Abstract
UV radiation (UVR) is a complete carcinogen that elicits a constellation of pathological events, including direct DNA damage, generation of reactive oxidants that peroxidize lipids and damage other cellular components, initiation of inflammation, and suppression of the immune response. Recent dramatic increases in the incidence of nonmelanoma skin cancers are largely attributable to higher exposure of an aging population to UVR. Therefore, the development of cellular strategies for intrinsic protection of the skin against the deleterious effects of UVR is imperative. Here we show that erythema resulting from UVR is a comprehensive and noninvasive biomarker for assessing UVR damage and can be precisely and easily quantified in human skin. Topical application of sulforaphane-rich extracts of 3-day-old broccoli sprouts up-regulated phase 2 enzymes in the mouse and human skin, protected against UVR-induced inflammation and edema in mice, and reduced susceptibility to erythema arising from narrow-band 311-nm UVR in humans. In six human subjects (three males and three females, 28–53 years of age), the mean reduction in erythema across six doses of UVR (300–800 mJ/cm2 in 100 mJ/cm2 increments) was 37.7% (range 8.37–78.1%; P = 0.025). This protection against a carcinogen in humans is catalytic and long lasting.
Keywords: erythema, nicotinamide:quinone oxidoreductase 1, skin tumor, chemoprotection
The incidence of skin cancer is rising, and skin cancers are now the most common types of cancer in the United States, where over 1 million new cases are reported each year (1). This steady increase in incidence is expected to continue because of depletion of stratospheric ozone, increased exposure to solar radiation, and longer life expectancy. UV radiation (UVR) is the principal etiological factor responsible for the majority of skin cancers. Several lines of evidence support this notion. First, UVR causes direct DNA damage, leading to the formation of unique DNA photoproducts (e.g., cyclobutane–pyrimidine dimers and pyrimidine–pyrimidone products) (2). The resulting CC–TT transitions are known as “signature mutations” and have been detected in protooncogenes (ras) and tumor suppressor genes (p53 and PTCH) isolated from skin cancer samples (3, 4). Individuals with impaired DNA repair capacity (e.g., xeroderma pigmentosum) who are deficient in nucleotide excision repair are much more sensitive to sunburn and develop skin tumors exclusively on sun-exposed areas. Overall, they are at 1,000-fold increased risk for developing skin cancer compared with the general population (5). Second, UVR can damage DNA indirectly by causing oxidative stress resulting from lipid peroxidation and formation of reactive oxygen and nitrogen intermediates (6, 7). Third, exposure to UVR causes inflammation, including erythema and edema (8), and chronic inflammation is a recognized risk factor for tumor development (9). Fourth, UVR causes immunosuppression, which raises tolerance to genetic instability (10). Thus, immunosuppressive agents that both increase the photosensitivity of the skin and create opportunities for viral infections also contribute substantially to the increased risk (≈200-fold) for skin cancer in solid organ transplant recipients (11). Therefore, the development of strategies for protection against UVR is an urgent need (12).
One possible strategy for protection is by enhancing the intrinsic protective mechanisms that all multicellular organisms have evolved, including an elaborate network of cytoprotective (phase 2) proteins that combat oxidants and electrophiles (13). There is convincing evidence that the genes encoding these cytoprotective proteins can be transcriptionally up-regulated by small molecules (inducers) by the Keap1–Nrf2–antioxidant response element (ARE) pathway (14, 15). Such induction is a highly effective strategy for achieving protection against toxicities and a variety of diseases in animal models (13). Importantly, inducers are found in edible plants, and the isothiocyanate sulforaphane (SF) (derived from broccoli) is a prominent example (16–19). We have reported that prior topical application of SF [delivered in the form of broccoli sprout extracts (BSEs)] to the skin of mice and healthy human subjects elevates NAD(P)H:quinone oxidoreductase 1 (NQO1) (20), a representative cytoprotective enzyme. We now show that such treatment reduces the skin sensitivity to erythema, a comprehensive and noninvasive biomarker for UVR-inflicted injury (21), and thus provides evidence that up-regulation of the intrinsic cytoprotective mechanisms of the skin protects against damage of a carcinogen that is ubiquitous in our environment and represents the principal etiological factor for the development of human skin cancers.
Results
The mechanisms and effectiveness of SF in protection against UVR erythema were elucidated by complementary experiments in mouse and human skins.
Response of Mouse Skin to UVR and Protection by SF.
Among rodents, the hairless but immunologically competent SKH-1 mouse, lacking a transcription factor essential for hair follicle regeneration, is a highly relevant model for human skin cancer. After 16–20 weeks of biweekly exposure to relatively low-dose UVR (30 mJ/cm2), this mouse develops multiple skin tumors during the subsequent 12–16 weeks (22). We have previously shown that topical application of SF-containing BSEs induced the cytoprotective (phase 2) response in the skins of mice and humans (20, 23). Furthermore, UV-induced skin tumor development (incidence, multiplicity, and total tumor burden) in SKH-1 mice was strongly suppressed by topical BSEs containing SF (23).
When the backs of SKH-1 female mice were exposed to single, but much higher, doses (700–1,200 mJ/cm2) of narrow-band 311-nm UVB, comparable with those used to determine skin erythema sensitivity in humans, the skin layers became much thicker and showed marked edema and inflammation within 24 h (Fig. 1A Left and Center). These damaging effects were substantially averted by prior treatment of mouse skin for 3 days with daily doses of 100 nmol/cm2 of SF delivered as a BSE (Fig. 1A Right). Skin myeloperoxidase (MPO) activity, which is localized in azurophilic granules of neutrophils and is a sensitive marker of inflammation intensity (24), increases in a dose-dependent manner upon UVR (>25-fold at 1,200 mJ/cm2) (Fig. 1B). Prior treatment with synthetic SF or BSE containing SF suppressed the increases of MPO protein and enzyme activity levels (Fig. 1 C and D). The specific activities of the prototypic phase 2 enzyme, NQO1, in homogenates of these SF- or BSE-treated mouse skins also were elevated as reported (23). UVR depressed these inductions slightly (Fig. 1E). Topical treatment with either pure SF or BSE containing equivalent amounts of SF showed quantitatively equivalent effects on the inductive increases in NQO1 and the inhibition of the UVR-dependent MPO activity. This finding strongly supports the conclusions that: (i) both the phase 2 inducer activity and the protective effects against UV-mediated edema and inflammation (and probably other aspects of UV damage) provided by BSE are entirely attributable to their SF content, and (ii) these effects do not arise from direct UVR absorption because SF has negligible absorption at 311 nm, whereas BSE are aqueous plant extracts and are colored.
Fig. 1.
SF and SF-rich BSEs protect mouse skin against edema and inflammatory effects of 311-nm UVR. The backs of SKH-1 hairless mice were treated topically with three doses at 24-h intervals of: (i) BSE containing 0.5 μmol of SF in 50 μl of 80% acetone/20% water (vol/vol) applied to the caudal area, and (ii) solvent applied to the rostral area. The animals received 700 mJ/cm2 of 311-nm UVR 24 h after the last dose and were euthanized 24 h later, and their dorsal skin was harvested. (A) Fresh frozen 9-μm-thick sections of skin were fixed with paraformaldehyde and stained with H&E. (Scale bar: 100 μm.) (B and C) Mice were irradiated with a range of doses of UVR and euthanized 24 h later. MPO-specific activity (B) was measured in supernatant fractions of total homogenates prepared from liquid nitrogen-frozen and pulverized dorsal skin, and its protein levels (C) were detected by Western blots with anti-MPO antibody (Hycult Biotechnology, Uden, The Netherlands). Uniformity of protein levels was confirmed by Coomassie blue staining of a parallel gel (data not shown). (D and E) MPO-specific (D) and NQO1-specific (E) activities were measured in supernatant fractions of total skin homogenates from mice treated with solvent (black bars), SF (gray bars), and BSE (white bars) and are expressed as ratios of each treatment to the nonirradiated control. Average values ± SD are shown. Eight animals were used in the control group, and four in each of the treatment groups. Treatment with either SF or BSE led to equivalent protection against the UVR-induced MPO elevation (SF, P = 0.005; BSE, P = 0.001), and restoration of the NQO1 levels depressed by UVR (SF, P = 0.003; BSE, P = 0.00001).
Measurement of UV Erythema and Design of a Template for Treatment and Radiation.
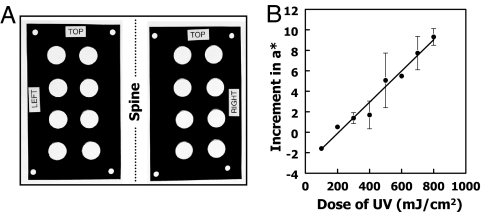
Translation of these findings from mice to humans required the development of highly quantitative and reproducible methods for evaluating UV-mediated damage of human skin. We chose to measure erythema as a noninvasive biomarker. We designed a reusable, adhesive vinyl template that could be precisely positioned on the skin to make repetitive measurements of red reflectivity at exactly the same areas (spots) that were treated with standardized 311-nm doses of UVR and with potential protectors. Two 10 × 17-cm rectangular, opaque vinyl templates, each with four pairs of 2.0-cm diameter circular windows, were attached on successive days in precisely the same paraspinal region of the backs of volunteers (Fig. 2A). The erythema of each spot was quantified under standardized conditions with a chromometer (model CR-400; Konica Minolta, Ramsey, NJ) that determines the erythema index, a*, a unitless ratio of the intensities of the red reflectivity of the skin to the emission of a xenon arc flash adjusted for chromaticity along the green–red axis (25, 26). Male and female volunteers (28–53 years of age) with skin type 1, 2, or 3 and no skin pathology were enrolled. They were asked to refrain from consuming cruciferous plants, including mustard, horseradish, wasabi, and condiments for 1 week before and during the study period, and they were told not to consume coffee or to exercise before each study visit, which took place at 1300 hours each day. No restrictions were placed on consumption of medications or dietary supplements. Subjects rested prone for 20 min, and measurements were begun on each spot 20 s after allowing skin to equilibrate under the weight of the chromometer (≈780 g). Eleven repetitive measurements were obtained on each spot in rapid succession (≈5-s intervals). The last eight values were used to calculate mean a* values and coefficients of variation (CVs) for each spot. All readings were obtained by a single trained operator.
Fig. 2.
Intensity of erythema depends linearly on the dose of UV radiation. (A) Adhesive vinyl templates placed on the back of the chest in the paraspinal regions. The apertures are 2.0-cm diameter and can be individually occluded to allow delivery of a range of UVR doses. The positions of the small holes at the four corners of each template are marked with a skin marker to locate the templates precisely in the same positions on successive days. (B) Intensity of erythema as a function of UVR dose. The erythema values (a*) were measured on 2.0-cm-diameter circles on the back of a male subject immediately before and 24 h after exposure to 100–800 mJ/cm2 of 311-nm UVR. Two pairs of adjacent spots were assigned to each UV dose. The mean changes in a* values after radiation are shown (filled circles), together with bars indicating the range of the duplicate values. The mean a* value for all 16 spots before radiation was 6.22 ± 1.91 (CV = 30.7%). The linear correlation coefficient (r2) of the increment of a* values with respect to UV dose is 0.986.
The reproducibility of the measuring procedure was validated by obtaining mean a* values for all 16 spots of five subjects on 5 consecutive days (on the 4 days before and 24 h after UV exposure). These measurements established the following: (i) the last eight repetitive chromometer measurements made on the same 16 individual spots during the 4 days before UVR had a CV of 3.79 (SEM = 0.19%; n = 320 measurements), whereas 24 h after UV exposure the CV of the now higher a* values of 16 spots in the same five subjects was 2.26 (SEM = 0.21%; n = 80). In other words, repetitive measurements of the higher a* values of radiated spots could be determined with greater precision (P < 0.0001); (ii) the initial mean a* values of the 16 spots in five individuals measured on 4 successive days before UVR were 4.52 ± 1.89 (n = 320). However, the variability of a* values among individual spots was considerably greater, ranging from 0.59–10.17. Although both the spot location and day of measurement significantly affected the basal a* values for a given individual, the CV, because of spot location alone, was 19.2%, whereas the CV from day to day was 6.2%. Thus, the differences in a* values among individual spots in a single subject were much larger than the variation between repetitive measurements on the same spot over time. This analysis of erythema index a* measurements led us to conclude that each spot of any individual must be considered an independent observational unit; (iii) the increase in erythema resulting from UVR (Δa*) varied inversely with the initial value of a* before UV exposure (P = 0.008), which is consistent with the view that lighter skin is more susceptible to erythema than darker skin; and (iv) the variation in UVR-induced erythema (Δa*) was random across the back, indicating no statistical bias to selecting vertically or horizontally adjacent control and treatment spots. On the basis of anatomical considerations (e.g., dermatomes and vasculature), horizontally adjacent spots were selected as treatment/control pairs.
UV Radiation Dose-Response Curve.
Having established a quantitative and reproducible system for assessing UV-mediated erythema, the relationship between UV dose and erythema response was examined in a 53-year-old single male with type 2 skin. Eight horizontally paired windows were exposed to UVR doses from 100–800 mJ/cm2 in 100 mJ/cm2 increments, and a* measurements were made just before and 24 h after UVR. This range of UVR doses is widely used by dermatologists to determine the minimum erythema dose. The increments in a* values rose linearly with UV doses in this range (Fig. 2B), and there was reasonable agreement among duplicate areas even when the initial a* values of each spot were quite different. Therefore, we express increases in erythema as arithmetic increments in a* values for each individual spot, rather than the ratio of the a* value after UVR to that before UVR.
Optimization of SF Scheduling for Induction of NQO1 in Human Skin.
We recently reported that topical application of SF-containing BSE (in 80% acetone:20% water) to the epidermis of SKH-1 female mice induced NQO1, GST A1, and heme oxygenase 1, which typify the phase 2 response. The specific activities of NQO1 also were increased 1.5- to 4.5-fold in biopsies of human skin. Single and multiple dermal applications (up to at least 340 nmol SF equivalent to a 1.0-cm diameter circle) of such extracts were well tolerated (20). To optimize the dosing schedule for the present studies of protection of human skin against UV erythema, we treated 1.0-cm circular areas on the lower backs of three volunteers with 5-μl aliquots of BSE containing 100 nmol of SF. The extract was applied at 24-h intervals on day 1, on day 3, on days 2 and 3, or on days 1, 2, and 3, and biopsies were obtained on day 4. Treatment on 3 successive days resulted in the largest induction, with mean elevations of NQO1-specific activities of 2.19-fold (range 1.76–3.24). Therefore, in the following experiments, we treated with BSE at 24-h intervals on 3 successive days before UVR.
Protection Against UV Erythema Depends on SF Dose.
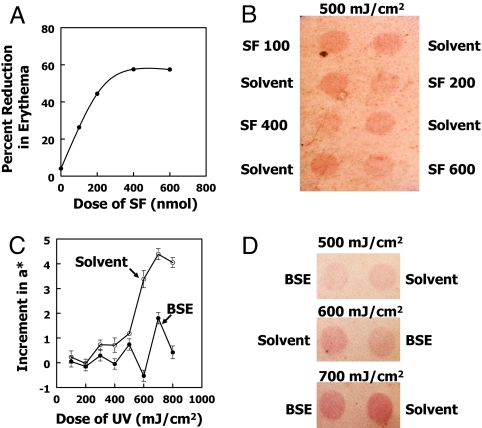
To optimize the protective doses of SF, one subject (male, age 53) received daily treatments with a range of doses of BSE (containing 100, 200, 400, or 600 nmol of SF) on 3 successive days and was irradiated with 500 mJ/cm2 of UV 24 h later. The increments in erythema a* values from before (mean of 4 days; 4.72 ± 0.871) to 24 h after radiation showed that SF treatment provided dose-dependent protection (Fig. 3 A and B). The increase in erythema was inhibited by 26.3%, 44.4%, 57.6%, and 57.5% at daily doses of 100, 200, 400, or 600 nmol of SF per 2.0-cm diameter spot, respectively. This degree of protection by SF as a function of dose was in reasonable agreement with the dose-dependence of NQO1 induction as previously established in human skin (20).
Fig. 3.
SF-rich BSEs protect human skin against erythema caused by 311-nm UVR. (A) Inhibition of skin erythema development by topical treatment of a male volunteer with a range of SF doses. The circular 2.0-cm-diameter spots received 100, 200, 400, or 600 nmol SF as BSE in 25 μl of 80% acetone/20% water on 3 days at 24-h intervals. Control spots received 25 μl of solvent only. Chromometer measurements of a* were obtained 4 days before radiation with 500 mJ/cm2 of UVR and 24 h after radiation. The 4-day mean a* value for the solvent-treated areas before radiation was 6.70 ± 1.16. Inhibition of erythema formation (%) was calculated from [a* (untreated) − a* (treated)/a* (untreated)] × 100. The untreated values (zero dose) were calculated from the increment of two areas that received 25 μl of BSE in 80% acetone/20% water containing 400 nmol of unhydrolyzed glucoraphanin (the inactive glucosinolate precursor of SF). (B) Photograph of four pairs of spots of individuals (described in A) who received 100, 200, 400, or 600 nmol doses of SF (as BSE) or solvent only. (C) Effect of topical treatment with SF-containing BSE on erythema response to a range of doses of UVR. With the use of 16-window template, horizontally adjacent pairs of spots were treated with either 200 nmol of SF in 25 μl of 80% acetone/20% water or solvent alone on 3 successive days at 24-h intervals and 24 h later were radiated with 100–800 mJ/cm2 of UVR. The increments in a* values for each spot after UVR with respect to their 4-day means before UVR are plotted as a function of UV dose. The visually determined minimum erythema dose was 600 mJ/cm2. (D) Photographs of pairs of BSE- and solvent-treated spots that received 500, 600, or 700 mJ/cm2 of UVR. The complete set of percent reduction values for this subject are shown in Table 1 (subject 2).
Protection Against UV Erythema by SF in Volunteers.
To examine the protective effects of treatment with SF-containing BSE on UV dose-dependent erythema, the extracts were applied topically inside the 2.0-cm diameter circles of the vinyl template. Treated spots received the BSE in 25 μl of 80% acetone/20% water, and horizontally paired spots were treated with solvent only. Measurements of a* values were made on 5 consecutive days: 3 days before UV exposure, on the day of exposure immediately before UVR, and 24 h after exposure. Each subject was studied at eight doses of UVR (100–800 mJ/cm2 in 100 mJ/cm2 increments), and a* values were obtained for treated and control spots at each UV dose level. The a* measurements for each spot obtained on 4 successive days before UVR were averaged, and the means were used as the a* (pre-UVR) values. Pilot experiments showed that the increments in a* values (Δa*) after UVR [i.e., a* ([post-UVR) − a* (pre-UVR)] were the most appropriate measures of changes in skin erythema of individual spots. Because the response of individual subjects to a given dose of UV varied significantly (P < 0.0001), as did the pre-UVR a* values, we expressed the protective effects as the fractional reduction (%) in erythema upon treatment, thus providing a method of subject- and skin region-specific normalization.
A typical result (Fig. 3 C and D) establishes that SF treatment inhibited erythema development by 84.3%, 41.6%, and 89.4% at 600, 700, and 800 mJ/cm2 doses of UVR, respectively, in spots that had been treated with BSE containing 200 nmol of SF on 3 successive days before radiation. We next examined the protective effect of SF in six volunteers who received BSE containing either 200 nmol (four subjects) or 400 nmol (two subjects) of SF on 3 successive days before radiation and were exposed to a range of eight doses of UVR (100–800 mJ/cm2). In our continued analysis (Table 1), we excluded the responses at 100 and 200 mJ/cm2 because the increment in a* at these low-UV doses was consistently smaller than their basal daily variations. The UV dose had an insignificant effect on the percent reduction of erythema (P = 0.50), and trend analysis of the fractional reduction in erythema with respect to UV dose indicated no significant association (P = 0.09). This finding suggests that the degree of protection is a relatively constant fraction of the erythema response irrespective of its magnitude. Therefore, SF probably protects against a relatively constant fraction of the multifactorial erythema response. Consequently, we chose to pool the data for all six subjects at six UV exposures to provide more power to the study. However, even without this restriction (n = 35; one observation was not available) of the values, including those at which no erythema was observed, there was a highly significant effect of treatment (P < 0.0001), and this finding was readily apparent visually. The data reveal levels of protection ranging from 8.37% to 78.1% for the six individual subjects across all six UVR doses (300–800 mJ/cm2). When the results were examined on an individual basis (i.e., across a row in Table 1), the mean degree of protection for all subjects across the six UVR doses administered was 37.7% (SEM = 11.2; n = 6), which was highly significant (P = 0.025; C.I. = 11.8–64.0%). The a* measurements, confirmed by visual inspection, provided evidence that, although SF treatment inhibited UV erythema in most observations (27 of 35 spots showed 8.7% or more protection), the response varied considerably both in individual subjects and among subjects.
Table 1.
Effect of treatment with sulforaphane (broccoli sprout extract) on the erythema induced by UV radiation
| Subject | Sex | Age, yr | Reduction in UVR-induced erythema at given UVB radiation dose, % |
Mean reduction in erythema, % | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 300 mJ/cm2 | 400 mJ/cm2 | 500 mJ/cm2 | 600 mJ/cm2 | 700 mJ/cm2 | 800 mJ/cm2 | |||||
| 1 | M | 53 | 66.8 | 32.3 | 33.1 | 16.6 | 48.8 | 32.1 | 38.3 | 0.0029 |
| 2 | F | 32 | 69.1 | −1.4 | −5.5 | 56.5 | 15.4 | 8.7 | 23.8 | 0.1220 |
| 3 | F | 28 | 30.1 | 1.7 | 1.5 | −5.7 | 22.2 | 0.4 | 8.37 | 0.2102 |
| 4 | M | 41 | 60.0 | 107.5 | 37.1 | 115.7 | 58.9 | 89.5 | 78.1 | 0.0016 |
| 5 | F | 29 | 52.0 | 72.5 | 87.0 | 64.5 | 26.7 | 20.9 | 53.9 | 0.0038 |
| 6 | M | 48 | N/A | 61.4 | 1.1 | 45.9 | 11.7 | −2.5 | 23.5 | 0.1390 |
| 37.7 ± 11.2 (± SEM) | 0.025 | |||||||||
The six subjects (three men and three women) were studied under identical conditions over a 5-day period, as described under Materials and Methods. The pairs of adhesive vinyl templates were applied in the same paraspinal positions on 4 successive days, at 24-h intervals, and erythema index (a*) values were determined with the chromometer on each of the 16 circular (2.0-cm diameter) windows at each session. The means of the last eight values of each set of measurements obtained on 4 days were averaged, and these means were assumed to be the a* values for each spot before UVR (Pre-UVR). Immediately after the last measurements, the subjects were exposed to a range of doses of UV (311 nm), such that the eight pairs of adjacent spots received 100–800 mJ/cm2 in 100 mJ/cm2 increments. Twenty-four hours after UVR, the chromometer a* measurements were repeated (Post-UVR). Only results for the 300–800 mJ/cm2 UVR are shown (see text). On the first 3 days, one of each pair of spots was treated with 25 μl of BSE containing 200–400 nmol SF (in 80% acetone/20% water), and the other received 25 μl of solvent only. The effects of treatment on UVR-induced erythema a* were derived from the change in a* values (Δa*), i.e., (a*Post-UVR − a*Pre-UVR) for BSE and solvent-treated spots, and the percentage change expressed as follows: [(Δa* of treated spot/Δa*of control spot) × 100]. The P values were calculated using a two-sided Student t test and represent the comparison between an individual subject's average percent reduction (i.e., across all UVR doses administered) and no protection (i.e., 0% reduction in erythema). For the purpose of the t test, we assumed the standard deviation associated with no protection (0% reduction) was the same as that calculated for each individual. Consequently, in determining the significance of the mean percent reduction for all six subjects, the standard deviation associated with a no protection value (0%) was assumed to be equal to that of the individual subject responses.
Protection Does Not Depend on Absorption of UV Radiation.
Three types of experiments provide convincing evidence that the protective effects of SF against photodamage were not mediated by absorption of the incident UV radiation. (i) Application of a sunscreen preparation (≈10 mg per spot of Neutrogena Ultrasheer, Sun Protection Factor 55) for 3 days on the same schedule as the BSE and UV radiation (500 mJ/cm2) resulted in negligible protection (3.5%; mean of two observations) 24 h after the last application. Because volunteers were encouraged to retain their personal hygiene, it seems highly unlikely that a UV-absorbing effect could have persisted for 24 h or longer. (ii) Application of a BSE preparation delivering 400 nmol of unhydrolyzed glucoraphanin (the inactive glucosinolate precursor of SF) (27) per spot provided negligible protection (4.7%; mean of two observations). These preparations were identical to the SF-containing BSE, except that the SF precursor had not been hydrolyzed by myrosinase. (iii) Treatment of one subject with BSE for 3 days according to the previous protocol, but delay of UV radiation for 48 or 72 h after the end of treatment, resulted in substantial continuing protection: 32.1% protection at 48 h and 10.3% at 72 h. These control experiments also shed light on the unique nature of a protective strategy that depends on transcriptional activation of a wide variety of enzymes. Thus, SF has a short tissue half-life (1–2 h), and yet its effects are clearly evident even 2–3 days after treatment because they depend on the synthesis of long-lived proteins. This long-lasting property has not been demonstrated for other topical skin protectors like sunscreens, melatonin, epigallocatechin gallate, and carotenes (12, 28, 29). Moreover, experiments on mouse skin strongly suggest that the UVR protective effects of BSE are equivalent to those of an equivalent dose of pure SF as shown previously. Because SF absorbs UV maximally near 240 nm and is almost transparent at 311 nm, this compound is unlikely to be decomposed or absorbed by UVR at 311 nm, in contrast to some of the other topical protective agents.
Discussion
The mean reduction in erythema by BSE in all subjects was 37.7% (range 8.37–78.1%) and was highly significant. Nonetheless, whereas the protection was highly significant in three subjects (mean 56.8%, range 38.3–53.9%, P < 0.004), it was not significant in the three other subjects (mean 19.2%, range 8.37–25.5%, P = 0.122–0.210) (Table 1). We believe that this lack of significant mean protection in these subjects is attributable to the apparently random lack of protective responses at some spots (Table 1). Although the reasons for this variability in protective responses are not explained by our experiments, a number of factors require consideration. Although exact positioning of the subject during UV radiation and the delivery of precisely equivalent doses of UV to all spots are difficult to control because of the uneven contours of the backs, especially among lean subjects, these factors are unlikely to contribute significantly to the uneven protective responses of some adjacent skin regions. A recent study has shown that the inductive responses of both phase 1 and 2 enzymes of skin vary considerably among individuals, although regional responses in skin were not examined (30).
Differences in protective responses among individuals may be related to the well known genetic polymorphisms among the genes encoding for phase 2 enzymes (31). Furthermore, Nrf2, the major transcription factor that is responsible for both basal and inducible expressions of phase 2 genes, and the AREs, the enhancer sequences to which Nrf2 binds to initiate transcription of phase 2 genes, are both polymorphic (32, 33). This finding is relevant because of the well recognized regional mosaicism in gene expression in the skin that seems to follow ectodermal development (Blaschko lines) (34). Furthermore, in mice, gender affects the UVR sensitivity and response of skin to carcinogens, inflammation, and DNA damage (35), and the incidence of skin carcinogenesis is different among men and women. Although gender is not likely to affect regional differences in skin responsiveness, such effects would not have been detected by our studies. Differences in responsiveness to protection among individuals also are probably related to the effects of diet and the intake of drugs, hormones, and dietary supplements, which were not strictly controlled in our studies.
The extensive and wide variety of efforts by many investigators to control and prevent photodamage to human skin attest to the great medical importance of this goal. A comprehensive review of such animal and human studies is not feasible here. Efforts have been made to achieve such protection by topical and dietary strategies. Most of these studies have been done in cell lines and mouse skin, demonstrating that both dietary and topical applications of a variety of plant products effectively block nearly all aspects of the UV response of the skin and skin-derived cells (28, 36). There are few topical protection studies (other than conventional sunscreens) in humans, and among these studies the use of topical carotenoids (β-carotene, lutein, and zeaxanthin) has been the most prominent (36, 37). Most closely related to our studies are the reports that topical carotenoids, including lycopene, reduce UV erythema in human skin (36). Other topical agents are melatonin, vitamin E, and epigallocatechin gallate (28, 29, 38). In some of these experiments, the contribution of possible absorption of UVR to protection has not been rigorously excluded. Carotenoids were examined because they are highly potent quenchers of certain types of reactive oxygen species (singlet oxygen). The targeting of specific damaging photoproducts generated by UVR has taken several directions. The use of direct quenchers of photoexcited states, and antioxidants that react directly with oxidants and are mostly destroyed by the process of protection, metal chelators, modulators of photooxidative signaling pathways, and others, have been reviewed (12). Up-regulation of the phase 2 cytoprotective response empowers the cell to counteract nearly all of the previously mentioned damaging processes. The complete range of the Nrf2-dependent phase 2 response is not precisely known, but typical proteins that are elevated include glutathione (GSH) transferases, NQO1, epoxide hydrolase, heme oxygenase 1, ferritin, thioredoxin reductase, and the rate-limiting enzyme in GSH synthesis (γ-glutamylcysteine synthetase), the induction of which results in elevation of GSH levels. The scope of protective mechanisms evoked in cells by SF and other stimulators of the Keap1–Nrf2–ARE pathway includes inhibition of the activation of procarcinogens, blocking of all stages of carcinogenesis, inhibition of neoangiogenesis and metastasis that are involved in later stages of carcinogenesis, disposal of damaged and potentially neoplastic cells by cell cycle arrest and apoptosis, and suppression of inflammatory responses (18). SF also protects the retina against oxidative stress and UVR and visible light damage (39).
Induction of the phase 2 response can, therefore, provide powerful protection against a wide variety of processes that damage living cells. Most of the same pathological processes occur in skin exposed to UVR. The advantage of using SF as an inducer is that it is a dietary component. Because it exerts most of its effects by the transcriptional enhancement of the synthesis of proteins, most of which are enzymes, its effects are long lasting, catalytic, and unlikely to interfere with vitamin D biosynthesis.
These experiments demonstate that SF provides direct protection against the pathophysiological effects of UVR in human skin.
Materials and Methods
Human Studies: Measurement of Erythema.
The pairs of eight-window vinyl templates used to locate the same regions of the skin of the backs of volunteers are described in the text. The windows could be occluded individually by easily removable vinyl shades (adhesive at the periphery, but nonadhesive over the windows) so that graded UV dosages could be delivered to the spots. Narrow-band UV (centered at 311 nm) was delivered in a Daavlin Full Body Phototherapy Cabinet with NB-UVB/TL01 lamps equipped with an integrated UVB dosimeter (Bryan, OH). The windows were used to produce either the same dose of UV to all windows or graded doses from 100–800 mJ/cm2 to selected pairs of horizontally adjacent windows. Subjects were of skin phototypes 1 (always burns, never tans), 2 (always burns, sometimes tans), or 3 (sometimes burns, always tans). The subjects rested prone for 20 min in a temperature-controlled room (25 ± 2°C) before measurements were made. The intensity of radiation was calibrated with an IL-1400 Radiometer (International Light, Newburyport, MA). The erythema of the human skin was determined with a chromometer (model CR-400; Konica Minolta). The chromometer was calibrated (with white and red tiles) before each measurement session. All human study protocols were approved by our Institutional Review Board. Informed consent was obtained from volunteers who were recruited by approved advertising and word of mouth.
Animal Studies.
Animal experiments were in compliance with the National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of Johns Hopkins University. The mice were radiated in ventilated cabinets equipped with UV lamps with the same specifications as those in the Daavlin Full Body Phototherapy cabinet. The topical application of SF and BSE to mouse skin is described in the legend of Fig. 1. Skin harvesting, processing, and determination of enzyme activity of NQO1 were done as described (20). MPO activity was measured according to Bradley (40).
Preparation and Standardization of BSEs.
Boiling water extracts of 3-day-old BSEs (17) were hydrolyzed with daikon sprout myrosinase, lyophilized, and redissolved in 80% acetone/20% water. Their isothiocyanate concentration, of which 90% was SF, was determined as described (19, 20).
Statistical Analysis.
Results are presented as means with SDs and CVs unless otherwise indicated. Statistical comparisons were performed by using two-tailed Student's t tests and two-way ANOVA. Potential associations were analyzed by nonparametric trend analysis (Wilcoxon). Values of P < 0.05 were considered significant. Statistical analysis was performed with Stata IC, version 10 (Stata, College Station, TX).
Acknowledgments
We thank Drs. Nanette Liégeois-Kwon, Warwick L. Morrison, Eric C. Vonderheid, Elliott Weiss, and S. Elizabeth Whitmore for invaluable advice on experimental design; Dr. Osmar Radom for carrying out UV radiation; K. K. Stephenson and K. L. Wade for expert technical assistance; and Delois E. Lee, Moneira Hawkins, Iman S. Jones, and Jessica L. Rudolph for logistical support. This work was supported by American Cancer Society Grant RSG-07-157-01-CNE, National Institutes of Health Grants CA06973 and CA93780, the American Institute for Cancer Research, and the Lewis B. and Dorothy Cullman Foundation.
Abbreviations
- ARE
antioxidant response element
- BSE
broccoli sprout extract
- C.I.
confidence interval
- CV
coefficient of variation
- GSH
glutathione
- MPO
myeloperoxidase
- NQO1
NAD(P)H:quinone oxidoreductase 1
- SF
sulforaphane
- UVR
ultraviolet radiation.
Footnotes
Conflict of interest statement: P.T. and J.W.F. are unpaid consultants to Brassica Protection Products, LLC (BPP), which licenses the technology to produce broccoli sprouts from The Johns Hopkins University. P.T., J.W.F., and The Johns Hopkins University are equity owners in BPP (whose chief executive officer is Antony Talalay, son of P.T.), and their stock is subject to certain restrictions under university policy. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Alam M, Ratner D. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 2.Setlow RB, Carrier WL. J Mol Biol. 1966;17:237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- 3.Daya-Grosjean L, Robert C, Drougard C, Suarez H, Sarasin A. Cancer Res. 1993;53:1625–1629. [PubMed] [Google Scholar]
- 4.Daya-Grosjean L, Sarasin A. Mutat Res. 2000;450:193–199. doi: 10.1016/s0027-5107(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 5.van Steeg H, Kraemer KH. Mol Med Today. 1999;5:86–94. doi: 10.1016/s1357-4310(98)01394-x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed NU, Ueda M, Nikaido O, Osawa T, Ichihashi M. Br J Dermatol. 1999;140:226–231. doi: 10.1111/j.1365-2133.1999.02653.x. [DOI] [PubMed] [Google Scholar]
- 7.Schneider LA, Bloch W, Kopp K, Hainzl A, Rettberg P, Wlaschek M, Horneck G, Scharffeller-Kochanek K. Br J Dematol. 2006;154:1147–1154. doi: 10.1111/j.1365-2133.2006.07192.x. [DOI] [PubMed] [Google Scholar]
- 8.Clydesdale GJ, Dandie G, Muller HK. Immun Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 9.Albini A, Sporn MB. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 10.Kripke ML. Immunol Rev. 1984;80:87–102. doi: 10.1111/j.1600-065x.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 11.Moloney FJ, Comber H, O'Lorcain P, O'Kelly P, Conlon PJ, Murphy GM. Br J Dermatol. 2006;154:498–504. doi: 10.1111/j.1365-2133.2005.07021.x. [DOI] [PubMed] [Google Scholar]
- 12.Wondrak GT. Curr Opin Investig Drugs. 2007;8:390–400. [PubMed] [Google Scholar]
- 13.Talalay P. BioFactors. 2001;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Yamamoto M. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Kensler TW, Wakabayashi N, Biswal S. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Talalay P, Cho CG, Posner GH. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahey JW, Zhang Y, Talalay P. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juge N, Mithen RE, Traka M. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 20.Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, Kerns ML, Talalay P. Cancer Epidemiol Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 21.Young AR, Sheehan JM, Chadwick CA, Potten CS. J Invest Dermatol. 2000;115:37–41. doi: 10.1046/j.1523-1747.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 22.Lu YP, Lou YR, Xie JG, Peng QY, Liao J, Yang CS, Huang MT, Conney AH. Proc Natl Acad Sci USA. 2002;99:12455–12460. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, Stephenson KK, Wade KL, Talalay P. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Klebanoff SJ. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 25.Farr PM, Diffey BL. Br J Dermatol. 1984;111:673–682. doi: 10.1111/j.1365-2133.1984.tb14150.x. [DOI] [PubMed] [Google Scholar]
- 26.Diffey BL, Farr PM. Clin Phys Physiol Meas. 1991;12:311–325. doi: 10.1088/0143-0815/12/4/001. [DOI] [PubMed] [Google Scholar]
- 27.Fahey JW, Zalcmann AT, Talalay P. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 28.Baliga M, Katiyar SK. Photochem Photobiol Sci. 2006;5:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 29.Bangha E, Elsner P, Kistler GS. Dermatology. 1997;195:248–252. doi: 10.1159/000245953. [DOI] [PubMed] [Google Scholar]
- 30.Smith G, Ibbotson SH, Comrie MM, Dawe RS, Bryden A, Ferguson J, Wolf CR. Br J Dermatol. 2006;155:275–281. doi: 10.1111/j.1365-2133.2006.07317.x. [DOI] [PubMed] [Google Scholar]
- 31.Lampe JW. Alter Ther Health Med. 2007;13:S108–S111. [PubMed] [Google Scholar]
- 32.Yamamoto T, Yoh K, Kobayashi A, Ishii Y, Kure S, Koyama A, Sakamoto T, Sekizawa K, Motohashi H, Yamamoto M. Biochem Biophys Res Commun. 2004;321:72–79. doi: 10.1016/j.bbrc.2004.06.112. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Tomso DJ, Chorley BN, Cho HY, Cheung VG, Kleeberger SR, Bell DA. Hum Mol Genet. 2007;16:1188–1200. doi: 10.1093/hmg/ddm066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paller AS. J Clin Invest. 2004;114:1407–1409. doi: 10.1172/JCI23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Cancer Res. 2007;67:3468–3474. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]
- 36.Sies H, Stahl W. Photochem Photobiol Sci. 2004;3:749–752. doi: 10.1039/b316082c. [DOI] [PubMed] [Google Scholar]
- 37.Palombo P, Fabrizi G, Ruocco V, Fluhr J, Roberst R, Morganti P. Skin Pharmacol Physiol. 2007;20:199–210. doi: 10.1159/000101807. [DOI] [PubMed] [Google Scholar]
- 38.Greul AK, Grundmann JU, Heinrich F, Pfitzner I, Bernhardt J, Ambach A, Biesalski HK, Gollnick H. Skin Pharmacol Appl Skin Physiol. 2002;15:307–315. doi: 10.1159/000064534. [DOI] [PubMed] [Google Scholar]
- 39.Gao X, Talalay P. Proc Natl Acad Sci USA. 2004;101:10446–10451. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley PP, Priebat DA, Christensen RD, Rothstein G. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]