Abstract
Malaria has been a major selective force on the human population, and several erythrocyte polymorphisms have evolved that confer resistance to severe malaria. Plasmodium falciparum rosetting, a parasite virulence phenotype associated with severe malaria, is reduced in blood group O erythrocytes compared with groups A, B, and AB, but the contribution of the ABO blood group system to protection against severe malaria has received little attention. We hypothesized that blood group O may confer resistance to severe falciparum malaria through the mechanism of reduced rosetting. In a matched case-control study of 567 Malian children, we found that group O was present in only 21% of severe malaria cases compared with 44–45% of uncomplicated malaria controls and healthy controls. Group O was associated with a 66% reduction in the odds of developing severe malaria compared with the non-O blood groups (odds ratio 0.34, 95% confidence interval 0.19–0.61, P < 0.0005, severe cases versus uncomplicated malaria controls). In the same sample set, P. falciparum rosetting was reduced in parasite isolates from group O children compared with isolates from the non-O blood groups (P = 0.003, Kruskal–Wallis test). Statistical analysis indicated a significant interaction between host ABO blood group and parasite rosette frequency that supports the hypothesis that the protective effect of group O operates through the mechanism of reduced P. falciparum rosetting. This work provides insights into malaria pathogenesis and suggests that the selective pressure imposed by malaria may contribute to the variable global distribution of ABO blood groups in the human population.
Keywords: ABO, erythrocyte, pathogenesis, rosette formation, virulence
Plasmodium falciparum malaria is a leading cause of death during childhood, therefore erythrocyte polymorphisms that provide protection against life-threatening malaria have risen to high frequencies in malaria-exposed populations (1, 2). Investigation of these polymorphisms and their effects on P. falciparum can provide insights into malaria pathogenesis, although in most cases, including hemoglobin S (HbS), α-thalassemia, and glucose-6-phosphate dehydrogenase deficiency, the mechanism of protection remains controversial (2). A polymorphism resulting in erythrocyte complement receptor (CR) 1 deficiency that is found at high frequency in Melanesian populations exposed to malaria is associated with reduced P. falciparum rosetting (spontaneous binding of infected erythrocytes to uninfected erythrocytes) (3) and protection against severe disease (4). The protective effects of thalassemia, sickle cell trait, and HbC may also be partly caused by reduced rosetting (5, 6). Rosetting is a known parasite virulence factor (7, 8) that is thought to contribute to the pathogenesis of severe malaria by obstructing microvascular blood flow (9). Previous studies have shown that rosetting is reduced in blood group O erythrocytes compared with the non-O blood groups (A, B, and AB) in P. falciparum laboratory strains (10, 11) and field isolates (8, 12). The A and B antigens are trisaccharide structures [A, GalNAcα1–3(Fucα1–2)Galβ1; and B, Galα1–3(Fucα1–2)Galβ1] attached to a variety of glycolipids and glycoproteins on the erythrocyte surface (13). Group O individuals lack the terminal glycosyltransferases necessary to produce the A or B antigens and carry the disaccharide H antigen (Fucα1–2Galβ1) (13). The A and B trisaccharides are thought to act as receptors for rosetting on uninfected erythrocytes (14), and direct binding between the parasite rosetting ligand PfEMP1 and the A antigen has been demonstrated (15). P. falciparum rosettes are able to form in group O cells, but these rosettes tend to be smaller and more easily disrupted than rosettes formed in group A, B, or AB erythrocytes (10, 14).
Given the known role of the A and B trisaccharides in rosette formation, we hypothesized that blood group O may be a protective factor against severe malaria because of its rosette-reducing effects. Earlier studies have found no consistent effect of the ABO blood group on the incidence of uncomplicated malaria, parasite density, or levels of anti-malarial antibody (for review, see ref. 16). The effect of the ABO blood group on severe falciparum malaria has received little attention, although previous studies have suggested that in African children, blood group A may predispose to severe malaria (17, 18), and in Southeast Asian adults, blood group O may confer resistance to the multiorgan failure form of severe disease (19). A rigorous study of the effect of ABO blood group on susceptibility to severe malaria using a matched case-control design with adjustment for known host protective factors such as hemoglobin variants has not been performed (20). The possibility of a rosette-mediated protective effect of blood group O has been raised previously (17–21); however, the effects of ABO blood group on rosetting and susceptibility to severe malaria have not yet been examined in a single study. We therefore investigated the effect of ABO blood group on rosetting and malaria severity in a case-control study of African children.
Results and Discussion
ABO blood group types were assessed on 567 blood samples from Malian children. Each severe malaria case (defined as unrousable coma, severe anemia, neurological impairment, repeated seizures, or evidence of hepatic or renal failure; for full details see Materials and Methods) was matched by age, ethnicity, and place of residence to an uncomplicated malaria control and a healthy control child (22). Cases of nonsevere hyperparasitemia (>500,000 infected erythrocytes per μl of blood) were also recruited and matched to uncomplicated malaria controls and healthy controls. Some researchers (e.g., refs. 18 and 23) include hyperparasitemia as a criterion for severe disease; however, we have found that children with hyperparasitemia and no other symptoms or signs of severe malaria have an excellent prognosis (0% mortality) and can thus be considered as having a form of uncomplicated malaria with very high parasite burdens (22, 24). The characteristics of the recruited children are shown in Table 1.
Table 1.
Malian patient characteristics
| Category | Age, months* | Hb, g/dl* | Log10 parasite density per μl blood* |
|---|---|---|---|
| Severe malaria cases and controls (124 matched triplets) | |||
| Severe malaria cases | 38.2 (27.3) | 8.1 (2.6) | 4.90 (0.83) |
| Uncomplicated malaria controls | 40.0 (27.8) | 9.6 (1.8) | 3.80 (0.70) |
| Healthy controls | 40.0 (28.0) | 10.7 (1.6) | NA‡ |
| P value† | 0.85 | <0.001 | <0.001 |
| Nonsevere hyperparasitemia cases and controls (65 matched triplets) | |||
| Nonsevere hyperparasitemia cases | 51.9 (29.7) | 10.0 (1.5) | 5.86 (0.12) |
| Uncomplicated malaria controls | 52.8 (32.0) | 9.9 (2.2) | 4.09 (0.62) |
| Healthy controls | 49.7 (30.5) | 11.0 (1.5) | NA‡ |
| P value† | 0.83 | <0.001 | <0.001 |
*Values shown are the means (± SD).
†ANOVA or Student's t test.
‡NA, not applicable.
We found that blood group O was present in only 21% of the severe malaria cases compared with 44–45% of their matched uncomplicated malaria controls and healthy controls [Fig. 1A and supporting information (SI) Table 4]. The effect of the ABO blood group on susceptibility to severe malaria was analyzed by conditional logistic regression. The statistical model was tested for the potential confounding effect of variables occurring in the Malian population that may protect against severe malaria, including HbC and HbS (25, 26) and the CR1–Knops blood group allele Sl2 (Vil) (3, 27–29). The influence of Hb variant and CR1 genotype on the final model fit for the analysis of ABO blood group and malaria severity is described in detail in Materials and Methods (Statistical Analysis), and the frequencies of Hb variants and CR1 genotypes are shown in SI Table 5.
Fig. 1.
ABO blood group frequencies in cases and controls and the effect of host ABO blood group on P. falciparum rosetting in Mali. (A) Distribution of ABO blood group frequencies in severe malaria cases and matched uncomplicated malaria controls and healthy controls (n = 124 triplets). Percentages are shown within each section of the pie. (B) Distribution of ABO blood group frequencies in nonsevere hyperparasitemia cases and matched uncomplicated malaria controls and healthy controls (n = 65 triplets). Percentages are shown within each section of the pie. (C) Box plot showing the effect of host ABO blood group on rosetting in Malian P. falciparum isolates (group A, n = 51; group AB, n = 12; group B, n = 66; group O, n = 76). Boxes indicate median (central line) and interquartile range. The 90th percentile is shown by the error bar, and points beyond the 90th percentile are shown as circles. Rosetting is significantly lower in isolates from blood group O patients compared with the non-O blood groups (P = 0.003, Kruskal–Wallis test).
Conditional logistic regression analysis showed that blood group O confers significant protection against severe malaria compared with the non-O blood groups (Table 2; P < 0.0005). The protective effect of group O was seen when the severe malaria cases were compared with either the uncomplicated malaria controls or the healthy controls (Table 2). The level of protection afforded by group O [severe malaria cases versus uncomplicated malaria controls, odds ratio (OR) 0.34, 95% confidence interval (CI) 0.19–0.61, P < 0.0005 for group O versus the non-O blood groups] is equivalent to that seen for a rosette-reducing CR1 deficiency polymorphism in Papua New Guinea (4) and is slightly lower than the protection against severe malaria conferred by HbS heterozygosity in previous studies (80–90% protection) (30, 31).
Table 2.
Effect of blood group O on resistance to severe malaria in Mali
| Blood groups | Odds ratio (95% CI) P value* |
|
|---|---|---|
| Severe malaria cases versus uncomplicated malaria controls | Severe malaria cases versus healthy controls | |
| O vs. A | 0.39 (0.21–0.75) P = 0.0048 | 0.28 (0.13–0.61) P = 0.0014 |
| O vs. B | 0.28 (0.14–0.57) P = 0.0005 | 0.29 (0.14–0.59) P = 0.0007 |
| O vs. AB | 0.31 (0.11–0.89) P = 0.0290 | 0.44 (0.17–1.17) P = 0.1000 |
| O vs. non-O | 0.34 (0.19–0.61) P = 0.0003 | 0.31 (0.17–0.58) P = 0.0003 |
*Conditional logistic regression analysis of matched cases and controls (n = 124 triplets).
The above statistical analysis was carried out on severe malaria cases composed of patients with a spectrum of clinical syndromes (see Materials and Methods). This approach is valid for the hypothesis to be tested here because previous studies have shown an association between P. falciparum rosetting and multiple clinical forms of severe malaria, including cerebral malaria (7, 8), severe anemia (32), neurological impairment (8), and hepatic or renal dysfunction (33). Therefore, if blood group O does protect against severe malaria through the mechanism of reduced rosetting, we would expect this protective effect to be demonstrated across all forms of severe disease. Analysis of the effect of ABO blood group within the subcategories of severe malaria showed reduced odds ratios for group O versus non-O, ranging from 0.11 for severe anemia to 0.54 for neurological impairment (SI Table 6); however, CIs for each subcategory overlap, and a larger study would be required to address whether the protective effect of group O differs significantly between distinct subcategories of severe malaria.
In contrast to the results for severe malaria, we found that the frequency of group O in the nonsevere hyperparasitemia cases did not differ markedly from that in their matched controls (Fig. 1B and SI Table 4). Conditional logistic regression analysis showed no significant effect of ABO blood group on susceptibility to the nonsevere hyperparasitemia form of clinical malaria (compared with uncomplicated malaria controls: OR 1.00, 95% CI 0.45–2.23, P = 1.0 for O versus non-O; compared with healthy controls: OR 0.94, 95% CI 0.46–1.90, P = 0.86 for O versus non-O). Interestingly, the ABO blood group frequencies of the controls matched to the nonsevere hyperparasitemia cases (Fig. 1B) appear to differ from the ABO blood group frequencies of the controls matched to the severe malaria cases (Fig. 1A). This result may be because the nonsevere hyperparasitemia cases and controls are older than the severe malaria cases and controls (Table 1), and they differ slightly in ethnic group composition (data not shown).
We also used conditional logistic regression to investigate the effect of ABO blood group on uncomplicated malaria compared with the healthy control children and found no significant effect of the ABO blood group on uncomplicated clinical disease (OR 0.97, 95% CI 0.58–1.61, P = 0.90 for O versus non-O using the severe malaria controls; OR 0.94, 95% CI 0.46–1.90, P = 0.86 for O versus non-O using the nonsevere hyperparasitemia controls). Therefore, blood group O only protects against severe, life-threatening malaria (Table 2) and not against uncomplicated clinical malaria with low or high parasite burdens. The number of deaths in the recruited children was too low for a reliable analysis of the effect of the ABO blood group on mortality from malaria. Fourteen children from the severe malaria group died, of which five (36%) were group A, one (7%) was group AB, six (43%) were group B, and two (14%) were group O.
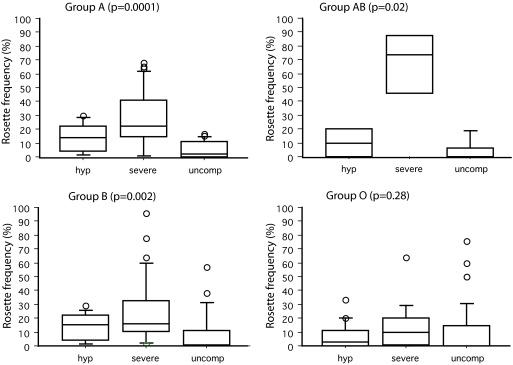
To determine whether reduced rosetting could be the explanation for the protective effect of blood group O against severe malaria in Mali, parasite rosette frequency (percentage of infected erythrocytes binding two or more uninfected erythrocytes) was assessed in the same set of samples. The rosette frequency varies from one parasite isolate to another, and the level of rosetting in a given isolate will depend partly on the variant surface antigens expressed by that isolate (i.e., whether rosetting or nonrosetting PfEMP1 variants are being expressed) (3) and partly on the phenotype of the host erythrocytes (i.e., whether rosetting receptors such as the A or B trisaccharides are present). Rosette frequencies were found to be significantly lower in parasite isolates from patients with blood group O compared with isolates from patients with groups A, B, and AB (Fig. 1C, P = 0.003, Kruskal–Wallis test). In addition, rosetting was significantly associated with severe malaria in patients with blood groups A, B, and AB (Fig. 2); however, this relationship was not apparent in patients with blood group O (Fig. 2).
Fig. 2.
Rosetting and severe malaria in Mali in relation to ABO blood group. The distributions of rosette frequencies in patients with nonsevere hyperparasitemia (hyp), severe malaria (severe), and uncomplicated malaria (uncomp) are shown as box plots (as in Fig. 1) for each ABO blood group type. High levels of rosetting were seen most frequently in severe malaria isolates in groups A, B, and AB, but not in group O (Kruskal–Wallis test; P value shown in parentheses above each graph). Numbers in each category are as follows: group A (hyp, 8; severe, 27; and uncomp, 16); group AB (hyp, 2; severe, 4; and uncomp, 5); group B (hyp, 11; severe, 29; and uncomp, 25); group O (hyp, 17; severe, 15; and uncomp, 43).
The results shown in Figs. 1 and 2 are consistent with the hypothesis that children with blood group O are protected against severe malaria through the mechanism of reduced P. falciparum rosetting. However, it remains possible that the ABO blood group could be independently associated with malaria resistance by a mechanism other than rosetting. To determine whether this explanation is a likely one for the data shown here, we investigated the interaction of host ABO blood group and parasite rosette frequency in relation to severe malaria using logistic regression. An unmatched analysis of the severe malaria cases and their uncomplicated malaria controls was carried out because the technical difficulties of assessing rosette frequency in all samples (see Materials and Methods, P. falciparum Culture) meant that matching reduced the available data from 168 to 82 observations. The reference case for this analysis was the group of children with non-O blood groups who were infected with parasite isolates with no rosetting or very low rosetting (≤5% rosette frequency). Compared with the reference children, we found that group O children infected with no/low rosetting parasite isolates were not significantly protected against severe malaria (OR 0.97, 95% CI 0.29–3.19, P = 0.96), which demonstrates that in the absence of rosetting, group O does not protect. Children with the non-O blood groups who were infected with parasite isolates showing marked rosetting (>5% rosette frequency) had a greatly increased risk of severe malaria compared with the reference group (OR 15.23, 95% CI 5.24–49.86, P < 0.0001). This observation indicates that rosetting is a risk factor for severe malaria in children with the non-O blood groups, as is consistent with the results shown in Fig. 2. Further analysis showed that there was a significant interaction between the ABO blood group and parasite rosette frequency (interaction OR 0.11, 95% CI 0.02–0.58, P = 0.01), which means that group O children infected with rosetting parasites (>5% rosette frequency) are still at risk of severe malaria (OR 1.63); however, this risk is substantially reduced compared with the non-O children infected with rosetting parasites (OR 15.23). In summary, this analysis shows that group O only protects in the presence of rosetting parasites and that rosetting is a strong risk factor for severe disease in children with non-O blood groups but a much weaker risk factor in group O children. These data support the hypothesis that the protective effect of group O operates through the mechanism of reduced P. falciparum rosetting. The cutoff rule used in the above analysis (no/low rosetting is ≤5% rosette frequency) is arbitrary because the exact rosette frequency at which significant pathogenic effects start to occur is unknown. However, varying the cutoff point to ≤2% or ≤10% rosette frequency did not materially alter the conclusions of the analysis (see SI Table 7).
Parasite density did not differ between the ABO types in the Mali study, indicating that the protective effect of group O is not caused by reduced parasite burden [log10 parasite density, mean (±SD) severe cases: group A, 4.94 (0.78); group AB, 4.80 (0.37); group B, 4.86 (0.91); and group O, 4.97 (0.93); uncomplicated malaria controls: group A, 3.87 (0.70); group AB, 3.71 (0.92); group B, 3.69 (0.71); and group O, 3.84 (0.68); P = 0.36, ANOVA].
To determine whether the relationship between the ABO blood group and resistance to severe malaria is found elsewhere, we retrospectively examined the effect of ABO blood group on malaria severity by using samples collected during two previous studies in Kilifi, Kenya, in 1993 and 2003 (8, 34). Rosetting was shown to be significantly lower in parasite isolates from patients with blood group O compared with the non-O blood groups in the 1993 study (8). In both studies, the ABO blood group was assessed on samples from children with uncomplicated and severe malaria (for clinical definitions and inclusion criteria, see Materials and Methods). The patient characteristics for the samples included in the ABO analysis are shown in SI Table 8. The Kenyan data are derived from small unmatched, retrospective studies that are not adjusted for potential confounding variables; however, they do provide preliminary data addressing whether blood group O confers protection against severe malaria in another population. As can be seen in Table 3, the ORs for severe malaria were reduced in group O patients compared with non-O patients for both Kenyan studies, but only the 1993 study was statistically significant.
Table 3.
Effect of blood group O on resistance severe malaria in Kenya
| Category | Blood group |
||||
|---|---|---|---|---|---|
| A | AB | B | O* | Non-O* | |
| 1993 | |||||
| Severe n = 19 | 5 (26.3) | 7 (36.8) | 3 (15.8) | 4 (21.1) | 15 (78.9) |
| Uncomplicated n = 36 | 9 (25.0) | 2 (5.6) | 6 (16.7) | 19 (52.8) | 17 (47.2) |
| 2003 | |||||
| Severe n = 20 | 7 (35.0) | 0 (0) | 6 (30.0) | 7 (35.0) | 13 (65.0) |
| Uncomplicated n = 19 | 3 (15.8) | 0 (0) | 4 (21.1) | 12 (63.2) | 7 (36.8) |
Shown are the numbers in each blood group with percentage in parentheses.
*Statistics for the comparison of group O vs. non-O patients: 1993: χ2, 5.15; OR, 0.24; 95% CI, 0.07–0.83; P = 0.043. 2003: χ2, 3.09; OR, 0.31; 95% CI, 0.09–1.14; P = 0.11.
Other work also supports the protective effect of group O against severe malaria in multiple countries, although the level of protection varies between sites, and some of these studies do not use matched controls or take into account potential confounding variables (refs. 17–19 and A. Fry, M. Griffiths, S. Auburn, M. Diakite, J. Forton, et al., unpublished work). Previous work has also suggested that group A and AB are greater risk factors for severe disease than group B (17, 18), which was not apparent in our study in Mali (Table 2). It has been shown previously that P. falciparum rosetting strains are either A-preferring (forming larger rosettes with group A or AB erythrocytes) or B-preferring (forming larger rosettes with group B or AB erythrocytes) (10), therefore it is possible that variation in parasite rosetting phenotypes in distinct geographical areas could influence the effect of blood group on severe malaria.
Previous experimental work has provided evidence that rosetting may be a parasite virulence factor because it causes microvascular obstruction (9), which is thought to be a key process in the pathogenesis of severe malaria (35). In an ex vivo model, P. falciparum rosettes were disrupted by high shear forces in the arterial side of the circulation but reformed in the postcapillary venules by adhesion of uninfected erythrocytes onto infected erythrocytes that were bound to endothelial cells (9). This combination of rosetting and cytoadherence occurring simultaneously, resulted in rosetting parasites causing greater obstruction to microvascular blood flow than isogenic cytoadherent nonrosetting parasites (9). The ABO determinants are present on endothelial cells and platelets as well as erythrocytes, and it seems likely that parasite isolates that bind to A or B determinants on erythrocytes to form rosettes may also bind A or B antigens on other cell types, which could enhance sequestration and increase pathogenic potential (20).
In this work we have shown that blood group O protects against severe malaria in a matched case-control study of Malian children, and we provided preliminary evidence for a similar protective effect of group O in Kenya. Statistical analysis of the Malian study indicates a significant interaction between the host ABO blood group and parasite rosette frequency that provides strong evidence to support the hypothesis that group O protects by the mechanism of reduced rosetting and sequestration. These findings indicate that blood group O provides a further example of an erythrocyte polymorphism that, similarly to CR1 deficiency, α-thalassemia, and HbC (4–6), is able to reduce the adhesion potential of P. falciparum-infected erythrocytes and consequently modify the virulence of the parasite.
One obvious question that arises from this work is why blood group O does not occur at higher frequency in all malaria endemic regions. It seems likely that this phenomenon represents an example of a balanced polymorphism in the human population because blood group O is thought to confer susceptibility to diseases such as cholera (36) and other diarrheal diseases (37–39) that may be a significant selective force in many malarious countries. The global distribution of ABO blood group types is complex and may be influenced by selection imposed by a variety of pathogenic microorganisms (40, 41). Our work indicates that malaria is likely to be a significant factor influencing ABO blood group frequencies in tropical and subtropical regions of the world.
Materials and Methods
Mali Study Site and Field Isolates.
Blood samples were collected in Bandiagara, Mali, an area with intense seasonal transmission of P. falciparum (20–60 infected bites per person per month at the peak of the July–December transmission season) (42). The samples were collected as part of the Bandiagara Malaria Project case-control study that has been described in detail previously (22). Blood samples were collected after informed consent from children's parents or guardians, and all protocols received institutional review board approval. The World Health Organization (WHO) criteria for severe malaria were applied (23), except that patients with hyperparasitemia (>500,000 parasites per μl of blood) and no other symptoms or signs of severe disease were analyzed as a separate group. Uncomplicated malaria controls were children with P. falciparum infection and fever but with no symptoms or signs of severe malaria and no hyperparasitemia. The healthy control children were asymptomatic and parasite-negative by thick blood smear (22). In classification of the severe malaria cases, cerebral malaria [unrousable coma with a Blantyre Coma Score (BCS) of ≤2 with other obvious causes of coma excluded] and severe anemia (Hb <5g/dl) were taken as primary defining criteria when they coexisted with other criteria, as described previously (22). The severe malaria cases (n = 124) consisted of 32 children (25.8%) with cerebral malaria, 10 children (8.1%) with severe anemia, 9 children (7.3%) with cerebral malaria and severe anemia, 38 children (30.6%) with neurological impairment (impaired consciousness or prostration) but with a BCS of >2, 26 children (21.0%) with repeated seizures but no lasting neurological impairment, and 9 children (7.3%) with no neurological abnormalities or anemia but with evidence of renal or hepatic failure (anuria, hematuria, jaundice).
Kenya Study Site and Field Isolates.
Blood samples were collected from patients with a P. falciparum parasitemia of 0.5% or higher, attending Kilifi District Hospital, Kenya, in two studies carried out in 1993 and 2003. The effect of ABO blood group on rosetting in the 1993 study has been reported previously (8). The invasion phenotypes of some of the samples recruited in 2003 have also been reported (34). At this site in Kenya, malaria transmission is seasonal (June–August and December–February), with the average number of infected bites per person estimated at ≈10–30 per year (43). In both 1993 and 2003, severe malaria was defined as cerebral malaria (as described above for Mali), prostration (inability to sit or in babies, to breast feed), or respiratory distress (abnormally deep breathing). This clinical definition identifies approximately the same group of children at risk of life-threatening malaria as those identified by the more comprehensive WHO criteria (23). Uncomplicated malaria controls were children with malaria presenting at the outpatient department of the same hospital during the same study period with no signs of severe disease, who were successfully treated as outpatients with oral antimalarial therapy. ABO blood group data were collected for 2 months of the 1993 study (June and July) and throughout the study period in 2003. All samples fulfilling the clinical definitions above for which ABO data were available were included in the analysis.
ABO Blood Group Typing.
Blood samples were typed for ABO blood group by standard hemagglutination techniques (44).
Hemoglobin Typing.
Hemoglobin types (HbA, HbS, HbC, and HbF) of the Malian samples were analyzed by cellulose acetate electrophoresis (25). In 141 samples, electrophoresis results were confirmed by PCR (26).
CR1 Genotyping.
Samples were genotyped for the CR1–Knops blood group alleles Sl1 and Sl2 by PCR and restriction digest as described (45).
P. falciparum Culture.
After removal of lymphocytes (46), the Malian samples were washed and then frozen in glycerolyte to −80°C and shipped to Edinburgh. The isolates were thawed and cultured for 18–36 h as described (46) to allow maturation from ring stage to the pigmented trophozoite stage at which rosetting occurs. No additional erythrocytes were added during this short culture step. Parasite maturity was monitored by Giemsa smear, and only those with normal morphology that matured to the pigmented trophozoite stage were assessed for rosetting. Of a possible 378 P. falciparum-infected samples, 272 were put into culture, the others having been lost during a freezer malfunction. Of those cultured, 13 did not grow, and 51 were excluded because the parasitemia was too low to assess rosetting (<0.5%). Therefore, rosette frequency was assessed for 208 samples (91 uncomplicated malaria, 40 nonsevere hyperparasitemia, and 77 severe malaria).
Rosetting Assays.
Rosette frequency was assessed in the first cycle of in vitro growth. An aliquot of culture suspension was stained with 25 μg/ml ethidium bromide for 5 min at 37°C. A wet preparation of the suspension (2% hematocrit) was viewed with a fluorescence microscope, and the number of mature infected erythrocytes binding two or more uninfected erythrocytes was counted. The rosette frequency is the percentage of infected erythrocytes in rosettes of 200 infected erythrocytes counted.
Statistical Analysis.
A conditional logistic regression model (the survival package within the R statistical system) (47) was used to estimate ORs and 95% CIs for the effect of ABO blood group on malaria severity in Mali and to test for the potentially confounding effect of hemoglobin variant (HbS and HbC) and CR1 Sl genotype. Candidate variables were initially identified by univariate logistic modeling using P < 0.2 as an inclusion criterion. Data for some hemoglobin classes were sparse, so this variable was reclassified to wild-type/abnormal hemoglobin before analysis. The final logistic regression models for the ABO analyses were built by manual inspection of overall fit, significance of coefficients, and the effect of additional variables on estimated ORs. Hb variant and CR1 Sl genotype were not included in the final model for the comparison of severe cases and the uncomplicated malaria controls because their inclusion did not have a marked effect on ABO ORs, and their parameter estimates were not statistically significant. Hb variant did improve model fit for the ABO analysis using severe cases and healthy controls and was therefore included in the final model, but CR1 Sl genotype was excluded because it did not improve model fit. Hb level (grams per deciliter) and parasite density were not included in the logistic regression analyses because these variables were used in the definition of cases and controls (see above).
Other analyses were carried out by using Statview (version 5, SAS Institute, Inc., Cary, NC) and http://statpages.org/ctab2x2.html. Differences in proportions between severe malaria cases and uncomplicated malaria controls for the Kenyan samples were analyzed by using χ2 tests, and CIs for ORs were calculated as described (48) (www.hutchon.net/ConfidOR.htm). For continuous variables, differences between means were tested by Student's t test or ANOVA. Parasite densities were normalized by log10 transformation. Rosette frequency data were not normally distributed and were analyzed by nonparametric tests (Kruskal–Wallis).
Supplementary Material
Acknowledgments
We are grateful to the Bandiagara Malaria Project team in Mali and the clinical, nursing, and laboratory staff at the KEMRI Unit in Kilifi for assistance with this work. We also thank the children and their parents/guardians at both study sites. This work was supported by a Wellcome Trust Ph.D. studentship (to A.-M.D.) and Senior Research Fellowship 067431 (to J.A.R.), National Institutes of Health Contract N01-AI-85346 and Grant AI 42367, and Fogarty International Center Training Grant D43TW001589.
Abbreviations
- CI
confidence interval
- CR
complement receptor
- OR
odds ratio.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705390104/DC1.
References
- 1.Weatherall DJ, Miller LH, Baruch DI, Marsh K, Doumbo OK, Casals-Pascual C, Roberts DJ. Hematology Am Soc Hematol Educ Program. 2002:35–57. doi: 10.1182/asheducation-2002.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Williams TN. Mol Biochem Parasitol. 2006;149:121–127. doi: 10.1016/j.molbiopara.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Rowe JA, Moulds JM, Newbold CI, Miller LH. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 4.Cockburn IA, Mackinnon MJ, O'Donnell A, Allen SJ, Moulds JM, Baisor M, Bockarie M, Reeder JC, Rowe JA. Proc Natl Acad Sci USA. 2004;101:272–277. doi: 10.1073/pnas.0305306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlson J, Nash GB, Gabutti V, al-Yaman F, Wahlgren M. Blood. 1994;84:3909–3914. [PubMed] [Google Scholar]
- 6.Fairhurst RM, Baruch DI, Brittain NJ, Ostera GR, Wallach JS, Hoang HL, Hayton K, Guindo A, Makobongo MO, Schwartz OM, et al. Nature. 2005;435:1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 7.Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, Wahlgren M. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 8.Rowe A, Obeiro J, Newbold CI, Marsh K. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul DK, Roth EFJ, Nagel RL, Howard RJ, Handunnetti SM. Blood. 1991;78:812–819. [PubMed] [Google Scholar]
- 10.Carlson J, Wahlgren M. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chotivanich KT, Udomsangpetch R, Pipitaporn B, Angus B, Suputtamongkol Y, Pukrittayakamee S, White NJ. Ann Trop Med Parasitol. 1998;92:45–56. doi: 10.1080/00034989860166. [DOI] [PubMed] [Google Scholar]
- 12.Udomsangpetch R, Todd J, Carlson J, Greenwood BM. Am J Trop Med Hyg. 1993;48:149–153. doi: 10.4269/ajtmh.1993.48.149. [DOI] [PubMed] [Google Scholar]
- 13.Daniels G. Transpl Immunol. 2005;14:143–153. doi: 10.1016/j.trim.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Barragan A, Kremsner PG, Wahlgren M, Carlson J. Infect Immun. 2000;68:2971–2975. doi: 10.1128/iai.68.5.2971-2975.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Heddini A, Barragan A, Fernandez V, Pearce SF, Wahlgren M. J Exp Med. 2000;192:1–10. doi: 10.1084/jem.192.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uneke CJ. Parasitol Res. 2007;100:759–765. doi: 10.1007/s00436-006-0342-5. [DOI] [PubMed] [Google Scholar]
- 17.Fischer PR, Boone P. Am J Trop Med Hyg. 1998;58:122–123. doi: 10.4269/ajtmh.1998.58.122. [DOI] [PubMed] [Google Scholar]
- 18.Lell B, May J, Schmidt-Ott RJ, Lehman LG, Luckner D, Greve B, Matousek P, Schmid D, Herbich K, Mockenhaupt FP, et al. Clin Infect Dis. 1999;28:794–799. doi: 10.1086/515193. [DOI] [PubMed] [Google Scholar]
- 19.Pathirana SL, Alles HK, Bandara S, Phone-Kyaw M, Perera MK, Wickremasinghe AR, Mendis KN, Handunnetti SM. Ann Trop Med Parasitol. 2005;99:119–124. doi: 10.1179/136485905X19946. [DOI] [PubMed] [Google Scholar]
- 20.Cserti CM, Dzik WH. Blood. 2007;110:2250–2258. doi: 10.1182/blood-2007-03-077602. [DOI] [PubMed] [Google Scholar]
- 21.Hill AVS. Trans R Soc Trop Med Hyg. 1992;1992:225–226. doi: 10.1016/0035-9203(92)90282-h. [DOI] [PubMed] [Google Scholar]
- 22.Lyke KE, Diallo DA, Dicko A, Kone A, Coulibaly D, Guindo A, Cissoko Y, Sangare L, Coulibaly S, Dakouo B, et al. Am J Trop Med Hyg. 2003;69:253–259. [PubMed] [Google Scholar]
- 23.World Health Organization. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–S90. [PubMed] [Google Scholar]
- 24.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, et al. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A, Guindo A, Cissoko Y, Taylor JG, Coulibaly D, Kone A, Kayentao K, Djimde A, Plowe CV, Doumbo O, et al. Blood. 2000;96:2358–2363. [PubMed] [Google Scholar]
- 26.Modiano D, Luoni G, Sirima BS, Simpore J, Verra F, Konate A, Rastrelli E, Olivieri A, Calissano C, Paganotti GM, et al. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 27.Moulds JM, Zimmerman PA, Doumbo OK, Kassambara L, Sagara I, Diallo DA, Atkinson JP, Krych-Goldberg M, Hauhart RE, Hourcade DE, et al. Blood. 2001;97:2879–2885. doi: 10.1182/blood.v97.9.2879. [DOI] [PubMed] [Google Scholar]
- 28.Thathy V, Moulds JM, Guyah B, Otieno W, Stoute JA. Malar J. 2005;4:54. doi: 10.1186/1475-2875-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmerman PA, Fitness J, Moulds JM, McNamara DT, Kasehagen LJ, Rowe JA, Hill AV. Genes Immun. 2003;4:368–373. doi: 10.1038/sj.gene.6363980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 31.Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, Marsh K. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newbold C, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N, Marsh K. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 33.Ringwald P, Peyron F, Lepers JP, Rabarison P, Rakotomalala C, Razanamparany M, Rabodonirina M, Roux J, Le Bras J. Infect Immun. 1993;61:5198–5204. doi: 10.1128/iai.61.12.5198-5204.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deans AM, Nery S, Conway DJ, Kai O, Marsh K, Rowe JA. Infect Immun. 2007;75:3014–3020. doi: 10.1128/IAI.00249-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White NJ. Acta Leidensia. 1987;56:27–47. [PubMed] [Google Scholar]
- 36.Swerdlow DL, Mintz ED, Rodriguez M, Tejada E, Ocampo C, Espejo L, Barrett TJ, Petzelt J, Bean NH, Seminario L, et al. J Infect Dis. 1994;170:468–472. doi: 10.1093/infdis/170.2.468. [DOI] [PubMed] [Google Scholar]
- 37.Black RE, Levine MM, Clements ML, Hughes T, O'Donnell S. Trans R Soc Trop Med Hyg. 1987;81:120–123. doi: 10.1016/0035-9203(87)90302-6. [DOI] [PubMed] [Google Scholar]
- 38.Blackwell CC, Dundas S, James VS, Mackenzie DA, Braun JM, Alkout AM, Todd WT, Elton RA, Weir DM. J Infect Dis. 2002;185:393–396. doi: 10.1086/338343. [DOI] [PubMed] [Google Scholar]
- 39.Hutson AM, Atmar RL, Graham DY, Estes MK. J Infect Dis. 2002;185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 40.Reid ME, Bird GW. Transfus Med Rev. 1990;4:47–55. doi: 10.1016/s0887-7963(90)70247-7. [DOI] [PubMed] [Google Scholar]
- 41.Moulds JM, Nowicki S, Moulds JJ, Nowicki BJ. Transfusion. 1996;36:362–374. doi: 10.1046/j.1537-2995.1996.36496226154.x. [DOI] [PubMed] [Google Scholar]
- 42.Lyke KE, Dicko A, Kone A, Coulibaly D, Guindo A, Cissoko Y, Traore K, Plowe CV, Doumbo OK. Vaccine. 2004;22:3169–3174. doi: 10.1016/j.vaccine.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 43.Kinyanjui SM, Howard T, Williams TN, Bull PC, Newbold CI, Marsh K. J Immunol Methods. 2004;288:9–18. doi: 10.1016/j.jim.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Vengelen-Tyler V. AABB Technical Manual. Bethesda: American Association of Blood Banks; 1996. pp. 609–610. [Google Scholar]
- 45.Moulds JM, Thomas BJ, Doumbo O, Diallo DA, Lyke KE, Plowe CV, Rowe JA, Birmingham DJ. Transfusion. 2004;44:164–169. doi: 10.1111/j.1537-2995.2004.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deans AM, Lyke KE, Thera MA, Plowe CV, Kone A, Doumbo OK, Kai O, Marsh K, Mackinnon MJ, Raza A, Rowe JA. Am J Trop Med Hyg. 2006;74:554–563. [PMC free article] [PubMed] [Google Scholar]
- 47.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 48.Bland JM, Altman DG. Br Med J. 2000;320:1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.