with the dual APL. Fig. 5B shows that the percentage of CD45RBlow+ cells that were gated on the CD4+CD25+ cell population was significantly elevated in both WT and CD8−/− mice after immunization with TAChR and treatment with the dual APL. Moreover, the dot plots in Fig. 5C demonstrate that the dual APL increased the expression of Foxp3 of the CD4+CD25+ cells in WT and CD8−/− mice. The mean percent of increase in CD4+CD25+ cells expressing Foxp3 of three individual experiments was significantly higher for the TAChR-immunized and dual APL-treated group as compared with the untreated group (7.4% ± 1.55 and 5.9% ± 0.4 increase for the WT and the CD8−/− mice, respectively; P ≤ 0.05). Thus, the dual APL induced the development and function of regulatory CD4+CD25+ cells in TAChR-immunized CD8−/− mice similarly to its effects in WT mice.
Fig. 5.
The dual APL-induced regulatory CD4+CD25+ cells in TAChR-immunized WT and CD8−/− mice. WT mice and CD8−/− mice were immunized with TAChR alone, concomitant with treatment with the dual APL, or with the reversed dual APL. Ten days later, LN cells of these mice were stained for CD4, CD25, CD45Rb, and Foxp3 and analyzed by FACS. (A) Mean staining of CD4+CD25+ cells out of CD4+ cells, as compared with the TAChR-immunized group defined as 100% (ranging from 8% to 16%) ± SD values of three experiments (*, P ≤ 0.05). (B) Mean staining of CD45Rblow+ cells gated on the CD4+CD25+ cell population, as compared with the TAChR-immunized group, defined as 100% (ranging from 52% to 68%) ± SD values of three experiments (*, P ≤ 0.05). (C) Dot plots of Foxp3+ cells gated on the CD4+CD25+ cell population. Results are from one representative experiment of three performed.
Discussion
The main findings of the present study are that treatment with the dual APL increased the fraction of regulatory CD8+CD28−Foxp3+ cells in parallel to up-regulating CD4+CD25+ regulatory cells. The ability of the dual APL to inhibit the TAChR-specific proliferation was abrogated in CD8−/−-immunized mice. In addition, the dual APL could not inhibit the secretion of IFN-γ by LN cells of CD8−/− mice immunized with the TAChR, in contrast to its inhibitory activity in their WT counterparts. However, the capacity of the dual APL to up-regulate the expression of IL-10 and TGF-β was similar regardless of the presence or absence of CD8+CD28− cells. Furthermore, the effect of the dual APL on the apoptotic markers caspases 3 and caspase 8, and the antiapoptotic marker Bcl-xL was the same in both WT and CD8−/− mice. Finally, the dual APL-induced CD4+CD25+Foxp3+ cells, which play an important role in the inhibitory effects of the dual APL, were up-regulated in CD8−/− mice. Thus, the CD8+CD28− regulatory cells appear to play a partial role in the mechanism of action by which the dual APL suppresses EAMG-associated T cell responses.
Like CD4+CD25+ regulatory cells, which express the functional factor Foxp3, CD8+CD28− regulatory cells are reported to express this marker (21). We demonstrated in the present study the up-regulation of CD8+CD28− cells that express Foxp3 after treatment with the dual APL. The importance of the regulatory CD8+CD28− cell population in the prevention of autoimmunity by maintaining peripheral tolerance has been reported in several in vitro studies, and limited studies have demonstrated the role of CD8+CD28− cells in autoimmune diseases (25, 26). Antibody depletion of CD8+ cells rendered CD28-deficient mice susceptible to experimental autoimmune encephalomyelitis. Moreover, adoptive transfer of CD8+CD28− cells into CD8−/− mice resulted in a significant suppression of the disease (22). In a study of a mouse model for inflammatory bowel disease (IBD), CD8+CD28− regulatory cells isolated from the spleen or gut prevented IBD induced by transfer of colitogenic T cells into immunodeficient hosts (27). In the present study, we demonstrated that the dual APL-induced regulatory CD8+CD28− cells play an important role in the inhibitory effect of T cell responses related to EAMG. Upon treatment with the dual APL, the ratio of CD8+CD28− to CD8+CD28+ cells shifted so that ≈90% of the CD8 cells were CD28− (Fig. 1C); therefore, it is most likely that the partial inability of the dual APL to inhibit EAMG-associated autoimmune responses is caused by the lack of CD8+CD28− cells.
CD8+ cells or their subsets may participate as both effectors and/or regulators of immune responses (22, 24, 28). Both CD4 and CD8 cells were reported to be involved in the induction of the disease as helper or effector cells (24). It is thus possible that the lack of CD8 effector cells from the cell milieu led to the decreased proliferation of TAChR from the CD4+ cells in the CD8−/− mice (Fig. 2A).
The mechanisms of action of the CD8+CD28− regulatory cells, especially in animal models, have not been completely elucidated. A few studies characterized the functions of CD8+CD28− regulatory cells in vitro (25, 26, 28). In human cultures, APCs exposed to CD8+CD28− regulatory T cells had impaired CD40-signaling pathways and could not up-regulate B7 molecules (25, 26, 29, 30). Furthermore, CD8+CD28− alloantigen-specific T suppressor cells induced the up-regulation of the ILT3 and ILT4 on APCs, rendering these cells tolerogenic (31). The impaired up-regulation of costimulatory molecules on APCs may prevent the efficient stimulation of CD4+ cells in the presence of CD8+CD28− T cells. This leads to decreased IFN-γ secretion, also known to up-regulate the expression of MHC-II (32) and costimulatory molecules on APCs (33).
In the present study, we showed that the dual APL could down-regulate the proliferation responses and the secretion of IFN-γ in WT mice, but not CD8−/− mice that were immunized with TAChR. CD8+CD28− regulatory cells from allospecific and xenospecific T cell lines were reported to inhibit T helper (Th) proliferation. In that case the mechanism was reported to be contact-dependent involving their interaction with APCs that were used for priming (34, 35). This finding indicates that CD8+CD28− regulatory cells inhibit Th proliferation by acting on APCs that coexpress the MHC class I and II antigens recognized by CD8+CD28− regulatory cells and Th (34, 35). Furthermore, APCs that were exposed to CD8+CD28− regulatory cells differentiated into professional inhibitory cells capable of inducing the differentiation of CD4+CD25+ cells (36–38), indicating that the CD8+CD28−-mediated suppression is a complex process.
IL-10 and TGF-β were previously shown to play a role in the dual APL-mediated suppression of MG-associated T cell responses (10). We demonstrated that treatment of TAChR-immunized CD8−/− mice with the dual APL up-regulated the expression of IL-10 and TGF-β mRNA levels, as observed for their WT counterparts.
In an experimental autoimmune encephalomyelitis disease model, CD8+ depletion in vivo and ex vivo did not affect the frequency of T cells producing Th2-type cytokines (22). It is thus suggested that the absence of CD8+CD28− regulatory cells affects mainly the Th1-dependent responses, rather than the Th2–Th3 responses.
We have previously demonstrated the importance of apoptosis in the mechanism of action of the dual APL, leading to inhibition of autoimmune T cell responses in mice that were immunized with the myasthenogenic peptides or TAChR (11, 20). We report here that the expression of caspase 3 and caspase 8 was similarly increased in WT and CD8−/− mice, and Bcl-xL was more significantly down-regulated in CD8−/− mice. It was reported that CD8+CD28− regulatory cells did not induce apoptosis in CD4+ cells in an experimental autoimmune encephalomyelitis model, and the addition of Fas- or FasL-blocking antibodies failed to reverse the suppression (22). Although the expression of Bcl-xL was lower in the CD8−/− mice than in the WT mice, it is likely that CD8+CD28− regulatory cells do not play a crucial role in the apoptosis of CD4 cells.
Both CD8+CD28− and CD4+CD25+ regulatory cells are important in inducing immune tolerance (17, 21, 22, 28). In our study, we demonstrated that both subsets are up-regulated in either myasthenogenic peptide- or TAChR-immunized WT mice after treatment with the dual APL (Figs. 1 and 5). We have previously reported that depletion of CD4+CD25+ regulatory cells abrogated the inhibition of EAMG-associated responses by the dual APL (19). In the present study, we demonstrated that CD4+CD25+-expressing Foxp3+ cells were developed in CD8−/− mice. However because treatment with the dual APL did not result in the down-regulation of proliferation and IFN-γ secretion in the CD8−/− mice (Fig. 2), it appears that the presence of CD8+CD28−Foxp3+ cells is required for the optimal function of CD4+CD25+ regulatory cells.
In summary, this study demonstrates the involvement of CD8+CD28−Foxp3+ regulatory cells in suppressing EAMG-associated responses by the dual APL. It is suggested that CD4+CD25+ regulatory cells, CD8+CD28− regulatory cells, and probably other regulatory cells, are required for an optimal down-regulation of EAMG-associated responses and an ongoing EAMG, which was previously shown to be ameliorated by the dual APL (9).
Materials and Methods
Mice.
Female mice of the inbred strains BALB/c, C57BL/6 (Harlan Breeders, Indianapolis, IN), and C57BL/6 CD8−/− (B6.129S2-CD8atm1Mak) (The Jackson Laboratory, Bar Harbor, ME) were used at the age of 8–12 weeks. The study was approved by the Animal Care and Use Committee of The Weizmann Institute of Science.
TAChR, Synthetic Myasthenogenic Peptide, and Dual APL.
AChR was purified from Torpedo californica as described (39). The myasthenogenic peptide p259-271 (VIVELIPSTSSAV) was synthesized and characterized as described (5). The dual APL (VIVKLIPSTSSAVDTPYLDITYHFVAQRLPL) (262Lys–207Ala) was designed as described (5) and synthesized (97% purity) by UCB Bioproducts (Brussels, Belgium). A peptide synthesized in the reversed order (reversed peptide) (LPLRQAVFHYTIDLYPTDVASSTSPILKVIV) of the dual APL was used as a control.
Immunization and Treatment of Mice.
BALB/c and C57BL/6 mice were immunized with 10 μg of p259-271 and TAChR, respectively, per mouse in complete Freund's adjuvant (Difco, Detroit, MI). Part of the mice was concomitantly injected with the dual or the reversed dual APL (s.c. 200 μg per mouse in PBS).
Proliferative Responses of LN Cells.
Popliteal LN cells (0.5 × 106 per well), obtained from mice 10 days after their immunization, were cultured in enriched RPMI medium 1640 supplemented with 1% normal mouse serum in the presence of various concentrations of TAChR for 96 h. [3H]thymidine (0.5 μCi of 5 Ci/mmol; Amersham Biosciences, Buckinghamshire, U.K.) was then added, and 16 h later, plates were harvested onto filter paper, and radioactivity was counted.
Secretion and Detection of IFN-γ.
LN cells (5 × 106/ml) from the tested mice were stimulated with TAChR (1 μM) for 48 h. Supernatants were collected and analyzed for IFN-γ levels by ELISA, using standard, capture, and detecting antibodies for IFN-γ (OptEIA Set; Pharmingen, San Diego, CA) according to the manufacturer's instructions.
Antibodies.
The following antibodies were used for fluorescence staining: anti-CD28-FITC antibody (clone 37.51), anti-CD25/IL-2R-FITC antibody (clone 7D4), anti-CD8-FITC (clone 53–6.7), rat anti-mouse CD4-APC (L3T3) antibody (clone 1B8), and their matched isotype controls (Southern Biotechnology Associates, Birmingham, AL) and anti-CD45Rb-PE (clone 16A), and its matched isotype control (Pharmingen).
Fluorescence Staining.
LN cells (5–10 × 105 cells per sample) were washed with 5% FCS/PBS, incubated with the relevant surface antibody, washed again, and analyzed by FACS. For Foxp3 intracellular staining, cells were further incubated with a fixation solution, washed, and resuspended in a permeabilization solution (eBioscience, San Diego, CA). Analysis of the triple-stained cells was assessed on the CD4+CD25+ gated population.
Real-Time RT-PCR.
Levels of mRNA expression of the genes were analyzed by quantitative real-time RT-PCR using LightCycler (Roche, Mannheim, Germany). Total RNA was isolated from LN cells and then reverse-transcribed to prepare cDNA by using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). The resulting cDNA was subjected to real-time RT-PCR, according to the manufacturer's instructions. Briefly, 20 μl of reaction volume contained 3 mM MgCl2, LightCycler HotStart DNA SYBR Green I mix (Roche), specific primer pairs, and 5 μl of cDNA. PCR conditions were as follows: 10 min at 95°C, followed by 35–50 cycles of 15 s at 95°C, 15 s at 60°C, and 15 s at 72°C. Primer sequences (forward and reverse, respectively) used were as follows: IL-10 (5′-aacctcgtttgtacctct-3′ and 5′-caccatagcaaagggc-3′), TGF-β (5′-gaacccccattgctgt-3′ and 5′-gccctgtattccgtct-3′), caspase 3 (5′-tctcgctctgtacgg-3′ and 5′-ggcagtagtcgcctct-3′), caspase 8 (5′-acataacccaactccgaa-3′ and 5′-gtgggataggatacagcaga-3′), Bcl-xL (5′-ggaccgcgtatcagag-3′ and 5′-gcattgttcccgtagag-3′), and β-actin (5′-gacgttgacatccgtaaag-3′ and 5′-ggccggactcatcgta-3′).
Statistical Analysis.
To evaluate the differences between groups, Student's t, alternate-Welch, and Mann–Whitney tests were used. Values of P ≤ 0.05 were considered significant.
Abbreviations
- AChR
acetylcholine receptor
- TAChR
Torpedo AChR
- APC
antigen-presenting cell
- APL
altered peptide ligand
- MG
myasthenia gravis
- EAMG
experimental autoimmune MG
- Foxp3
forkhead box P3
- ILT
Ig-like transcript
- LN
lymph node
- Th
T helper.
Footnotes
The authors declare no conflict of interest.
References
- 1.Drachman DB. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom J, Shelton D, Fuji Y. Adv Immunol. 1994;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 3.Brocke S, Brautbar C, Steinman L, Abramsky O, Rothbard J, Neumann D, Fuchs S, Mozes E. J Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brocke S, Dayan M, Rothbard J, Fuchs S, Mozes E. Immunology. 1990;69:495–500. [PMC free article] [PubMed] [Google Scholar]
- 5.Katz-Levy Y, Paas-Rozner M, Kirshner S, Dayan M, Zisman E, Fridkin M, Wirguin I, Sela M, Mozes E. Proc Natl Acad Sci USA. 1997;94:3200–3205. doi: 10.1073/pnas.94.7.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz-Levy Y, Dayan M, Wirguin I, Fridkin M, Sela M, Mozes E. J Neuroimmunol. 1998;85:78–86. doi: 10.1016/s0165-5728(97)00265-8. [DOI] [PubMed] [Google Scholar]
- 7.Kirshner SL, Zisman E, Fridkin M, Sela , Mozes E. Scand J Immunol. 1996;44:512–521. doi: 10.1046/j.1365-3083.1996.d01-330.x. [DOI] [PubMed] [Google Scholar]
- 8.Zisman E, Katz-Levy Y, Dayan M, Kirshner SL, Paas-Rozner M, Karni A, Abramsky O, Brautbar C, Fridkin M, Sela M, et al. Proc Natl Acad Sci USA. 1996;93:4492–4497. doi: 10.1073/pnas.93.9.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paas-Rozner M, Dayan M, Paas Y, Changeux JP, Wirguin Y, Sela M, Mozes E. Proc Natl Acad Sci USA. 2000;97:2168–2173. doi: 10.1073/pnas.040554597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paas-Rozner M, Sela M, Mozes E. Proc Natl Acad Sci USA. 2001;98:12642–12647. doi: 10.1073/pnas.221456798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-David H, Sela M, Mozes E. Proc Natl Acad Sci USA. 2005;102:2028–2033. doi: 10.1073/pnas.0409549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badiga-Venkata A, Sela M, Mozes E. Proc Natl Acad Sci USA. 2005;102:10285–10290. doi: 10.1073/pnas.0504578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 14.Sakagushi S. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak T, Sakaguchi S. J Exp Med. 2000;192:303–309. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Nomura T, Sakaguchi S. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 18.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paas-Rozner M, Sela M, Mozes E. Proc Natl Acad Sci USA. 2003;100:6676–6681. doi: 10.1073/pnas.1131898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aruna BV, Sela M, Mozes E. J Neuroimmunol. 2006;177:63–75. doi: 10.1016/j.jneuroim.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Shevach EM. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Najafian N, Chitnis T, Salama AD, Zhu B, Benou C, Yuan X, Clarkson MR, Sayegh MH, Khoury SJ. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlad G, Cortesini R, Suciu-Fuca N. J Immunol. 2005;174:5907–5914. doi: 10.4049/jimmunol.174.10.5907. [DOI] [PubMed] [Google Scholar]
- 24.Zhang GX, Xiao BG, Bakhiet M, Van-der Meide P, Wigzell H, Link H, Olsson T. J Exp Med. 1996;184:349–356. doi: 10.1084/jem.184.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. J Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- 26.Colovai AI, Liu Z, Ciubotariu R, Lederman S, Cortesini R, Suciu-Foca N. Transplantation. 2000;69:1304–1310. doi: 10.1097/00007890-200004150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Menager-Marcq I, Pomine C, Romagnoli P, Vad-Meerwijk JP. Gastroenterology. 2006;131:1775–1785. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Eur J Immunol. 2005;35:2896–2908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Tugulea S, Cortesini R, Suciu-Fuca N. Int Immunol. 1998;10:775–783. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Tuguela S, Cortesini R, Lederman S, Suciu-Foca N. Hum Immunol. 1999;60:568–574. doi: 10.1016/s0198-8859(99)00044-0. [DOI] [PubMed] [Google Scholar]
- 31.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovani AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, et al. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 32.Corthay A. Scand J Immunol. 2006;64:93–96. doi: 10.1111/j.1365-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 33.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Tuguela S, Cortesini R, Suciu-Foca N. Int Immunol. 1998;10:775–783. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 35.Ciubotariu R, Colovani AI, Pennesi G, Liu Z, Smith D, Berlocco P, Cortesini R, Suciu-Foca N. J Immunol. 1998;161:5193–5302. [PubMed] [Google Scholar]
- 36.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 37.Thorton AM, Shevach EM. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorton AM, Shevach EM. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 39.Elliot J, Blanchard SG, Wu W. Biochem J. 1980;185:667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]