Abstract
An inducible chloroplast gene expression system was developed in Chlamydomonas reinhardtii by taking advantage of the properties of the copper-sensitive cytochrome c6 promoter and of the nucleus-encoded Nac2 chloroplast protein. This protein is specifically required for the stable accumulation of the chloroplast psbD RNA and acts on its 5′ UTR. A construct containing the Nac2 coding sequence fused to the cytochrome c6 promoter was introduced into the nac2-26 mutant strain deficient in Nac2. In this transformant, psbD is expressed in copper-depleted but not in copper-replete medium. Because psbD encodes the D2 reaction center polypeptide of photosystem II (PSII), the repression of psbD leads to the loss of PSII. We have tested this system for hydrogen production. Upon addition of copper to cells pregrown in copper-deficient medium, PSII levels declined to a level at which oxygen consumption by respiration exceeded oxygen evolution by PSII. The resulting anaerobic conditions led to the induction of hydrogenase activity. Because the Cyc6 promoter is also induced under anaerobic conditions, this system opens possibilities for sustained cycling hydrogen production. Moreover, this inducible gene expression system is applicable to any chloroplast gene by replacing its 5′ UTR with the psbD 5′ UTR in the same genetic background. To make these strains phototrophic, the 5′ UTR of the psbD gene was replaced by the petA 5′ UTR. As an example, we show that the reporter gene aadA driven by the psbD 5′ UTR confers resistance to spectinomycin in the absence of copper and sensitivity in its presence in the culture medium.
Keywords: copper, cytochrome c6, inducible promoter, photosystem II, RNA processing
Although the mechanisms of plastid gene expression have been studied intensively (1), few efforts have been devoted to establish an inducible chloroplast gene expression system that would be applicable to any plastid gene. Such a system would be of considerable interest for several reasons. First, an inducible chloroplast gene expression system would prevent problems arising from constitutive high-level expression of foreign proteins of commercial interest in transplastomic plants and algae. Some of these proteins, e.g., membrane proteins, may be toxic to the cells and could impair their growth. Second, the assembly of photosynthetic complexes could be studied in a new way because, by blocking the synthesis of a chloroplast-encoded core subunit, the entire complex would be degraded. Its assembly could be reinitiated de novo by restoring expression of the core subunit in the context of preformed thylakoid membranes. Third, the expression of essential chloroplast genes could be blocked, and the impact on cell function could be examined before the cells die. Besides genes involved in chloroplast protein synthesis, some plastid genes of unknown function such as ycf1 and ycf2 are thought to be essential because it has not been possible to obtain homoplasmic disruptions of these genes (1, 2). Fourth, such a system would allow one to control photosynthetic activity and the oxygen level within the cell by regulating photosystem II (PSII) accumulation. This system would open new possibilities for inducing anaerobic metabolism, e.g., hydrogen evolution under controlled conditions. An inducible chloroplast gene expression system was developed earlier in tobacco. The phage T7 RNA polymerase expressed from a nuclear inducible promoter was targeted to the chloroplast for expressing genes under the control of the phage T7 promoter (3–5). However this system has not been used widely and has not been developed in Chlamydomonas.
Genetic analysis in Chlamydomonas, maize, and Arabidopsis has revealed a large set of nucleus-encoded factors which act mostly at posttranscriptional steps of chloroplast gene expression such as RNA processing and stability, splicing, and translation (1). Among these proteins, the Nac2 protein of Chlamydomonas reinhardtii is of particular interest for establishing an inducible chloroplast gene expression system. This protein is specifically required for the stable accumulation of the psbD mRNA encoding the D2 reaction center polypeptide of PSII (6, 7). Here, we have taken advantage of the properties of this protein and of the Cyc6 promoter of the cytochrome c6 gene, which is induced by copper depletion (8) as well as by anaerobiosis (9), to develop an inducible gene expression system that is applicable to any chloroplast gene.
This inducible chloroplast gene expression system may open new possibilities for producing hydrogen in Chlamydomonas. It is known since the early work of Gaffron and Rubin (10) that this alga can produce hydrogen under anaerobic conditions in the light. However, this production is transient because the hydrogenase is rapidly inactivated by oxygen produced by photosynthesis (11). To circumvent this problem, Melis et al. (12) used sulfur deprivation, which leads to the gradual inactivation of PSII. Under these conditions, oxygen evolution ceases and oxygen is depleted through respiration. This process leads to anaerobiosis, which in turn induces the synthesis of hydrogenase. Moreover, sulfur starvation leads to the accumulation of carbohydrates, which is important for sustained hydrogen production in the long term (13, 14). It also leads to the inhibition of the Calvin–Benson cycle and thereby removes an important electron sink, thus favoring hydrogen production (15). However, cells can survive only for a few days in sulfur-depleted medium and will eventually die. We have tested whether the copper-repressible system in Chlamydomonas can be used to turn off PSII activity and thereby create anaerobic conditions suitable for hydrogen production.
Results
Inducible Chloroplast Gene Expression System Based on Nac2.
No natural inducible chloroplast gene expression system is available for Chlamydomonas. To develop such a system, we have taken advantage of the properties of the nucleus-encoded chloroplast Nac2 protein. This protein is required for processing and stable accumulation of the psbD mRNA, which encodes the D2 reaction center polypeptide of PSII (6). The target site of Nac2 is comprised within the 74 nucleotide psbD 5′ UTR (7). Fusion of this 5′ UTR to another coding sequence renders expression of this gene dependent on Nac2. We have fused the Nac2 coding sequence to the Cyc6 promoter of the cytochrome c6 gene, whose expression is induced by copper depletion and anaerobiosis and also by addition of nickel, but which is repressed under copper-replete conditions (8, 9, 16). Because of the specificity of Nac2 for the psbD 5′ UTR, this system can be used in principle for the inducible expression of any chloroplast gene by fusing its coding sequence to the psbD 5′ UTR.
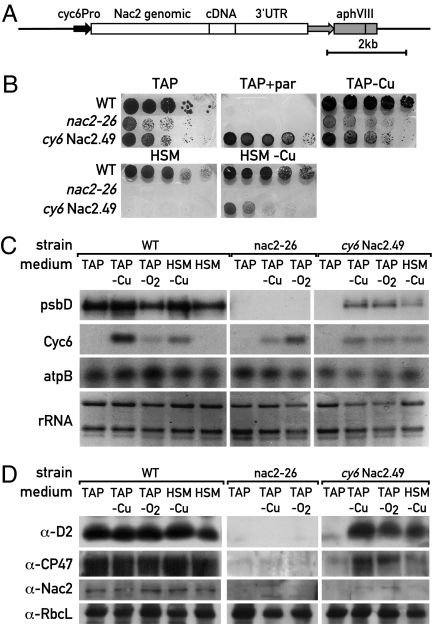
The Cyc6-Nac2 construct was inserted into a plasmid containing the aphVIII gene conferring resistance to paromomycin (17) (Fig. 1A). This plasmid was used for transformation of the Chlamydomonas nac2-26 mutant using paromomycin resistance for selection. Among 55 transformants tested, two displayed proper control of Nac2 expression by copper. The growth properties of one of these transformants (cy6Nac2.49), of WT, and of the nac2-26 mutant are shown in Fig. 1B. As expected, all three strains grow on Tris-acetate-phosphate (TAP) medium with and without copper, and the transformants also grow in the presence of paromomycin because they contain the selectable marker aphVIII. Only WT cells grow on minimal medium containing copper. However, growth of the cy6Nac2.49 strain is restored on minimal medium lacking copper. Growth can also be restored by adding nickel because the Cyc6 promoter is induced by this metal (16) (data not shown). The level of psbD expression was determined by RNA blot hybridization under different growth conditions (Fig. 1C). As expected, psbD RNA is undetectable in the nac2-26 mutant strain. In contrast, in the cy6Nac2.49 strain, expression of psbD follows that of Cyc6 and is induced in the absence of copper or under anaerobic conditions (Fig. 1C). The level of the psbD product D2 was examined by immunoblotting using D2 antiserum (Fig. 1D). D2 protein is undetectable in nac2-26 cells grown on TAP plates under all conditions. However, in cy6Nac2.49, it accumulates to 20% of WT levels when cells are grown in the absence of copper or under anaerobic conditions [supporting information (SI) Fig. 6]. On minimal medium, the induction of D2 is slightly lower. As expected, other PSII proteins such as CP47 follow a similar pattern as D2 because it is known that these proteins are unstable in the absence of the D2 protein (18). In contrast, the level of the ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) protein (RbcL) is not affected in nac2-26 (Fig. 1D).
Fig. 1.
Inducible expression of the chloroplast psbD gene. (A) Map of the Cyc6-Nac2 construct with the paromomycin resistance cassette. (B) Growth properties of the cy6Nac2.49 strain. Cells from WT, nac2-26, and cy6Nac2.49 (nac2-26 transformed with Cyc6-Nac2) were grown on TAP medium, TAP with paromomycin, TAP lacking copper, HSM (minimal) medium, and HSM lacking copper. In each case, 5 μl of a liquid culture were spotted on agar plates with 5-fold serial dilutions. Cells were grown under 60 μmol·m−2·s−1 light. (C) RNA blot analysis. RNA was extracted from cells of WT, nac2-26, and cynac2.49, fractionated by agarose gel electrophoresis, and hybridized with the gene probes indicated on the left. (D) Immunoblot analysis. Proteins from WT, nac2-26, and cyNac2.49 were fractionated by PAGE and immunoblotted with antibodies indicated on the left.
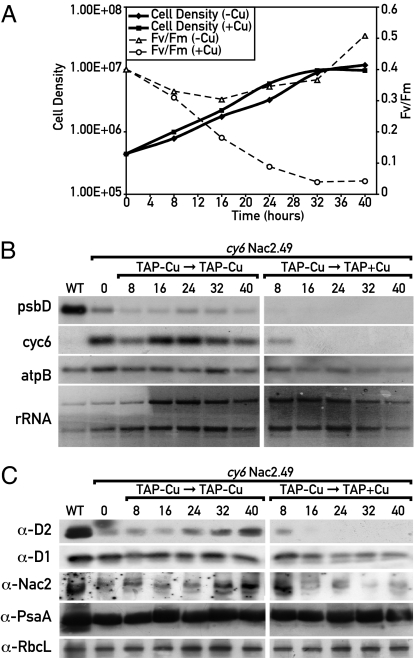
To assess the time required to deplete cells of PSII upon arrest of Nac2 synthesis, cells of cy6Nac2.49 were first grown in copper-depleted TAP medium. Under these conditions, PSII is synthesized and accumulates. The culture was split in half, and one culture was maintained under copper deprivation whereas copper was added to the other culture. The time course of cell density and the FV/FM ratio (variable/maximal fluorescence), which provides an estimate of PSII quantum yield, was determined at various time points (Fig. 2A). In the presence of copper, the FV/FM ratio declined to a minimal value within 32 h. During this period, cells divided 3- to 4-fold under both conditions and reached stationary phase. Cell extracts were prepared at various times for RNA and protein analysis. The levels of Cyc6 and psbD RNA were significantly decreased 8 h after copper addition and were undetectable thereafter (Fig. 2B). Other chloroplast RNAs (atpB, rRNA) were stable under these conditions. Immunoblotting revealed that the amount of D2 diminished after copper addition with a lag compared with the decrease of its mRNA (Fig. 2C) and the other PSII core protein D1 also decreased. As expected, a decrease in Nac2 was also observed although the low amount of this protein made its detection difficult. In contrast, chloroplast proteins from PSI (PsaA) and Rubisco were stable (Fig. 2C).
Fig. 2.
Time course of copper-mediated repression of PSII synthesis in cy6Nac2.49. (A) Cells were grown in TAP medium depleted of copper to a cell concentration of 2 × 106 cells per ml. At time 0, copper was added, and FV/FM and cell concentration were determined at different time points. (B) RNA was isolated from cells at different times, fractionated by agarose gel electrophoresis, blotted, and hybridized with the gene probes indicated. (Left) Cells grown in TAP lacking copper. (Right) Cells were grown in TAP lacking copper; at time, 0 copper was added to the culture. (C) Immunoblot analysis of proteins from cyNac2.49 was performed as described in Fig. 2C. The antibodies used are indicated on the left.
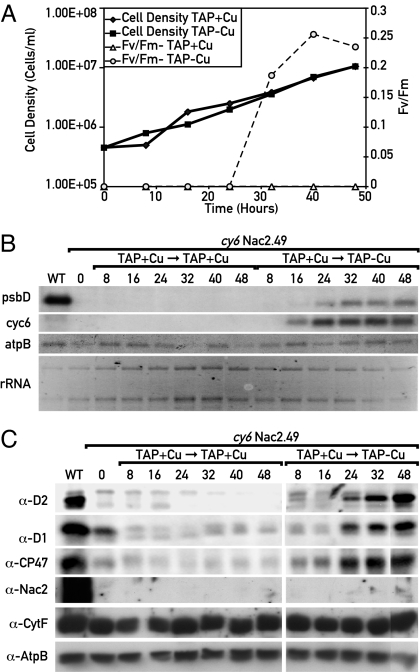
In a reciprocal experiment, cells grown in the presence of copper were transferred to TAP medium lacking copper, and the time course of cell density and of FV/FM was determined. FV/FM started to increase only after a lag of 25 h, which is presumably due to the time needed to deplete the internal cellular copper reserve (Fig. 3A). RNA and protein from cell extracts at different time points were examined by RNA blot analysis and protein immunoblotting (Fig. 3 B and C). Although the Cyc6 RNA was detectable after 16 h, there was a delay in psbD RNA accumulation and PSII activity presumably because of the fact that a threshold level of Nac2 is required for the accumulation of psbD mRNA and D2 synthesis.
Fig. 3.
Time course of accumulation of PSII in cy6Nac2.49 after copper depletion. (A) Cells were grown in TAP medium to a concentration of 2 × 106 cells per ml, centrifuged, and resuspended in TAP medium depleted of copper. FV/FM and cell concentration were determined at different time points. (B) RNA blot analysis. RNA was extracted from cells at different times under either copper-replete (left) or copper-deficient (right) conditions and subjected to RNA blot analysis. The probes used are indicated on the left. (C) Immunoblot analysis of proteins from the samples described in B. The antibodies used are indicated on the left.
Inducible Expression of Chloroplast Genes Unrelated to PSII.
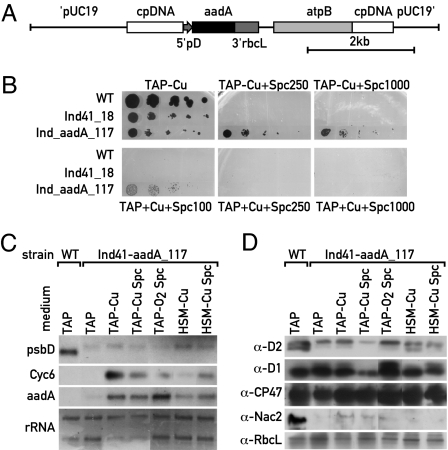
Although the Nac2 system can be used to deplete PSII in a reversible manner, we tested whether it can be extended to any other chloroplast gene. This extension is in principle possible because the Nac2 protein acts specifically on the psbD 5′ UTR and can drive chimeric psbD 5′ UTR reporter genes (7). Therefore, it should suffice to fuse the psbD promoter and 5′ UTR to the gene of interest. However, under those conditions, PSII does no longer accumulate because of the nac2-26 mutation, which leads to the loss of psbD RNA. To circumvent this problem, the petA promoter and 5′ UTR were fused to the psbD coding sequence, and this construct was introduced into a modified version of the p108-14 chloroplast transformation vector (7) (for details, see Materials and Methods). In this vector, the recyclable aadA cassette (19) is inserted upstream of the psbD gene, which is driven by the petA promoter and 5′ UTR (SI Fig. 7A). This DNA was inserted into the chloroplast genome by biolistic transformation using the aadA cassette as selectable marker. In this way the endogenous psbD gene was replaced by the petA-psbD construct, and thus accumulation of its transcript was no longer dependent on Nac2. Transformants were restreaked three times on spectinomycin plates, and the homoplasmicity was tested by DNA blot and PCR analysis (data not shown), and one of the transformants, Ind41, was selected. The aadA cassette used was flanked by two repeats. To allow for the excision of the cassette (19), the homoplasmic transformant Ind41 was plated repeatedly on medium lacking spectinomycin. In this way, a strain was obtained, Ind41_18, which is sensitive to spectinomycin because it lacks the aadA cassette. The growth properties of Ind41, Ind41_18 and cy6Nac2.49 were tested on different media (SI Fig. 7B). As expected, Ind41 grows in the presence of spectinomycin in contrast to Ind41_18, which is sensitive to the antibiotic. Moreover, both Ind41 and Ind41_18 grow on high-salt minimal (HSM) medium with or without copper. RNA blot analysis revealed that the chimeric petA-psbD RNA in Ind41_18 accumulates under all conditions independent of the Cyc6 RNA level (SI Fig. 7C). The psbD RNA is larger because the size of the petA 5′ UTR exceeds that of the psbD 5′ UTR. Immunoblot analysis revealed that D2 and D1 proteins accumulate to the same level under all conditions tested, in particular when Nac2 is not expressed (SI Fig. 7D).
Next, the aadA cassette fused to the psbD promoter, and 5′ UTR was introduced into this strain by transformation using the cg12 vector (7) (Fig. 4A). Growth of the transformants Ind_aadA-117 and of Ind41_18 was tested on TAP plates containing increasing amounts of spectinomycin with and without copper (Fig. 4B). All strains grow in the absence (Fig. 4B) or presence (data not shown) of copper. As expected, the Ind_aadA-117 grows on spectinomycin plates at concentrations of 250 μg/ml or higher only in the absence of copper. A faint growth was also observed in the presence of copper on plates containing 100 μg/ml spectinomycin. RNA blot analysis revealed that aadA RNA accumulates only under inducing conditions for the Cyc6 promoter (Fig. 4C). Protein levels of D2, D1, CP47, and RbcL were largely unaffected, but Nac2 was detected only under inducing conditions (Fig. 4D). Because of a lack of reliable AadA antibody, the amount of this protein was assayed by measurements of aminoglycoside adenyl transferase activity. The activity was significantly elevated under conditions when Nac 2 is expressed (Table 1).
Fig. 4.
Expression of the psbD-aadA gene is induced by copper depletion and repressed by copper in the Ind_aadA-117 transformants. (A) Map of aadA construct in the atpint vector. (B) Cells of the transformants were grown with or without copper on plates with increasing amounts of spectinomycin (0, 100, 250, and 1,000 μg). (C) RNA blot analysis of Ind_aadA-117 grown under different conditions as indicated. The probes are shown on the left. (D) Immunoblot analysis of the same samples as in C with the antibodies indicated on the left.
Table 1.
Aminoglycoside adenyl transferase activity in Ind41_aadA-117 under inducing and repressing conditions
| Strain | Activity, cpm/μg of protein |
|
|---|---|---|
| +Cu | −Cu | |
| WT-aadA | 207.0 ± 49.5 (3) | 192.6 ± 51.4 (4) |
| Ind41_18 | 9.2 ± 4.1 (4) | 12.2 ± 7.9 (4) |
| Ind41_117 | 24.2 ± 12.5 (4) | 274.3 ± 90.6 (7) |
Extracts from WT-aadA, Ind41_18, and Ind41_aadA-117strains were assayed for aadA activity and for total protein content as described in Materials and Methods. The activity is indicated as cpm incorporated per μg protein. The number of independent measurements is indicated in parentheses.
The Inducible Chloroplast Gene Expression System Can Be Used to Trigger Hydrogen Production.
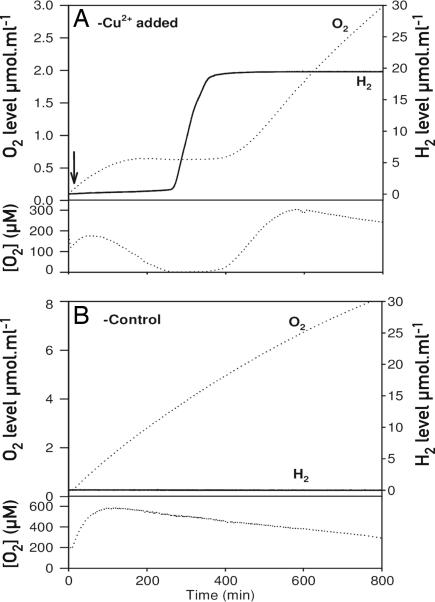
Chlamydomonas is able to induce hydrogenase and produce hydrogen under anaerobic conditions in the light. It was therefore tempting to test whether the inducible Nac2 system could be used to turn off PSII activity and O2 evolution, so that respiration would lead to anaerobic conditions suitable for induction of hydrogen production. Cells of cy6Nac2.49 were grown in TAP medium lacking copper to a concentration of 2 × 106 cells per ml. Copper was added, and the cells were transferred into a chamber connected to a mass spectrometer and illuminated with white light (250 μE·m−2·s−1) [E (einstein) = mol of photons]. In this system, the bottom of the chamber is sealed with a polypropylene membrane, which allows dissolved gases to diffuse directly into the ion source of the mass spectrometer (20). In this way, the abundance of O2 and H2 could be measured at discrete time intervals. Because the chamber was closed and because copper repressed the synthesis of PSII, O2 evolution diminished. Within a period of 200 min, the O2 was consumed by respiration. An anaerobic state was reached, which led to the synthesis of active hydrogenase and H2 production (Fig. 5A). The maximal rate of hydrogen production ranged between 1 and 3.1 mmol H2 mol−1 Chl s−1 (between 1 and 3.1 mmol of H2 per mol of Chl per s), slightly lower than that obtained with sulfur-starved cells (12), and of much shorter duration (≈1.5 h vs. 3–4 days).
Fig. 5.
Hydrogen production in the cy6nac2-49 strain. (A) Cells of cy6nac2-49 were grown in TAP medium lacking copper. The arrow indicates the time at which copper was added to the culture to inhibit Nac2 expression and PSII synthesis. Measurements of O2 and H2 were performed in the liquid phase by diffusion of the gases through a polypropylene membrane into the ion source of a mass-spectrometer. The calculated accumulation of the gases (i.e., the amounts that have actually been produced by the cells) is shown in the Upper panels of A and B; it is derived from the concentration measurements by taking into account the slight consumption of gases by the mass spectrometer. During one cycle, 20 μmol H2/liter was produced corresponding to a maximal rate of 1 mmol H2 mol−1 Chl s−1. These rates varied from one experiment to another and reached in some cases 3.1 mmol H2 mol−1 Chl s−1. Under conditions permissive for photosynthesis (Cu-deprived medium), the net rate of oxygen evolution was 23 mmol O2 mol−1 Chl s−1 in cy6Nac2.49 cells. The decline in O2 because of respiration causes anaerobiosis, which leads to hydrogen production after 250 min. Because of anaerobiosis, the Cyc6 promoter is turned on later, leading to PSII synthesis and oxygen evolution and inactivation of the hydrogenase. (B) Control: cy6Nac2.49 cells without copper treatment. For oxygen in A and B, both the calculated accumulation (Upper) and the concentration in the medium (Lower) are given. (Lower) Indicates when anaerobiosis is attained.
An interesting feature of the Cyc6 promoter is that it is also induced under anaerobic conditions even in the presence of copper (9). One would therefore expect that, once anaerobic conditions have been reached and hydrogenase is induced in cy6Nac2.49 cells, Nac2 synthesis resumes, PSII is synthesized, and oxygen levels rise, thus inactivating hydrogenase and blocking hydrogen production. This process was indeed observed. PSII synthesis was switched on during the anaerobic hydrogen production phase with concomitant O2 evolution and inactivation of hydrogenase so that the hydrogen levels remained constant (Fig. 5A). As control, the same cell culture was examined without addition of copper. Under these conditions, no hydrogen was produced (Fig. 5B) with a constant production of O2 and a gradual decrease of CO2 as observed in the copper-treated cells (data not shown). Because the Cyc6 promoter is expected to be switched off under aerobic conditions in copper-replete medium, a new cycle of hydrogen production would be expected. To test this possibility further, cy6Nac2.49 cells were grown in TAP medium lacking copper in a sealed vessel for 50 h, and measurement of hydrogen and oxygen were performed by mass spectrometry. The results suggest that two successive phases of hydrogen and oxygen production occurred (SI Fig. 8). It remains to be seen whether the Cyc6-Nac2 system can be further improved for sustained cyclic hydrogen production in Chlamydomonas.
Discussion
Inducible Chloroplast Gene Expression System.
In this work, we have developed an inducible chloroplast gene expression system in Chlamydomonas. Several attempts have been made in land plants for expressing specific nucleus-encoded chloroplast proteins with inducible promoters. The gene of a chloroplast omega-3 fatty acid desaturase was fused to a cold-inducible promoter and introduced into tobacco plants (21). The transgenic plants displayed enhanced cold tolerance. In another study, several glycine betaine-producing transgenic lines were produced in which a bacterial choline oxidase gene fused to a chloroplast targeting sequence was expressed under the control of an ABA-inducible promoter, which is induced under salt stress (22).
T7 RNA polymerase-dependent plastid gene transcription has been developed in land plants (3, 4). This system was used for expressing a single chain camel antibody fragment from a plastid transgene whose expression was driven by a plastid targeted T7 RNA polymerase under the control of a light-inducible promoter (23). However, expression of the plastid transgene occurred even under noninducible conditions, and the plants were pale-green and their growth was severely affected. One problem with this system seems to be the high specific activity of T7 RNA polymerase and its great stability in chloroplasts (5). Therefore, even a very low level of expression of T7 RNA polymerase can lead to significant accumulation of T7 RNA polymerase-dependent transcripts in chloroplasts.
The chloroplast-inducible gene expression system developed in this study takes advantage of the properties of the nucleus-encoded Nac2 factor of C. reinhardtii, whose expression is driven by the Cyc6 promoter. This promoter is repressed by copper and induced by copper deprivation or in an anaerobic environment (8, 9). It is at present the tightest inducible promoter in C. reinhardtii. The Nac2 protein is specifically required for the accumulation of the psbD mRNA encoding the reaction center polypeptide D2 of PSII. In this system, PSII synthesis can be stopped in a reversible manner while maintaining all other photosynthetic complexes in the thylakoid membrane. Upon shut-off of the expression of Nac2, it takes 20 h to deplete cells of PSII (Fig. 3). This period depends on the half-lives of the Nac2 mRNA, of the Nac2 protein, and of PSII, which depends on the light conditions as high light is known to enhance D1 turnover. It is also possible to start with cells lacking PSII and to study its de novo synthesis and assembly in fully developed thylakoid membranes. However, in this case, the copper-containing medium needs to be replaced by a copper-free medium, and there is a lag of 24 h before the internal copper is exhausted.
The Cyc6-Nac2 System Can Act as a General Inducible Chloroplast Gene Expression System.
The Nac2 factor is specifically required for the stable accumulation of the psbD RNA encoding the PSII reaction center subunit D2. Moreover, its target site is comprised within the psbD 5′ UTR because fusion of this leader sequence to any coding sequence renders the stability of this chimeric transcript dependent on Nac2. Because of this feature, the Cyc6-Nac2 system can be extended in principle to any chloroplast gene or transgene simply by fusing its coding sequence to the 230-nt psbD promoter-5′ UTR region. However, one limitation is that the strain used in this work will be PSII-deficient under noninducible conditions. To circumvent this problem, we have replaced the psbD 5′ UTR by the petA 5′ UTR in the strain Ind41-18, thus abolishing the dependence of psbD RNA accumulation on Nac2.
To test whether the Cyc6-Nac2 system can be indeed used for any chloroplast gene fused to the psbD 5′ UTR, we have tested this property by inserting a chimeric psbD 5′ UTR-aadA construct into the chloroplast genome of the Ind41-18 strain. As expected, the transformants express aadA and are resistant to spectinomycin in the absence of copper. In copper-replete medium, aadA is no longer expressed, and the cells are sensitive to spectinomycin. In this respect, it is noticeable that the inducible system works with AadA, which is known to have a high specific activity. Thus, there is only a small amount of leaky expression under noninducing conditions. This feature may be very important for expression of toxic foreign proteins in the chloroplast. This system thus provides a tool for probing the function of plastid genes and for expressing new proteins in the chloroplast of Chlamydomonas.
A similar strategy might be possible for establishing an inducible gene expression system in land plants. Indeed, nucleus-encoded plastid factors that act similarly to Nac2 have been identified in Arabidopsis. One of these proteins, HCF107, is specifically required for the intercistronic processing of the psbH 5′ UTR or the stabilization of 5′ processed psbH transcripts arising from the psbB-psbT-psbH-petB-petD operon. HCF107 seems to be the orthologue of Mbb1, a chloroplast protein of C. reinhardtii, which is also specifically involved in the processing and stability of the psbB-psbH transcripts, and the target site of this protein is within the psbB 5′ UTR (24). It is interesting that Nac2, Mbb1, and HCF107 contain many TPR (tetratricopeptide)-like repeats. Because chloroplast transformation is not yet possible in Arabidopsis, the target site of HCF107 is not known. Once this system is established, the same strategy as in Chlamydomonas could be used for Arabidopsis.
Inducible Hydrogen Production in Chlamydomonas.
An interesting property of the Cyc6-Nac2 system is that it allows one to arrest PSII synthesis by adding copper to the growth medium. Loss of PSII activity leads to the decrease of oxygen evolution below the rate of oxygen consumption by respiratory processes, allowing the culture to reach anoxia in the light. Further, as a result of the tight interplay between photosynthesis and respiration, and as long as PSII activity remains lower than respiration, all of the oxygen that might be produced by residual PSII is consumed through mitochondrial activity, thus leading to anoxic conditions, which are required for hydrogenase function (15). This property is at the basis of this study and of other experimental designs for sustainable evolution of hydrogen by Chlamydomonas. One advantage of our system is that anaerobiosis can be achieved simply by adding copper to the culture medium and the cells remain healthy. This procedure thus differs from the classical method in which PSII is inactivated through sulfur depletion, a condition that leads to impairment of cell growth and eventually to cell death. The maximal rates of hydrogen production obtained with the cy6Nac2.49 strain are slightly lower than those achieved through sulfur deprivation (Fig. 5 and SI Materials and Methods). However, this production is transient because the Cyc6 promoter is induced by anaerobiosis and as a consequence PSII recovers and reinitiates an aerobic phase.
This apparent limitation may turn out to be an advantage, as it opens the possibility of establishing a cycling hydrogen producing system, based on alternate expression and repression of Nac2, without the need for removing copper from the medium. Such a system would accumulate carbon reserves during the oxygenic phases, a part of which would be converted to hydrogen during the anaerobic phases before PSII activity has been restored to a level that is sufficient for inhibiting hydrogenase. A system of this type would be particularly interesting in the context of hydrogen production because it would avoid changes of growth media, which consume energy, and it could operate in the absence of micronutrient starvation, which may limit cell viability.
Further improvements of the system need to take into account that hydrogen production depends on other factors besides the turn-off of PSII activity, namely starch accumulation, which feeds electrons downstream of PSII into the photosynthetic electron transport chain and sustains respiration required for consuming the remaining O2 produced by the residual activity of PSII (13–15). Additionally, there is competition of hydrogen production with other electron sinks such as the Calvin–Benson cycle and cyclic electron flow. It is in principle possible to modify genetically Chlamydomonas to circumvent these limitations.
Materials and Methods
Strains and Media.
The nac2-26 mutant strain has been described (6, 7). The cyc6Nac2.49 strain contains a trans-gene consisting of the Cyc6 promoter fused to the Nac2 midi-gene inserted into the nuclear genome of the nac2-26 mutant (Δnac2::cy6proNac2). Ind41 was derived from cy6Nac2.49 by replacing the psbD promoter and 5′ UTR with a 675 fragment containing the petA promoter and 5′ UTR (25) (Δnac2::cy6proNac2::5′petA-psbD). Ind41-18 is related to the Ind41 strain, except that the aadA cassette in the Ind41-18 strain has been completely excised from the chloroplast DNA and the strain is therefore sensitive to spectinomycin (Δnac2::cy6proNac2::5′petA-psbD[SpcS]). Ind_aadA_117 was derived from Ind41-18 and contains the aadA cassette driven by the psbD promoter and 5′ UTR inserted downstream of the atpB gene (Δnac2::cy6proNac2::5′petA-psbD::5′psbD-aadA).
All strains were maintained on TAP medium supplemented with 1.5% Bacto-agar (26) at 25°C under dim light. In experiments where copper-supplemented or copper-deficient (−Cu+2) solid agar and liquid TAP and HSM medium was used, medium was prepared according to Quinn and Merchant (27). TAP and TAP-Cu+2 media were supplemented with 100 μg/ml spectinomycin (Sigma–Aldrich) or 20 μg/ml paromomycin (Sigma–Aldrich) where necessary. In experiments where cells were deprived of oxygen, liquid cultures where bubbled with N2 gas with 150 rpm/min agitation and constant light illumination (20 μE·m−2·s−1). Cell density was determined by using a hemacytometer.
Plasmid Construction.
Standard techniques were used to manipulate and analyze all plasmid constructs (28). Sequencing of constructs was carried out by using BigDye terminator sequencing kit (Applied Biosystems, La Jolla, CA) and an ABI Prism 377 automated sequencing machine. The bacterial host used for cloning in Escherichia coli was DH10B (GE Healthcare, Oelfingen, Switzerland). All oligonucleotides were ordered from Microsynth GmbH (Balgach, Switzerland).
Transformation of Chlamydomonas Cells.
Nuclear transformation of C. reinhardtii strains nac2-26 was performed by electroporation essentially as described (29). For details, see SI Materials and Methods.
Chlorophyll and Oxygen Evolution Rate Measurements.
Oxygen evolution and respiration rates were determined by using a Clark-type oxygen electrode attached to an X-type light source at 25°C (Hansatech Instruments, Norfolk, U.K.).
Hydrogen Measurements and Calculations.
All liquid phase and gas phase measurements of N2, O2, H2, and CO2 were performed as follows. Continuous monitoring of dissolved gases was made by using a sealable, thermo-stated Clark-type vessel as described (30) where gases were fed into the ion source of a mass spectrophotometer (model MM 880; VG Instruments, Cheshire, U.K.) through a polypropylene membrane under continuous agitation and constant illumination using a fiber-optic illuminator (model KL 1500; Schott, Mainz, Germany) (30). In experiments where transcription of the Cy6Nac2 transgene was repressed, 12 μM copper was added to the growth medium within the vessel (TAP-Cu+2 to TAP). Calibration of the mass spectrometer before all gas phase time points was achieved through injection of air and pure hydrogen gas samples.
Assay for aadA Activity.
Assays for aadA activity were carried out on WT, Ind41-18, and Ind_aadA_117 strains essentially as described (31), except that 32P-labeled dATP was used in place of the radiolabeled rATP used in the original experiments.
Supplementary Material
Acknowledgments
We thank N. Roggli for artwork and M. Goldschmidt-Clermont for helpful comments. This work was supported by Swiss National Foundation Grant 3100-0667763.02 and by the European Commission (6th Framework Programme, New and Emerging Science and Technology STREP SOLAR-H contract 516510).
Abbreviations
- PSII
photosystem II
- TAP
Tris-acetate-phosphate
- FV/FM
variable/maximal fluorescence
- HSM medium
high-salt minimal medium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704205104/DC1.
References
- 1.Boudreau E, Turmel M, Goldschmidt-Clermont M, Rochaix JD, Sivan S, Michaels A, Leu S. Mol Gen Genet. 1997;253:649–653. doi: 10.1007/s004380050368. [DOI] [PubMed] [Google Scholar]
- 2.Drescher A, Ruf S, Calsa T, Jr, Carrer H, Bock R. Plant J. 2000;22:97–104. doi: 10.1046/j.1365-313x.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 3.McBride KE, Schaaf DJ, Daley M, Stalker DM. Proc Natl Acad Sci USA. 1994;91:7301–7305. doi: 10.1073/pnas.91.15.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heifetz PB. Biochimie. 2000;82:655–666. doi: 10.1016/s0300-9084(00)00608-8. [DOI] [PubMed] [Google Scholar]
- 5.Magee AM, Kavanagh TA. J Exp Bot. 2002;53:2341–2349. doi: 10.1093/jxb/erf108. [DOI] [PubMed] [Google Scholar]
- 6.Kuchka M, Mayfield SP, Rochaix JD. EMBO J. 1988;7:319–324. doi: 10.1002/j.1460-2075.1988.tb02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nickelsen J, van Dillewijn J, Rahire M, Rochaix JD. EMBO J. 1994;13:3182–3191. doi: 10.1002/j.1460-2075.1994.tb06617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchant S, Bogorad L. J Biol Chem. 1987;262:9062–9067. [PubMed] [Google Scholar]
- 9.Quinn JM, Eriksson M, Moseley JL, Merchant S. Plant Physiol. 2002;128:463–471. doi: 10.1104/pp.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffron H, Rubin J. J Gen Physiol. 1942;26:219–240. doi: 10.1085/jgp.26.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghirardi ML, Zhang L, Lee JW, Flynn T, Seibert M, Greenbaum E, Melis A. Trends Biotechnol. 2000;18:506–511. doi: 10.1016/s0167-7799(00)01511-0. [DOI] [PubMed] [Google Scholar]
- 12.Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M. Plant Physiol. 2000;122:127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posewitz MC, Smolinski SL, Kanakagiri S, Melis A, Seibert M, Ghirardi ML. Plant Cell. 2004;16:2151–2163. doi: 10.1105/tpc.104.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouchard S, Hemschemeier A, Caruana A, Pruvost J, Legrand J, Happe T, Peltier G, Cournac L. Appl Environ Microbiol. 2005;71:6199–6205. doi: 10.1128/AEM.71.10.6199-6205.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melis A, Happe T. Plant Physiol. 2001;127:740–748. [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn JM, Kropat J, Merchant S, Eriksson M, Moseley JL. Eukaryot Cell. 2003;2:995–1002. doi: 10.1128/EC.2.5.995-1002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sizova I, Fuhrmann M, Hegemann P. Gene. 2001;277:221–229. doi: 10.1016/s0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 18.Erickson JM, Rahire M, Malnoe P, Girard-Bascou J, Pierre Y, Bennoun P, Rochaix JD. The EMBO J. 1986;5:1745–1754. doi: 10.1002/j.1460-2075.1986.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer N, Stampacchia O, Redding K, Rochaix JD. Mol Gen Genet. 1996;251:373–380. doi: 10.1007/BF02172529. [DOI] [PubMed] [Google Scholar]
- 20.Cournac L, Guedeney G, Peltier G, Vignais PM. J Bacteriol. 2004;186:1737–1746. doi: 10.1128/JB.186.6.1737-1746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khodakovskaya M, McAvoy R, Peters J, Wu H, Li Y. Planta. 2006;223:1090–1100. doi: 10.1007/s00425-005-0161-4. [DOI] [PubMed] [Google Scholar]
- 22.Su J, Hirji R, Zhang L, He C, Selvaraj G, Wu R. J Exp Bot. 2006;57:1129–1135. doi: 10.1093/jxb/erj133. [DOI] [PubMed] [Google Scholar]
- 23.Magee AM, Coyne S, Murphy D, Horvath EM, Medgyesy P, Kavanagh TA. Transgenic Res. 2004;13:325–337. doi: 10.1023/b:trag.0000040019.35147.a4. [DOI] [PubMed] [Google Scholar]
- 24.Vaistij FE, Boudreau E, Lemaire SD, Goldschmidt-Clermont M, Rochaix JD. Proc Natl Acad Sci USA. 2000;97:14813–14818. doi: 10.1073/pnas.97.26.14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choquet Y, Stern DB, Wostrikoff K, Kuras R, Girard-Bascou J, Wollman F-A. Proc Natl Acad Sci USA. 1998;95:4380–4385. doi: 10.1073/pnas.95.8.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris EH. The Chlamydomonas Source Book: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic; 1989. [DOI] [PubMed] [Google Scholar]
- 27.Quinn JM, Merchant S. Methods Enzymol. 1998;297:263–279. doi: 10.1016/s0076-6879(98)97020-3. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 29.Shimogawara K, Fujiwara S, Grossman A, Usuda H. Genetics. 1998;148:1821–1828. doi: 10.1093/genetics/148.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jouanneau Y, Kelley BC, Berlier Y, Lespinat PA, Vignais PM. J Bacteriol. 1980;143:628–636. doi: 10.1128/jb.143.2.628-636.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldschmidt-Clermont M. Nucleic Acids Res. 1991;19:4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.