Abstract
Calcium/calmodulin-dependent protein kinase II (CaMKII) has important roles in many processes in the central nervous system. It is enriched at the post-synaptic density (PSD), a localization which is thought to be critical for many of its proposed neuronal functions. In order to better understand the mechanisms that regulate association of CaMKII with the PSD, we compared the levels of autophosphorylation between PSD-associated kinase and kinase in other parts of the neuron. We were surprised to find that αCaMKII in a PSD-enriched fraction prepared from recovered hippocampal CA1-minislices had a relatively low level of threonine 286 (T286) phosphorylation and a relatively high level of threonine 305 (T305) phosphorylation. Furthermore, when the minislices were subjected to a treatment that mimics ischemic conditions, there was a significant translocation of αCaMKII to the PSD-enriched fraction accompanied with a dramatic reduction in T286 phosphorylation levels throughout the neuron. These findings have important implications for our understanding of the role of autophosphorylation in the localization of CaMKII.
Keywords: CaMKII, Post-Synaptic Density, Long Term Potentiation, Hippocampus, Ischemia, Phosphorylation
1. Introduction
Calcium/calmodulin-dependent protein kinase II (CaMKII) is a highly expressed neuronal protein that is involved in many neuronal systems. In particular, this kinase is widely believed to be a critical mediator of long term potentiation (LTP), a cellular mechanism that is thought to underlie learning and memory (Bliss and Collingridge, 1993). Several studies have demonstrated that CaMKII is necessary for the induction of LTP (Malinow et al., 1989; Malenka et al., 1989; Silva et al., 1992; Giese et al., 1998), and that introduction of activated CaMKII is sufficient to potentiate synaptic responses in neurons (Pettit et al., 1994; Lledo et al., 1995; McGlade-McCulloh et al., 1993). CaMKII is enriched at the post-synaptic density (PSD) (Kennedy et al., 1983; Suzuki et al., 1994; Petersen et al., 2003), a protein-dense specialization at the post-synaptic membrane that is composed primarily of scaffolding proteins, ion channels, and signal transduction enzymes (Ziff, 1997). Localization of CaMKII at the PSD has been proposed to be critical for its role in LTP (Lisman et al., 2002; Barria and Malinow, 2005). Furthermore, several different forms of neuronal activation, including treatments that lead to synaptic potentiation, have been shown to induce translocation of CaMKII to post-synaptic sites (Strack et al., 1997b; Shen and Meyer, 1999; Otmakhov et al., 2004; Dosemeci et al., 2001; Bayer et al., 2001). Accordingly, a more complete knowledge of the molecular mechanisms that regulate CaMKII binding to the PSD is likely to be crucial for our understanding of the biochemical events that occur during LTP.
CaMKII is activated when increases in intracellular Ca2+ concentration lead to the binding of Ca2+/calmodulin complexes to the kinase, relieving auto-inhibition (Hudmon and Schulman, 2002). If the Ca2+ signal is strong enough, and two adjacent subunits in the CaMKII holoenzyme are activated, then an intersubunit autophosphorylation can occur on threonine 286 (T286) of the α subunit (threonine 287 of the β, γ, and δ subunits) (Hanson et al., 1994; Rich and Schulman, 1998; Mukherji and Soderling, 1994; Miller et al., 1988). Phosphorylation of this residue sterically prevents the kinase from returning to its auto-inhibited state (Yang and Schulman, 1999), thus rendering it autonomously active following the Ca2+ stimulus (Saitoh and Schwartz, 1985; Miller and Kennedy, 1986). T286 phosphorylation is increased following LTP induction (Fukunaga et al., 1995; Barria et al., 1997; Ouyang et al., 1997), and LTP is impaired in mice in which T286 has been mutated to an amino acid that cannot be phosphorylated (Giese et al., 1998). Aside from promoting Ca2+-independent activity of CaMKII, T286 phosphorylation has also been shown to regulate its protein-protein interactions. Phosphorylation of this residue is thought to enhance CaMKII binding to isolated PSDs (Strack et al., 1997b), densin-180 (Strack et al., 2000), and the N-methyl-D-aspartate receptor (NMDAR) subunits NR2B (Strack and Colbran, 1998; Bayer et al., 2001; Leonard et al., 2002), NR1 (Leonard et al., 2002), and NR2A (Gardoni et al., 1999). Furthermore, T286 phosphorylation has been reported to decrease the dissociation rate of CaMKII that has been bound to the PSD (Shen et al., 2000; Bayer et al., 2006; Yoshimura and Yamauchi, 1997; Dosemeci et al., 2002) (but see (Strack et al., 1997b)). These studies suggest that phosphorylation of T286 is an important regulatory mechanism controlling αCaMKII association with the PSD, and that PSD-associated αCaMKII is most likely more heavily phosphorylated on T286 than αCaMKII in other parts of the neuron.
Following T286 phosphorylation and dissociation of Ca2+/calmodulin, CaMKII can further autophosphorylate on threonines 305 and 306 (T305/6) of the α subunit (threonines 306 and 307 of the β, γ, and δ subunits) in an intrasubunit reaction (Colbran and Soderling, 1990; Patton et al., 1990; Mukherji and Soderling, 1994). Phosphorylation of these residues, which lie within the calmodulin binding domain, prevent further calmodulin binding and thus inactivate the kinase (Hashimoto et al., 1987). Like T286 phosphorylation, T305/6 phosphorylation has been shown to modulate protein-protein interactions of CaMKII. Phosphorylation of these residues inhibits kinase binding to isolated PSDs (Strack et al., 1997b), α-actinin (Robison et al., 2005), and the NMDAR subunits NR1 and NR2B (Leonard et al., 2002). In addition, T305/6 phosphorylation increases the dissociation rate of CaMKII from the PSD (Shen et al., 2000), and mimicking phosphorylation on these residues prevents while inhibiting phosphorylation enhances PSD association of the kinase (Elgersma et al., 2002). Furthermore, CaMKII interaction with NR2B, which is enriched at the PSD, has been shown to suppress T305/6 phosphorylation (Bayer et al., 2001), and T306 phosphorylation leads to CaMKII dissociation from the synapse associated protein Camguk in Drosophila (Lu et al., 2003). These studies suggest that phosphorylation of T305/6 is an important inhibitory mechanism controlling αCaMKII association with the PSD, and that αCaMKII localized at the PSD is most likely less phosphorylated on T305/6 than αCaMKII in other parts of the neuron.
In this study, we compared T286 and T305 phosphorylation levels on αCaMKII between different subcellular fractions obtained from recovered hippocampal CA1-minislices from adult rats. This was done under basal conditions and following an in vitro ischemia treatment that has previously been shown to alter the localization of CaMKII in hippocampal slices (Kolb et al., 1995). To our surprise, T286 phosphorylation was significantly lower in the PSD-enriched fraction as compared to the cytosolic fraction and the microsome-enriched fraction. Also surprising was the finding that T305 phosphorylation was similar between the PSD-enriched fraction and the microsome-enriched fraction, and it was higher in the PSD-enriched fraction when compared to the cytosolic fraction and the extra-synaptic membrane-enriched fraction. Furthermore, the ischemic treatment induced a translocation of αCaMKII from the cytosolic fraction to the PSD-enriched fraction. This redistribution of the kinase was accompanied by a dramatic decrease in T286 phosphorylation throughout the neuron with no change in T305 phosphorylation.
2. Results
Previous studies have demonstrated that the conditions encountered during sacrifice and hippocampal slice preparation lead to alterations in T286 phosphorylation (Lengyel et al., 2001; Ho et al., 2004), an increase in CaMKII concentration in PSD-enriched fractions (Suzuki et al., 1994), and CaMKII cluster formation (Dosemeci et al., 2000; Tao-Cheng et al., 2002). Because of this, all of the CA1-minislices used in this study were allowed to recover for at least 90 minutes following sacrifice. Recovery protocols have been shown to restore T286 phosphorylation to what are believed to be physiological levels (Lengyel et al., 2001) and reverse sacrifice-induced CaMKII clustering (Tao-Cheng et al., 2002). Recovery of hippocampal slices is also essential for the recovery of normal membrane potential and evoked synaptic responses.
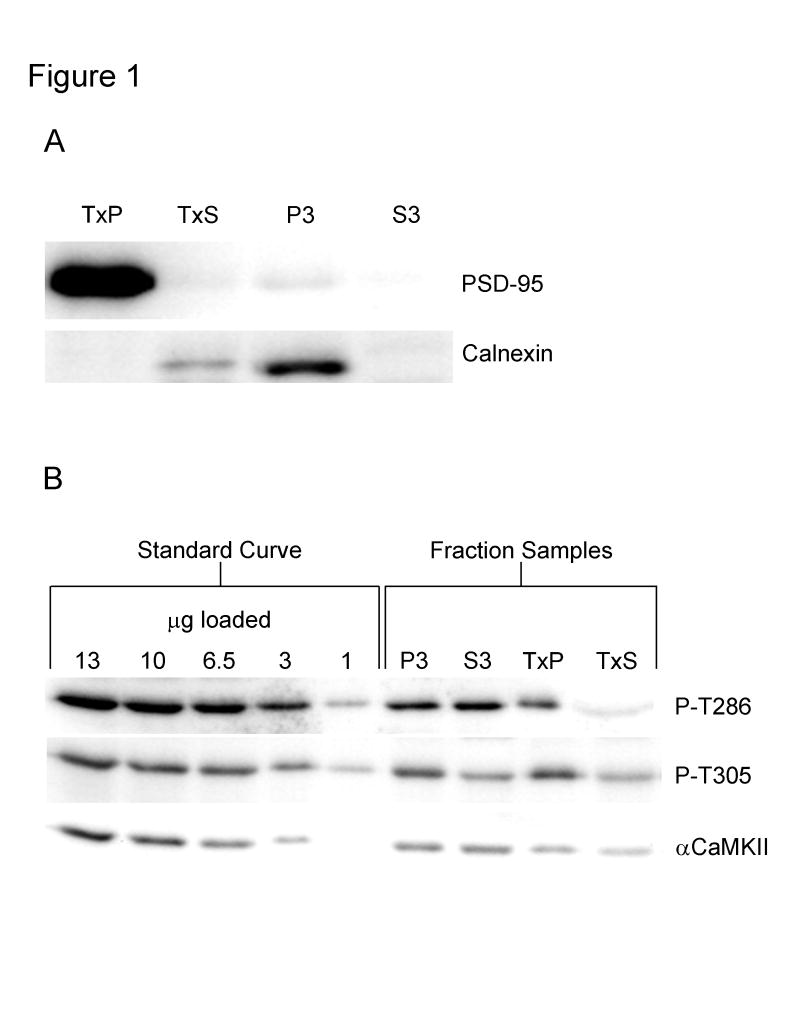
In order to separate different parts of the neuron, a modified subcellular fractionation protocol was used (Carlin et al., 1980; Huttner et al., 1983; Cho et al., 1992; Goebel et al., 2005; Smith et al., 2006). This protocol, which consisted of differential centrifugation combined with a detergent extraction using the non-ionic detergent Triton X-100, yielded a PSD-enriched fraction which we termed the TxP, an extra-synaptic membrane-enriched fraction which we termed the TxS, a microsome-enriched fraction which we termed the P3, and a cytosolic/soluble fraction which we termed the S3. As expected, PSD-95, which is a marker for the post-synaptic density, was found almost exclusively in the TxP fraction, and calnexin, which is a marker for the endoplasmic reticulum, was found predominantly in the P3 fraction with some labeling in the TxS fraction (Fig. 1A). Calnexin labeling in the TxS fraction might be explained by the finding that this protein is expressed in the plasma membrane in various cell types (Okazaki et al., 2000).
Fig. 1. Characterization of the subcellular fractionation procedure and semi-quantitative Western blotting.
A, Western blots demonstrating the efficiency of the subcellular fractionation procedure. Recovered CA1 minislices were homogenized and subjected to the subcellular fractionation protocol. Equal amounts of total protein from each of the fractions were then analyzed by Western blot using antibodies specific for the post-synaptic density marker PSD-95 and the endoplasmic reticulum marker calnexin. TxP: PSD-enriched fraction, TxS: extra-synaptic membrane-enriched fraction, P3: microsome-enriched fraction, and S3: cytosolic fraction. B, Examples of Western blots used for semi-quantitative analysis. An identical standard curve, which contained points of 13, 10, 6.5, 3, and 1 μg of total protein, was run on each blot. Blots were labeled with an antibody specific for T286 phosphorylated αCaMKII (top blot), an antibody specific for T305 phosphorylated αCaMKII (middle blot), and a pan-αCaMKII antibody (bottom blot). For the pan-αCaMKII and P-T305 blots; 5 μg of P3, 10 μg of S3, 1.5 μg of TxP, and 12 μg of TxS were loaded. For the P-T286 blot; 1.75 μg of P3, 3.5 μg of S3, 1.5 μg of TxP, and 34 μg of TxS were loaded.
Measurement of total and phosphorylated αCaMKII in the subcellular fractions was accomplished by semi-quantitative Western blotting. A 5-point standard curve of known total protein concentration was prepared from recovered whole hippocampal slices and run on every blot. The total amount of protein loaded for each of the fractions was adjusted such that the signal for each fraction was within the standard curve. A line was generated from the standard curve by plotting the integrated density value (IDV) vs. the total amount of protein loaded for each curve point. Using this line, the IDV of each sample was used to calculate its immunoreactivity, which was measured in arbitrary immunoreactive (AIR) units. This number was then divided by the total protein loaded in micrograms for the sample to obtain the AIR units/μg, which is a measure of relative concentration that can be compared between subcellular fractions. Examples of Western blots used for analysis are shown in Fig. 1B. Note that different amounts of total protein were loaded for each of the fractions so that the signals were within the standard curve. In subsequent figures, the same amount of protein was loaded for each of the fractions (or conditions) so that differences can be easily visualized. These blots were not used for analysis.
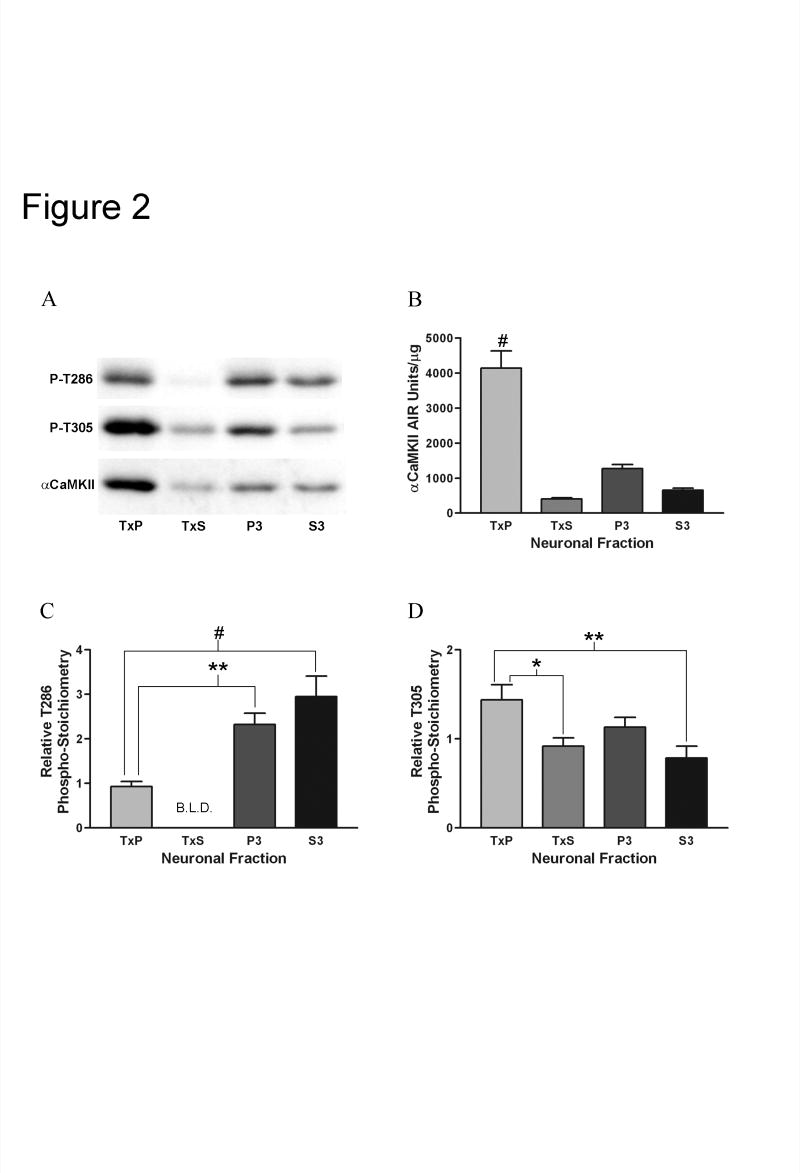
The relative concentration of total αCaMKII in each of the fractions under basal conditions was determined by semi-quantitative Western analysis using an antibody that recognizes all forms of αCaMKII (pan-αCaMKII) (Fig. 2A,B). In this case, the AIR units/μg measurement reflects the concentration of αCaMKII relative to all other proteins in the fraction. Although it has been demonstrated that self-associated CaMKII clusters contaminate PSD-enriched fractions (Dosemeci et al., 2000), CaMKII clusters have been shown to be virtually absent in recovered hippocampal slices (Tao-Cheng et al., 2002). Because of this, we assume that the αCaMKII in our TxP fraction represented predominantly PSD-associated kinase. The TxP fraction contained the highest relative concentration of αCaMKII, indicating that, as expected based on previous results (Kennedy et al., 1983; Petersen et al., 2003; Suzuki et al., 1994), αCaMKII is enriched at the post-synaptic density in recovered CA1-minislices from adult rats.
Fig. 2. Comparison of the relative concentration of αCaMKII and the relative phospho-stoichiometries of T286 and T305 between the subcellular fractions under basal conditions.
A, Representative Western blots with equal amounts of total protein loaded from each of the fractions and labeled with an antibody specific for T286 phosphorylated αCaMKII (top blot), an antibody specific for T305 phosphorylated αCaMKII (middle blot), and a pan-αCaMKII antibody (bottom blot). B, Quantitation of the relative concentration of αCaMKII for the TxP (n=12), TxS (n=11), P3 (n=12), and S3 (n=12) (for explanation of n see Experimental Procedure section). Bonferroni’s post-hoc analysis indicated that the TxP fraction had a significantly greater relative αCaMKII concentration than the other three fractions (#: p<0.001 for all three comparisons). C, Quantitation of the relative phospho-stoichiometry of T286 on αCaMKII for the TxP (n=12), P3 (n=12), and S3 (n=12). All bands except for one for the TxS were below the limit of linear detection (B.L.D.) when probed with the antibody specific for T286 phosphorylated αCaMKII. Since only one value was obtained for the TxS (0.073), this fraction was left out of the analysis. The relative phospho-stoichiometry for the TxP was significantly lower than the P3 (**: p<0.01) and the S3 (#: p<0.001) as determined by Bonferroni’s post-hoc analysis. D, Quantitation of the relative phospho-stoichiometry of T305 on αCaMKII for the TxP (n=12), TxS (n=11), P3 (n=12), and S3 (n=12). The relative phospho-stoichiometry for the TxP was significantly higher than the TxS (*: p<0.05) and the S3 (**: p<0.01) as determined by Bonferroni’s post-hoc analysis. Values represent the mean ± S.E.M.
If phosphorylation of T286 positively regulates constitutive αCaMKII localization at the post-synaptic density, then one would expect PSD-associated αCaMKII to be more highly phosphorylated on T286 than αCaMKII in other parts of the neuron. In order to test this hypothesis, we measured the relative phospho-stoichiometry of T286 on αCaMKII in the four subcellular fractions (Fig. 2A,C). The relative phospho-stoichiometry was calculated by dividing the AIR units/μg obtained using an antibody that only recognizes T286 phosphorylated αCaMKII by the AIR units/μg obtained using the pan-αCaMKII antibody. Importantly, this number is not a direct measure of the percentage of the kinase that is T286 phosphorylated, but it can be used to compare levels of phosphorylation between samples. To our surprise, the relative phospho-stoichiometry for T286 was significantly lower in the TxP fraction compared to the P3 and S3 fractions, which had approximately 2.5 and 3.2 fold higher relative phospho-stoichiometries, respectively. Most bands for the TxS fraction labeled with the phospho-T286 antibody were below the limits of linear detection, meaning that relative phospho-stoichiometry was extremely low in this fraction. This result suggests that αCaMKII that is at the PSD is less phosphorylated on T286 than αCaMKII that is soluble or associated with intracellular membranes.
If phosphorylation of T305 negatively regulates constitutive αCaMKII localization at the post-synaptic density, then one would expect PSD-associated αCaMKII to be predominantly unphosphorylated on T305 compared to αCaMKII in other parts of the neuron. To test this hypothesis, relative T305 phospho-stoichiometry was measured in the same manner as for T286 (Fig. 2A,D). To our surprise, the relative T305 phospho-stoichiometry was similar between the TxP and P3 fractions and significantly higher in the TxP fraction compared to the TxS and S3 fractions (approximately 1.6 and 1.8 times higher, respectively). This result suggests that PSD-associated αCaMKII is not relatively unphosphorylated on T305 compared to αCaMKII in other parts of the neuron. Not only is this result interesting in its own right, but it also suggests that the T286 result described above is not a general autophosphorylation artifact of the procedure. Furthermore, including additional protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) inhibitors in the fractionation buffers did not yield different results, suggesting that the T286 and T305 phosphorylation patterns we observed are not due to fraction-specific dephosphorylation during the fractionation procedure (data not shown).
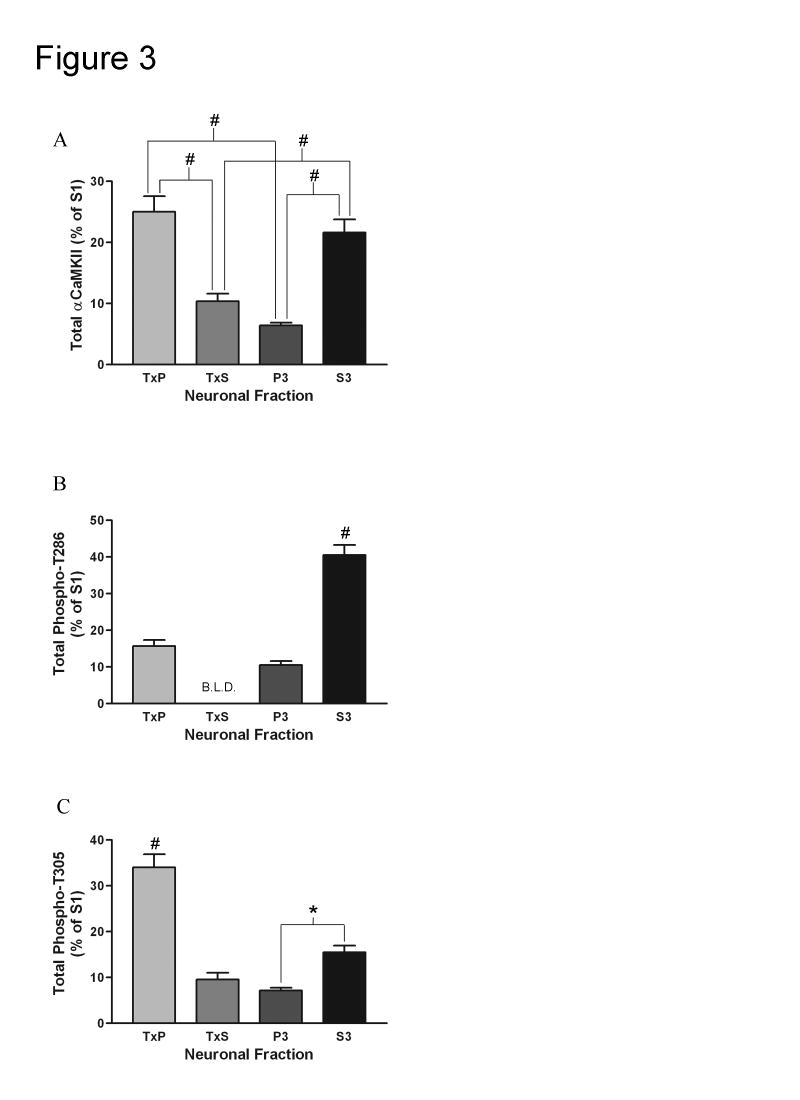
While it is important to know the relative concentration of αCaMKII and the relative phospho-stoichiometries of T286 and T305, it is also useful to calculate the total amount of total αCaMKII, T286 phosphorylated αCaMKII, and T305 phosphorylated αCaMKII in the fractions. This is achieved by multiplying the AIR units/μg of a sample (from figure 2) by the total protein concentration of the fraction and by the total volume of the fraction. By performing this calculation, the total AIR units of a fraction can be determined. This data is reported in figure 3. While the relative concentration of αCaMKII is much higher in the TxP fraction compared to the other fractions (Fig. 2B), there is actually a similar level of total αCaMKII in the TxP fraction as the S3 fraction (Fig. 3A). Furthermore, by performing this calculation, it becomes apparent that most of the T286 phosphorylated αCaMKII is in the S3 fraction (Fig. 3B), while most of the T305 phosphorylated αCaMKII is in the TxP fraction (Fig. 3C). The data in figure 3 is reported as the percent of the parent fraction (S1). It should be noted that in all 3 cases the individual fractions add up to only roughly 65% of the S1. This discrepancy is the result of certain practices during the fractionation procedure that are performed to enhance the purity of the fractions. For every centrifugation step during the fractionation, a substantial amount of the supernatant is left at the bottom of the tube and then discarded. Furthermore, every pellet that is obtained is washed with homogenization buffer, which also reduces the yield of the fractions.
Fig. 3. Comparison of the total amounts of total αCaMKII, T286 phosphorylated αCaMKII, and T305 phosphorylated αCaMKII between the subcellular fractions under basal conditions.
A, Quantification of the total amount (AIR units/μg x total protein concentration of fraction x volume of fraction) of total αCaMKII for the TxP (n=12), TxS (n=11), P3 (n=12), and S3 (n=12) (for explanation of n see Experimental Procedure section). Bonferroni’s post-hoc analysis indicated that the TxP contained more total αCaMKII than the TxS and P3 (#: p<0.001 for both comparisons) and the S3 contained more total αCaMKII than the TxS and P3 (#: p<0.001 for both comparisons). B, Quantification of the total amount of T286 phosphorylated αCaMKII for the TxP (n=12), P3 (n=12), and S3 (n=12). All bands except for one for the TxS were below the limit of linear detection (B.L.D.) when probed with the antibody specific for T286 phosphorylated αCaMKII (meaning that there was a very low level of T286 phosphorylated αCaMKII in this fraction). Because of this, the TxS was left out of analysis. Bonferroni’s post-hoc analysis indicated that the S3 contained significantly more T286 phosphorylated αCaMKII than the TxP and P3 (#: p<0.001 for both comparisons). C, Quantification of the total amount of T305 phosphorylated αCaMKII for the TxP (n=12), TxS (n=11), P3 (n=12), and S3 (n=12). Bonferroni’s post-hoc analysis indicated that the TxP contained more T305 phosphorylated αCaMKII than the other three fractions (#: p<0.001 for all three comparisons) and the S3 contained more T305 phosphorylated αCaMKII than the P3 (*: p<0.05). Values represent the mean ± S.E.M.
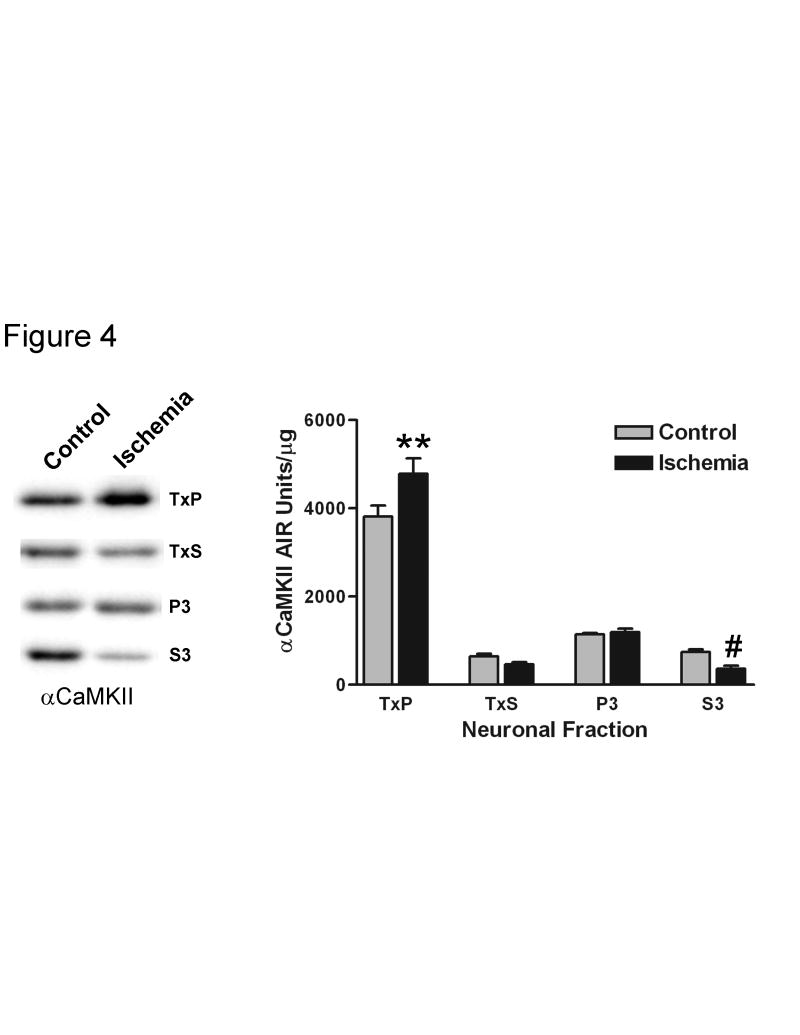
If phosphorylation of T286 is directly correlated with the PSD association of αCaMKII, then one would expect that any increase in the amount of kinase at the PSD should coincide with an increase in phosphorylation of this residue. Several models of ischemia have previously been shown to increase the amount of CaMKII in PSD-enriched fractions (Kolb et al., 1995; Aronowski et al., 1992; Shackelford et al., 1993; Hu et al., 1995). We subjected recovered CA1-minislices to an in vitro model of ischemia (a 10 minute anoxic/aglycemic insult (Kolb et al., 1995)), and then allowed them to recover for 30 minutes under standard incubation conditions. A previous study demonstrated that 10 minutes of anoxic/aglycemic insult resulted in a translocation of CaMKII from a supernatant fraction to a pellet fraction, and that this redistribution was associated with a loss in Ca2+-independent activity throughout the neuron (Kolb et al., 1995). We also found that the ischemic insult followed by a 30 minute recovery period produced a redistribution of the kinase, inducing a significant increase in the relative αCaMKII concentration in the TxP fraction (approximately 25%) and a significant decrease in the S3 fraction (approximately 51%) (Fig. 4). There was also a decrease in the TxS fraction (approximately 28%), but this change was not statistically significant. This result indicates that, upon ischemic treatment, αCaMKII translocates from the cytosol to Triton X-100 insoluble structures. A previous study found that a treatment that mimics ischemia leads to increases in CaMKII labeling at the PSD and CaMKII cluster formation in cultured hippocampal neurons (Dosemeci et al., 2000). Because of this, it is likely that the increase in relative αCaMKII concentration in the TxP that we observed represents both translocation to the PSD and the formation of self-associated clusters.
Fig. 4. A 10-minute ischemic treatment followed by a 30-minute recovery alters the subcellular distribution of αCaMKII.
Recovered CA1 minislices were subjected to a 10 minute anoxic/aglycemic insult (Ischemia) or kept in standard incubation conditions (Control), and then were allowed to recover in standard incubation conditions for 30 minutes. Left – individual representative Western blots with equal amounts of total protein loaded for each condition. The band on the left represents control and the band on the right represents the ischemic treatment for each fraction. Right – quantitation of the relative concentration of αCaMKII for ischemic and control conditions for the TxP (n=8), TxS (n=6), P3 (n=8), and S3 (n=8) (for explanation of n see Experimental Procedure section). Two-way ANOVA analysis indicated significant differences between the fractions (p<0.0001) but not a significant effect of the ischemic treatment (most likely because the treatment had effects in different directions depending on the fraction). Furthermore, the effect of the treatment was different among the fractions (interaction: p<0.01). Bonferroni’s post-hoc analysis indicated a significant increase in the TxP (**: p<0.01) and a significant decrease in the S3 (#: p<0.001) upon ischemic insult. Values represent the mean ± S.E.M.
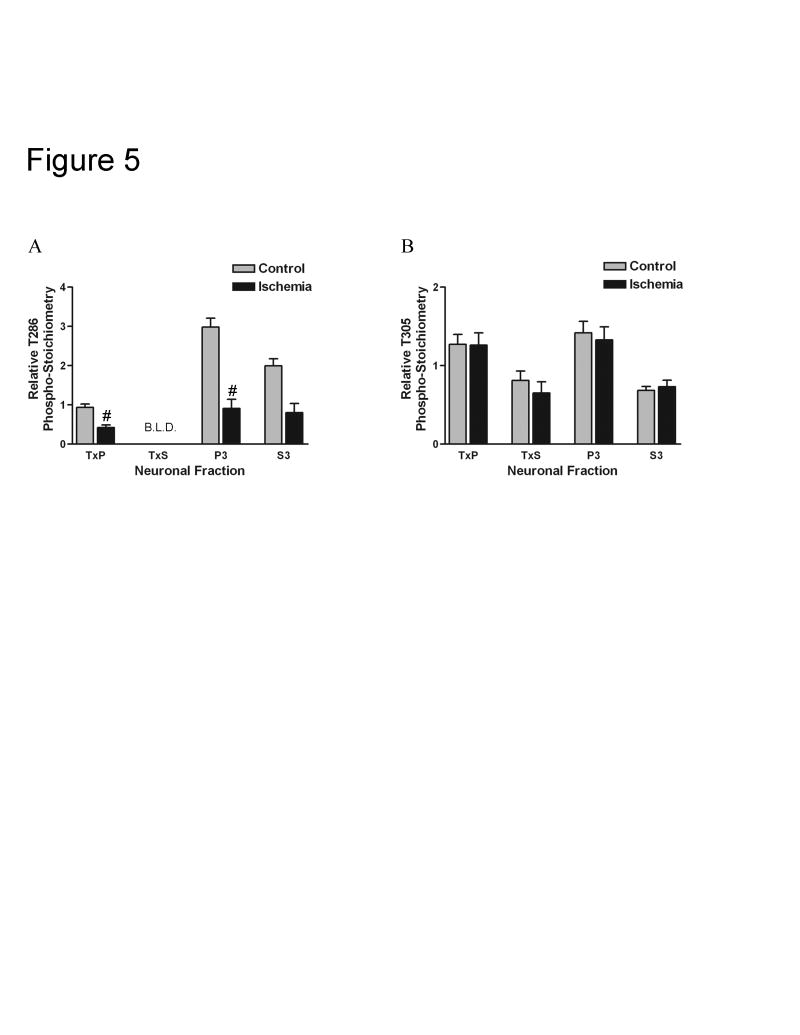
Surprisingly, the redistribution of αCaMKII following the ischemic treatment was associated with a dramatic decrease in T286 phosphorylation throughout the neuron (Fig. 5A). Again, we measured relative phospho-stoichiometry, meaning that the phosphorylation changes seen in each of the fractions was corrected for changes in the total amount of αCaMKII in those fractions. The relative T286 phospho-stoichiometry was significantly decreased in the TxP (approximately 55%) and the P3 (approximately 70%) fractions. There was also a large decrease in relative T286 phospho-stoichiometry in the S3 fraction (approximately 62%), but this change was not statistically significant. Most bands for the TxS fraction labeled with the phospho-T286 antibody were below the limits of linear detection, and therefore changes in relative phospho-stoichiometry for this fraction could not be statistically analyzed. No significant changes in the relative T305 phospho-stoichiometry were observed in any of the fractions (Fig. 5B). These results suggest that the translocation of αCaMKII following the ischemic treatment and recovery is associated with a selective dephosphorylation of T286.
Fig. 5. A 10-minute ischemic treatment followed by a 30-minute recovery leads to a dramatic decrease in T286 phosphorylation throughout the neuron but no change in T305 phosphorylation.
Recovered CA1 minislices were subjected to a 10 minute anoxic/aglycemic insult (Ischemia) or kept in standard incubation conditions (Control), and then were allowed to recover in standard incubation conditions for 30 minutes. A, Relative T286 phospho-stoichiometry was calculated for ischemic and control conditions for the TxP (n=8), P3 (n=8), and S3 (n=5) (for explanation of n see Experimental Procedure section). Most bands for the TxS were below the limit of linear detection (B.L.D.) when probed with the antibody specific for T286 phosphorylated αCaMKII. Relative T286 phospho-stoichiometry in the TxS could only be calculated for both ischemic and control conditions for an n=2, with means of 0.097 and 0.078, respectively. Because of this, this fraction was left out of analysis. Two-way ANOVA analysis indicated significant differences between the fractions (p<0.0001) and a significant effect of the ischemic treatment (p<0.0001). Furthermore, the effect of the treatment was different among the fractions (interaction: p<0.01). Bonferroni’s post-hoc analysis indicated a significant decrease in the TxP (#: p<0.001) and P3 (#: p<0.001) upon ischemic insult. The decrease in the S3 was large (62%), but it was not significant with an adjusted p-value. B, Relative T305 phospho-stoichiometry was calculated for ischemic and control conditions for the TxP (n=8), TxS (n=6), P3 (n=8), and S3 (n=8). Two-way ANOVA analysis indicated significant differences between the fractions (p<0.0001) but not a significant effect of the ischemic treatment. Relative T305 phospho-stoichiometry was not significantly different in any of the fractions upon ischemic insult as determined by Bonferroni’s post hoc analysis. Values represent the mean ± S.E.M.
3. Discussion
It is now widely accepted that CaMKII plays an essential role in long term potentiation. Several models of the function of CaMKII during LTP place high importance on its localization at the post-synaptic density (Lisman et al., 2002). Furthermore, CaMKII translocation to the PSD has been proposed to create a ‘synaptic tag’ that distinguishes potentiated from unpotentiated synapses (Frey and Morris, 1997; Lisman et al., 2002). Because of this, information regarding the mechanisms that regulate CaMKII binding to the PSD is critical for our understanding of LTP at the molecular level. In this study, we found that αCaMKII in the PSD-enriched fraction had a lower level of T286 phosphorylation than kinase in the cytosolic fraction and the microsome-enriched fraction. We also demonstrated that αCaMKII in the PSD-enriched fraction had a higher level of T305 phosphorylation than kinase in the cytosolic fraction and the extra-synaptic membrane-enriched fraction (although these differences were smaller in magnitude compared to differences in T286 phosphorylation) and a similar level of T305 phosphorylation as kinase in the microsome-enriched fraction. Furthermore, we observed that an in vitro ischemia treatment resulted in a translocation of αCaMKII from the cytosolic fraction to the PSD-enriched fraction, and that this redistribution of the kinase correlated with a global decrease in T286 phosphorylation.
Several studies have indicated that αCaMKII autophosphorylation of T286 enhances binding of the kinase to the PSD and several of its constituents (Bayer et al., 2001; Strack et al., 1997b; Strack and Colbran, 1998; Gardoni et al., 1999; Leonard et al., 2002; Strack et al., 2000). In addition, T286 phosphorylation has been shown to decrease the dissociation rate of the kinase from the PSD (Shen et al., 2000; Bayer et al., 2006; Yoshimura and Yamauchi, 1997; Dosemeci et al., 2002). It has also been observed that autophosphorylation of T305/6 inhibits binding of αCaMKII to the PSD and several PSD proteins (Strack et al., 1997b; Leonard et al., 2002; Robison et al., 2005). Furthermore, it has been demonstrated that T305/6 phosphorylation increases the dissociation rate of the kinase from the PSD (Shen et al., 2000). Given these results, it was surprising to find that αCaMKII in the PSD-enriched fraction had a relatively low level of T286 phosphorylation and a relatively high level of T305 phosphorylation. It is important, however, to take into consideration the fact that CaMKII exists in vivo as multimeric holoenzymes consisting of 12-14 individual subunits (Kolodziej et al., 2000; Hoelz et al., 2003). Using our techniques, we were not able to determine specifically which subunits within each holoenzyme were phosphorylated. It may be the case that the subunit(s) that are directly bound to PSD proteins are phosphorylated on T286 and unphosphorylated on T305 and that the remaining (unbound) subunits are predominantly unphosphorylated on T286 and phosphorylated on T305. This scenario would still be interesting, because it suggests that αCaMKII that is at the PSD is kept in a preferentially inactivated state under basal conditions. A possible mechanism for this is differential control by phosphatases at the post-synaptic density compared to elsewhere in the neuron, and indeed it has been demonstrated that T286 is dephosphorylated by different phosphatases depending on if the kinase is PSD-associated or soluble (Strack et al., 1997a). Whatever the explanation of these results, it is clear that our data are incompatible with a model in which T286 phosphorylated αCaMKII is preferentially localized to the PSD and T305 phosphorylated αCaMKII is excluded from the PSD. Another conclusion that can be made from our findings is that T286 phosphorylation alone is not sufficient to induce binding of αCaMKII to the PSD in adult animals. If this were the case, then one would not expect relatively highly T286 phosphorylated kinase to exist in the cytosolic fraction, because unbound kinase that is phosphorylated would eventually bind to the PSD.
Keeping PSD-associated αCaMKII relatively unphosphorylated on T286 and relatively highly phosphorylated on T305 may be beneficial in several ways. First, it may be a mechanism to prevent unwanted kinase activity at the synapse in the absence of an activating stimulus. Not only would autonomous activity be minimal, but T305 would need to be dephosphorylated for activation to occur. Second, this might be a way in which the neuron establishes a threshold for CaMKII activation at the PSD, such that weak or non-repetitive stimuli are insufficient to activate the kinase. Third, this state of phosphorylation might promote dynamic localization of the kinase at the synapse. If the unbound subunits were highly T286 phosphorylated and unphosphorylated on T305, then unbinding of one subunit in the holoenzyme would most likely lead to the immediate binding of another subunit to another CaMKII anchoring protein in the PSD. This would create a situation in which it would be difficult to regulate the PSD association of the kinase.
As has been shown with other in vivo (Aronowski et al., 1992; Shackelford et al., 1993; Hu et al., 1995) and in vitro (Kolb et al., 1995) ischemic treatments, we observed a significant translocation of αCaMKII from the cytosolic fraction to the PSD-enriched fraction following in vitro ischemia. Originally, increases in particulate/PSD-enriched fractions were thought to result exclusively from kinase translocation to the post-synaptic density. However, ischemic conditions have since been shown to induce the formation of self-associated CaMKII clusters in a variety of systems (Dosemeci et al., 2000; Tao-Cheng et al., 2002; Hudmon et al., 1996), and it has been demonstrated that these clusters contaminate PSD-enriched fractions (Dosemeci et al., 2000). Since it has been shown that in vivo ischemia increases the thickness of the PSD (Hu et al., 1998), and that an in vitro ischemic treatment induces both CaMKII clustering and an increase in CaMKII labeling at the PSD (which correlates with an increase in PSD thickness) (Dosemeci et al., 2000), it is likely that the increase in CaMKII concentration in PSD-enriched fractions that we and others have observed represents both CaMKII translocation to the PSD and cluster formation.
The disconnect between αCaMKII PSD-association and its phosphorylation on T286 under basal conditions was also seen following the ischemia induced translocation of the kinase. We observed that an increase in the relative αCaMKII concentration in the PSD-enriched fraction temporally coincided with a large decrease in T286 phosphorylation with no change in T305 phosphorylation. This result is incompatible with models in which reductions in T286 phosphorylation lead to dissociation of CaMKII from the PSD. However, a dramatic decrease in T286 phosphorylation may facilitate CaMKII cluster formation, because it has been demonstrated that when T286 is mutated to alanine (an amino acid that cannot be phosphorylated) cluster formation is enhanced and when T286 is mutated to aspartate (an amino acid that mimics phosphorylation) cluster formation is inhibited (Hudmon et al., 2005). The decrease in T286 phosphorylation that we observed may explain why Kolb et al., saw a decrease in Ca2+-independent activity with a similar treatment (Kolb et al., 1995), but it is at odds with several reports demonstrating an increase in T286 phosphorylation following in vivo ischemia (Meng and Zhang, 2002; Tang et al., 2004; Meng et al., 2003). However, it has also been reported that sub-lethal ischemia followed by reperfusion in rats leads to a decrease in T286 phosphorylation (Shamloo et al., 2000), so our in vitro model of ischemia may more closely mimic sub-lethal as opposed to lethal in vivo ischemia.
The present study suggests that there are multiple factors controlling the PSD association of CaMKII in the rat brain, and that the scenario is much more complicated than a situation in which association with the PSD is controlled exclusively by phosphorylation. Support for this conclusion can also be found in work showing that αCaMKII in which T286 has been mutated to alanine can still translocate to (Shen and Meyer, 1999; Bayer et al., 2001) and be constitutively localized at the synapse (Bayer et al., 2006), and αCaMKII in which T286 has been mutated to aspartate still requires neuronal activation to initially translocate to the PSD (Shen et al., 2000). Clearly, much remains to be elucidated regarding the localization and trafficking of CaMKII to the post-synaptic density.
4. Experimental Procedure
CA1-minislice preparation and treatment
All of the experiments used in this study were approved by the Institutional Animal Care and Use Committee at the University of Colorado Health Sciences Center. Seven to ten week old male Sprague-Dawley rats were used for all experiments. After sacrifice, the brain was immediately removed and placed in ice-cold oxygenated artificial cerebrospinal fluid (ACSF: 124mM NaCl, 4mM KCl, 1mM MgCl2, 2.5mM CaCl2, 10mM dextrose, 1mM KH2PO4, 25.7mM NaHCO3). Both hippocampi were carefully dissected out and unrolled along the hippocampal fissure. Subsequently, area CA1 was isolated by two cuts and then sliced into 400μm minislices using a McIlwain tissue chopper. The minislices were then incubated at interface in 32°C ACSF (that had been bubbled with a 95% O2 / 5% CO2 mix) and humidified with 95% O2 / 5% CO2. Since the conditions encountered during sacrifice and minislice preparation are known to alter T286 phosphorylation (Lengyel et al., 2001; Ho et al., 2004), increase CaMKII concentration in PSD-enriched fractions (Suzuki et al., 1994), and induce CaMKII cluster formation (Dosemeci et al., 2000; Tao-Cheng et al., 2002), the minislices were recovered at interface for at least 90 minutes (Ho et al., 2004), with ACSF being replaced every 15 minutes. Similar protocols using submersion recovery techniques have been shown to restore T286 phosphorylation to what are believed to be physiological levels (Lengyel et al., 2001) and reverse the sacrifice induced clustering of the kinase (Tao-Cheng et al., 2002). For basal experiments, the recovery period was followed immediately by homogenization. For anoxic/aglycemic treatment, following recovery, slices were incubated at interface in ‘ischemic ACSF’ (in which the 10mM dextrose was replaced with 10mM sucrose and bubbled with a 95% N2 / 5% CO2 mix) and humidified with 95% N2 / 5% CO2 for 10 minutes. Following this treatment, slices were returned to the regular ACSF and 95% O2 / 5% CO2 condition for 30 minutes before homogenization. Control slices for the anoxic/aglycemic treatment were incubated in the regular ACSF and 95% O2 / 5% CO2 condition during the 10-minute treatment and subjected to the same 30-minute recovery. The average number of minislices that could be harvested from one animal (both hippocampi) was approximately 31 with a minimum of 27. For basal experiments, each n represents all of the minislices that could be harvested from one animal, i.e. n=12 means that 12 animals (~31 minislices per animal) were used. For ischemia experiments, each n represents all of the minislices that could be harvested from two animals divided equally between ischemia and control conditions, i.e. n=8 means that 16 animals (~31 minislices per animal) were used.
Subcellular fractionation
Minislices from all conditions were harvested in ice-cold homogenization buffer (50 μl per slice) containing 320mM sucrose, 10mM Tris (pH 7.4), 100μM Na3VO4, 40mM NaF, 300nM Okadaic acid, and 1mM EDTA. Following harvesting, the slices were immediately homogenized in a glass grinding vessel by a Teflon pestle rotating at 1000 RPM. Subsequently, a modified subcellular fraction protocol was employed (Carlin et al., 1980; Huttner et al., 1983; Cho et al., 1992; Goebel et al., 2005; Smith et al., 2006). Due to the limited amount of tissue, the commonly used sucrose density gradient centrifugation step(s), which are utilized to obtain a more pure synaptic fraction, were omitted from our fractionation protocol. The homogenate was spun at 1000 X g for 10 min and the pellet (P1), which contains nuclei and incompletely homogenized cells, was discarded. The supernatant (S1) was then spun at 10,000 X g for 15 min. The pellet from this spin (P2) was resuspended in homogenization buffer containing 0.5% Triton X-100, homogenized by 20 passes with a glass dounce homogenizer, incubated on ice for 40 minutes, and then spun at 32,000 X g for 20 minutes. The pellet from this spin, TxP, is the PSD-enriched fraction and was resuspended in homogenization buffer. The supernatant from this spin, TxS, is the extra-synaptic membrane-enriched fraction. The supernatant from the second spin (S2) was spun at 100,000 X g for one hour. The pellet from this spin, P3, is the microsome-enriched fraction and was resuspended in homogenization buffer. The supernatant from this spin, S3, is the cytosolic fraction. For every centrifugation step above, a small amount of supernatant was left behind to ensure that the collected supernatant was not contaminated by the pellet. Furthermore, after the remainder of the supernatant was discarded, the pellet was washed once with homogenization buffer to ensure that it was not contaminated by the supernatant. The TxS and S3 fractions were subjected to acetone precipitation to concentrate the protein. Eight volumes of acetone was added to these supernatants which were then incubated at -20°C overnight. The precipitate was collected by centrifugation at 3000 X g for 15 minutes. The pellets from these spins were dried and then resuspended in water. All of the above spins were conducted at 4°C to inhibit enzyme action. The four final pellets (TxP, TxS, P3, and S3) were sonicated and boiled in 1% SDS, 1mM EDTA, and 10mM Tris (pH 8) for 5 minutes and kept at -80°C. The final resuspension volumes (per animal or per ~31 minislices) are as follows: TxP = 30 μl, TxS = 30 or 35 μl, P3 = 30 μl, S3 = 100 μl.
Semi-quantitative Western blotting
Protein concentrations of the subcellular fractions were determined using the BCA™ protein assay kit from Pierce with bovine serum albumin (BSA) as a standard. Samples were prepared for gel loading such that they contained a known amount of total protein and 1X SDS-PAGE sample buffer (62.5mM Tris, 2% SDS, 10% glycerol, bromophenol blue, and 5% β-mercapoethanol). A 5-point standard curve of known total protein concentration was prepared as above from SDS homogenized recovered whole hippocampal slices. The samples along with the standard curve were run through 10% polyacrylamide gels at 150-200 volts for 45-60 minutes. Every blot contained the same standard curve. Separated proteins were transferred to Poly-Screen polyvinylidene diflouride (PVDF) membranes. Blots were then blocked in 5% milk or 3% BSA in Tris-buffered saline with Tween (TBST: 140mM NaCl, 20mM Tris (pH 7.6), 0.1% Tween 20) for 1 hour and then incubated overnight at 4°C with primary antibody in 1% milk or 1% BSA. Following three 10-minute washes in TBST, blots were incubated in secondary antibody conjugated to horseradish peroxidase in 1% milk or 1% BSA at room temperature for 1 hour. After three 10-minute washes in TBST, bands were detected using Pierce SuperSignal® chemiluminescence kits and the Alpha Innotech ChemiImager 4400 imaging system. In some cases, blots were stripped with Restore™ Western Blot Stripping Buffer from Pierce for 60 minutes at 60°C, and then subjected to six 10-minute washes in TBST, followed by blocking and antibody incubation. Primary antibodies were as follows: pan-αCaMKII (prepared in our laboratory) used at 1:1000 in milk, phospho-T286 (PhosphoSolutions) used at 1:2000 in milk, phospho-T305 (PhosphoSolutions) used at 1:1000 in BSA, PSD-95 (Affinity Bioreagents) used at 1:5000 in BSA, and Calnexin (Santa Cruz) used at 1:1000 in BSA. To ensure that the samples were within the standard curve, different amounts of total protein were loaded depending on the fraction. If a particular sample lied outside of the standard curve, the total amount of protein loaded was altered and a subsequent Western blot was performed. However, due to limitations in the total volume that could be loaded on a gel and limitations in the yield of certain fractions, it was impossible to obtain a value that was within the curve for all of the samples. These samples were left out of analysis. For basal experiments, all of the fractions from a single animal were run on one gel. For ischemia experiments, ischemia and control conditions for each fraction were run side by side on gels.
Data-analysis
Quantitation of Western blots was performed using AlphaEase software. An integrated density value (IDV) for samples and standards was determined as the sum of the intensity of the pixels constituting each band. A line was generated from the standard curve by plotting the IDV vs. the total amount of protein loaded for each curve point. Using this line, the IDV of each sample was used to calculate its immunoreactivity, which was measured in arbitrary immunoreactive (AIR) units. This number was then divided by the amount of total protein loaded in micrograms for the sample to obtain the AIR units/μg. The AIR units/μg is a measure of relative concentration that can be compared between subcellular fractions. Relative phospho-stoichiometry was calculated by dividing the AIR units/μg determined with the phospho-antibody by the AIR units/μg determined with the pan-αCaMKII antibody. To calculate the total AIR units in the fractions, the AIR units/μg value was multiplied by the total protein concentration of the fraction and by the total volume of the fraction. Only samples with an IDV within the standard curve were used for analysis.
Acknowledgments
We would like to thank Dr. K. Ulrich Bayer, Dr. Lisa Hines, Ms. Susan Goebel, and Ms. Tianna Hicklin for helpful discussions. This work was supported by NIH research grants AA014691 and AA09675.
The abbreviations used are
- CaMKII
calcium/calmodulin-dependent protein kinase II
- αCaMKII
the alpha subunit of CaMKII
- PSD
post-synaptic density
- T286
threonine 286 on αCaMKII
- T305
threonine 305 on αCaMKII
- T305/6
threonines 305 and 306 on αCaMKII
- LTP
long term potentiation
- NMDAR
N-methyl-D-aspartate receptor
- NR2B
type 2B of the NMDAR
- NR2A
type 2A of the NMDAR
- NR1
type 1 of the NMDAR
- ACSF
artificial cerebrospinal fluid
- EDTA
ethylenediamine-tetraacetic acid
- BSA
bovine serum albumin
- SDS
sodium dodecyl sulfate
- PAGE
polyacrylamide gel electrophoresis
- PVDF
polyvinylidene diflouride
- IDV
integrated density value
- AIR
arbitrary immunoreactive
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronowski J, Grotta JC, Waxham MN. Ischemia-induced translocation of Ca2+/calmodulin-dependent protein kinase II: potential role in neuronal damage. J Neurochem. 1992;58:1743–1753. doi: 10.1111/j.1471-4159.1992.tb10049.x. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Bayer KU, Lebel E, McDonald GL, O’Leary H, Schulman H, De Koninck P. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and Characterization of Postsynaptic Densities from Various Brain-Regions - Enrichment of Different Types of Postsynaptic Densities. Journal of Cell Biology. 1980;86:831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The Rat-Brain Postsynaptic Density Fraction Contains A Homolog of the Drosophila Disks-Large Tumor Suppressor Protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- Dosemeci A, Reese TS, Petersen J, Tao-Cheng JH. A novel particulate form of Ca(2+)/calmodulin-dependent [correction of Ca(2+)/CaMKII-dependent] protein kinase II in neurons. J Neurosci. 2000;20:3076–3084. doi: 10.1523/JNEUROSCI.20-09-03076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Tao-Cheng JH, Vinade L, Winters CA, Pozzo-Miller L, Reese TS. Glutamate-induced transient modification of the postsynaptic density. Proc Natl Acad Sci U S A. 2001;98:10428–10432. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A, Vinade L, Winters CA, Reese TS, Tao-Cheng JH. Inhibition of phosphatase activity prolongs NMDA-induced modification of the postsynaptic density. J Neurocytol. 2002;31:605–612. doi: 10.1023/a:1025735410738. [DOI] [PubMed] [Google Scholar]
- Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Miyamoto E. Increased Phosphorylation of Ca2+/Calmodulin-Dependent Protein-Kinase-Ii and Its Endogenous Substrates in the Induction of Long-Term Potentiation. Journal of Biological Chemistry. 1995;270:6119–6124. doi: 10.1074/jbc.270.11.6119. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Schrama LH, van Dalen JJ, Gispen WH, Cattabeni F, Di Luca M. AlphaCaMKII binding to the C-terminal tail of NMDA receptor subunit NR2A and its modulation by autophosphorylation. FEBS Lett. 1999;456:394–398. doi: 10.1016/s0014-5793(99)00985-0. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Goebel SM, Alvestad RM, Coultrap SJ, Browning MD. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor is enhanced in synaptic membrane fractions of the adult rat hippocampus. Brain Res Mol Brain Res. 2005;142:65–79. doi: 10.1016/j.molbrainres.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Meyer T, Stryer L, Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Schworer CM, Colbran RJ, Soderling TR. Autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. Effects on total and Ca2+-independent activities and kinetic parameters. J Biol Chem. 1987;262:8051–8055. [PubMed] [Google Scholar]
- Ho OH, Delgado JY, O’Dell TJ. Phosphorylation of proteins involved in activity-dependent forms of synaptic plasticity is altered in hippocampal slices maintained in vitro. J Neurochem. 2004;91:1344–1357. doi: 10.1111/j.1471-4159.2004.02815.x. [DOI] [PubMed] [Google Scholar]
- Hoelz A, Nairn AC, Kuriyan J. Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol Cell. 2003;11:1241–1251. doi: 10.1016/s1097-2765(03)00171-0. [DOI] [PubMed] [Google Scholar]
- Hu BR, Kamme F, Wieloch T. Alterations of Ca2+/calmodulin-dependent protein kinase II and its messenger RNA in the rat hippocampus following normo- and hypothermic ischemia. Neuroscience. 1995;68:1003–1016. doi: 10.1016/0306-4522(95)00213-3. [DOI] [PubMed] [Google Scholar]
- Hu BR, Park M, Martone ME, Fischer WH, Ellisman MH, Zivin JA. Assembly of proteins to postsynaptic densities after transient cerebral ischemia. J Neurosci. 1998;18:625–633. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Aronowski J, Kolb SJ, Waxham MN. Inactivation and self-association of Ca2+/calmodulin-dependent protein kinase II during autophosphorylation. J Biol Chem. 1996;271:8800–8808. doi: 10.1074/jbc.271.15.8800. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, Decamilli P. Synapsin-I (Protein-I), A Nerve Terminal-Specific Phosphoprotein .3. Its Association with Synaptic Vesicles Studied in A Highly Purified Synaptic Vesicle Preparation. Journal of Cell Biology. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB, Bennett MK, Erondu NE. Biochemical and immunochemical evidence that the “major postsynaptic density protein” is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983;80:7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb SJ, Hudmon A, Waxham MN. Ca2+/calmodulin kinase II translocates in a hippocampal slice model of ischemia. J Neurochem. 1995;64:2147–2156. doi: 10.1046/j.1471-4159.1995.64052147.x. [DOI] [PubMed] [Google Scholar]
- Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains. J Biol Chem. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- Lengyel I, Cammarota M, Brent VA, Rostas JA. Autonomous activity and autophosphorylation of CAMPK-II in rat hippocampal slices: effects of tissue preparation. J Neurochem. 2001;76:149–154. doi: 10.1046/j.1471-4159.2001.00058.x. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Bayer KU, Merrill MA, Lim IA, Shea MA, Schulman H, Hell JW. Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and alpha-actinin. J Biol Chem. 2002;277:48441–48448. doi: 10.1074/jbc.M205164200. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Reviews Neuroscience. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CS, Hodge JJ, Mehren J, Sun XX, Griffith LC. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron. 2003;40:1185–1197. doi: 10.1016/s0896-6273(03)00786-4. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989;340:554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993;362:640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- Meng F, Guo J, Zhang Q, Song B, Zhang G. Autophosphorylated calcium/calmodulin-dependent protein kinase II alpha (CaMKII alpha) reversibly targets to and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in cerebral ischemia and reperfusion in hippocampus of rats. Brain Res. 2003;967:161–169. doi: 10.1016/s0006-8993(02)04267-1. [DOI] [PubMed] [Google Scholar]
- Meng F, Zhang G. Autophosphorylated calcium/calmodulin-dependent protein kinase II alpha induced by cerebral ischemia immediately targets and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in hippocampus of rats. Neurosci Lett. 2002;333:59–63. doi: 10.1016/s0304-3940(02)00961-8. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Miller SG, Patton BL, Kennedy MB. Sequences of autophosphorylation sites in neuronal type II CaM kinase that control Ca2(+)-independent activity. Neuron. 1988;1:593–604. doi: 10.1016/0896-6273(88)90109-2. [DOI] [PubMed] [Google Scholar]
- Mukherji S, Soderling TR. Regulation of Ca2+/calmodulin-dependent protein kinase II by inter- and intrasubunit-catalyzed autophosphorylations. J Biol Chem. 1994;269:13744–13747. [PubMed] [Google Scholar]
- Okazaki Y, Ohno H, Takase K, Ochiai T, Saito T. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J Biol Chem. 2000;275:35751–35758. doi: 10.1074/jbc.M007476200. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Kantor D, Harris KM, Schuman EM, Kennedy MB. Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J Neurosci. 1997;17:5416–5427. doi: 10.1523/JNEUROSCI.17-14-05416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990;265:11204–11212. [PubMed] [Google Scholar]
- Petersen JD, Chen X, Vinade L, Dosemeci A, Lisman JE, Reese TS. Distribution of postsynaptic density (PSD)-95 and Ca2+/calmodulin-dependent protein kinase II at the PSD. J Neurosci. 2003;23:11270–11278. doi: 10.1523/JNEUROSCI.23-35-11270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Perlman S, Malinow R. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science. 1994;266:1881–1885. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- Rich RC, Schulman H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1998;273:28424–28429. doi: 10.1074/jbc.273.43.28424. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Bartlett RK, Bass MA, Colbran RJ. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-D-aspartate receptor NR2B subunits and alpha-actinin. J Biol Chem. 2005;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Schwartz JH. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985;100:835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DA, Yeh RY, Zivin JA. Inactivation and subcellular redistribution of Ca2+/calmodulin-dependent protein kinase II following spinal cord ischemia. J Neurochem. 1993;61:738–747. doi: 10.1111/j.1471-4159.1993.tb02180.x. [DOI] [PubMed] [Google Scholar]
- Shamloo M, Kamme F, Wieloch T. Subcellular distribution and autophosphorylation of calcium/calmodulin-dependent protein kinase II-alpha in rat hippocampus in a model of ischemic tolerance. Neuroscience. 2000;96:665–674. doi: 10.1016/s0306-4522(99)00586-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nature Neuroscience. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Smith KE, Gibson ES, Dell’acqua ML. cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J Neurosci. 2006;26:2391–2402. doi: 10.1523/JNEUROSCI.3092-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997a;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. Journal of Biological Chemistry. 1997b;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. Journal of Biological Chemistry. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Strack S, Robison AJ, Bass MA, Colbran RJ. Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J Biol Chem. 2000;275:25061–25064. doi: 10.1074/jbc.C000319200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Okumura-Noji K, Tanaka R, Tada T. Rapid translocation of cytosolic Ca2+/calmodulin-dependent protein kinase II into postsynaptic density after decapitation. J Neurochem. 1994;63:1529–1537. doi: 10.1046/j.1471-4159.1994.63041529.x. [DOI] [PubMed] [Google Scholar]
- Tang K, Liu C, Kuluz J, Hu B. Alterations of CaMKII after hypoxia-ischemia during brain development. J Neurochem. 2004;91:429–437. doi: 10.1111/j.1471-4159.2004.02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Vinade L, Pozzo-Miller LD, Reese TS, Dosemeci A. Calcium/calmodulin-dependent protein kinase II clusters in adult rat hippocampal slices. Neuroscience. 2002;115:435–440. doi: 10.1016/s0306-4522(02)00451-7. [DOI] [PubMed] [Google Scholar]
- Yang E, Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1999;274:26199–26208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Yamauchi T. Phosphorylation-dependent reversible association of Ca2+/calmodulin-dependent protein kinase II with the postsynaptic densities. J Biol Chem. 1997;272:26354–26359. doi: 10.1074/jbc.272.42.26354. [DOI] [PubMed] [Google Scholar]
- Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]