Abstract
Recombinant and non-recombinant modified vaccinia virus Ankara (MVA) strains are currently in clinical trials as human immunodeficiency virus-1 (HIV) and attenuated smallpox vaccines, respectively. Here we tested the ability of a recombinant MVA delivered by alternative needle-free routes (intramuscular, intradermal, or into the palatine tonsil) to protect against immunodeficiency and orthopoxvirus diseases in a non-human primate model. Rhesus macaques were immunized twice one month apart with MVA expressing 5 genes from a pathogenic simian human immunodeficiency virus (SHIV)/89.6P and challenged intrarectally 9 months later with the pathogenic SHIV/89.6P and intravenously 2.7 years later with monkeypox virus. Irrespective of the route of vaccine delivery, binding and neutralizing antibodies and CD8 responses to SHIV and orthopoxvirus proteins were induced and the monkeys were successively protected against the diseases caused by the challenge viruses in unimmunized controls as determined by viral loads and clinical signs. These non-human primate studies support the clinical testing of recombinant MVA as an HIV vaccine and further demonstrate that MVA can provide long term poxvirus immunity, essential for use as an alternative smallpox vaccine.
Keywords: SHIV/89.6P, recombinant modified vaccinia virus Ankara, monkeypox virus, smallpox, rhesus macaque
Introduction
The development of safe and effective vaccines against existing and emerging pathogens is a major focus of medical research. Since the onset of the AIDS epidemic more than two decades ago, enormous efforts have been directed to making a vaccine that will protect against human immunodeficiency virus-1 (HIV); an effective vaccine is thought to require the induction of cellular and humoral responses (Douek et al., 2006; Letvin, 2005; Mascola, 2003). Vaccine candidates have included a variety of HIV immunogens delivered as DNA (Barouch et al., 2001a; Otten et al., 2005; Rao et al., 2006), attenuated poxviruses (Franchini et al., 2004; Ourmanov et al., 2000; Van Rompay et al., 2005), adenoviruses (Barouch et al., 2004; Peng et al., 2005; Shiver et al., 2002), vesicular stomatitis virus (Egan et al., 2004), proteins (Earl et al., 2001; Xu et al., 2006), and various combinations thereof (Hu, 2005). Our efforts to design an HIV vaccine have focused on modified vaccinia virus Ankara (MVA) as a vector (Moss, 1996). During repeated passages in chick embryo fibroblasts (Mayr et al., 1975b), MVA acquired several large deletions and small mutations including the loss of immune defense genes restricting efficient replication to chick embryo and baby hamster kidney cells (Antoine et al., 1998; Blanchard et al., 1998; Carroll and Moss, 1997; Drexler et al., 1998; Meyer et al., 1991). MVA was demonstrated to be safe in immunocompromised mice and monkeys and tested without incident in humans (Mayr et al., 1975a; Stickl et al., 1974; Stittelaar et al., 2001; Wyatt et al., 2004). Because the host restriction involves a late block in morphogenesis, foreign genes can be expressed at high levels by use of an appropriate viral promoter (Carroll and Moss, 1997) (Sutter and Moss, 1992). Furthermore, recombinant MVA (rMVA) induces strong cellular and humoral immunity in animal models and can be administered by numerous routes (Sutter et al., 1994).
In the present study we evaluated an rMVA, expressing HIV (HIV 89.6P Env and Tat) and SIV (SIVmac239 Gag, Pol and Nef) proteins, administered by three alternative routes. Rather than traditional needle delivery systems for intramuscular (IM) and intradermal (ID) inoculations, we used the needle-free Biojector, which has been reported to enhance immunogenicity of a DNA vaccine in humans (Wang et al., 2001). In addition, a study in mice demonstrating immunogenicity of a DNA vaccine injected into the inguinal lymph nodes (Maloy et al., 2001) prompted us to test this concept by direct delivery of rMVA into organized lymphoid tissue of the palatine tonsils (PT) via the Syrijet Mark II, a device used for subtopical delivery of anaesthesia in pediatric dentistry. Humoral and cellular immune responses were determined and protection against a SHIV/89.6P (Reimann et al., 1996) challenge was demonstrated after only two closely spaced rMVA injections without a DNA prime.
Recently, there has been increased interest in smallpox vaccines because of perceived terrorists threats (Henderson, 1999). Variola virus, the causative agent of smallpox, is closely related to vaccinia virus (VACV), which was successfully used to eradicate smallpox (Fenner et al., 1988). However, live VACV may be unsafe for a large segment of the world population (Enserink, 2002; Fulginiti et al., 2003) renewing interest in attenuated vaccine strains such as MVA, which had never been tested for efficacy. Monkeypox virus (MPXV), which causes a lethal human disease in parts of Africa, is closely related to both variola and VACV and has been used as an animal model for smallpox (Zaucha et al., 2001) (LeDuc et al., 2002). Recent studies showed that two immunizations with MVA protected monkeys against a lethal challenge of MPXV (Earl et al., 2004; Stittelaar et al., 2005), although the duration of immunity was not determined. Because the monkeys in the present study had maintained normal CD4 counts and appeared healthy despite the SHIV infection, we decided to challenge them with MPXV at more than two and a half years after vaccination with rMVA in order to test the longevity of poxvirus immunity. The results indicate that durable protection against monkeypox, and by analogy smallpox, can be achieved by MVA and would also be a collateral effect of a recombinant MVA vaccine.
Results
Construction and characterization of MVA/KB9-5
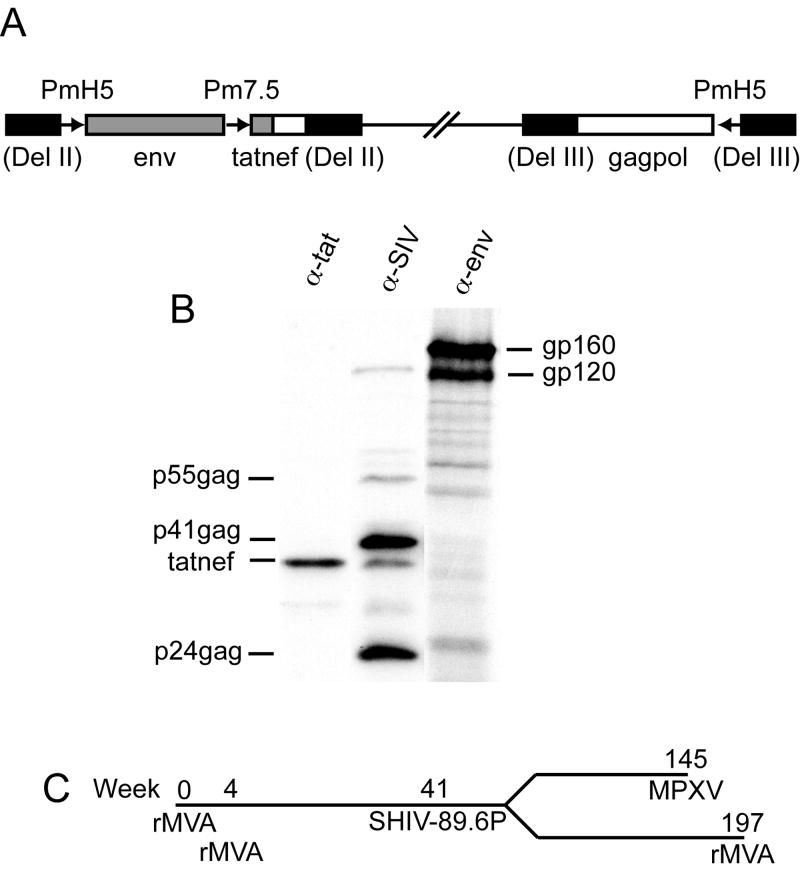
The products of the env and gag genes are the primary immunogens in most HIV vaccines. However, since regulatory proteins are also targets of cytotoxic T cells (CTL), we included a tatnef chimeric gene as well as env and gagpol genes corresponding to those of the clonal isolate SHIV/KB9 derived from the pathogenic SHIV/89.6P (Karlsson et al., 1997) in a new rMVA construct called MVA/KB9-5 (Fig. 1A). Expression of Env, Gag and Tatnef proteins was analyzed by immunoprecipitation. The Env precursor gp160 was produced and partially cleaved to gp120 (Fig. 1B). In addition, the Env protein was shown to be biologically functional in a fusion assay with cells expressing CD4 and CCR5 or CXCR4 (not shown). Gag precursor p55 as well as cleavage products p41 and p24 were also made (Fig. 1B) and assembled into particles as demonstrated by sedimentation of the medium of infected cells through a 20% sucrose cushion (not shown). Tatnef was immunoprecipitated by both anti-tat antibody and serum from a SIV-infected monkey and migrated at the predicted mass of 40.5 kDa (Fig. 1B). Localization of Tatnef in the cytoplasm of infected cells was demonstrated by immunofluorescence (not shown).
Fig. 1.
Structure of MVA/KB9-5, expression of SHIV genes, and study protocol. (A) Env and tat from SHIV/KB9 (gray boxes) and gagpol and nef from SIVmac239 (white boxes) were inserted into deletions II and III (black boxes) of MVA to form MVA/KB9-5. Chimeric Tatnef protein was expressed under control of the modified P7.5 promoter; Env and Gagpol were expressed under control of the modified H5 promoter. (B) SHIV antigens were immunoprecipitated from cells infected with MVA/KB9-5 with: monoclonal antibody T8 (α-env), SIV polyclonal serum E544 (α-SIV), and HIV-1BH10 Tat antiserum (α-tat). Precursor and cleavage products for Env and Gag are shown. The Tatnef protein was precipitated by both Tat and Nef (E544) antibodies. (C) Study timeline.
Study design
The study timeline is shown in Fig. 1C. Thirty rhesus macaques were immunized with 2 × 108 infectious units of either MVA/KB9-5 or non-recombinant MVA at weeks 0 and 4 using needle-free methods of administration. The Biojector was used for IM and ID immunizations and the Syrijet Mark II for delivery into the PT. To minimize variability, all MVA doses were delivered into two sites on each animal i.e. into both legs or both PTs. Eight monkeys were placed in each of the ID and IM groups; seven were placed in each of the PT and control groups. Control animals were subdivided such that three received non-recombinant MVA via the IM or ID route and two received it in the PT. Macaques used in the study were pre-screened and 15 MamuA*01+ animals were selected to allow analysis of CD8+ T cell responses to the immunodominant gag epitope CM9. Four MamuA*01+ animals were assigned to each of the MVA/KB9-5 groups and three to the control MVA group. The SHIV challenge was intrarectal, as the mucosal route mimics the predominant method of human HIV transmission, and was performed nine months after immunization to allow immune responses to recede into memory.
At two years after SHIV challenge, all but one of the vaccinated animals was alive: plasma SHIV RNA was undetectable and CD4 cell counts were in the normal range. At this time, we assessed the ability of the memory immune responses in these SHIV-infected monkeys to protect against MPXV. Thus, six monkeys (two from each immunization group) were challenged intravenously with a potentially lethal dose of MPXV. To determine the ability of rMVA to boost immune responses in SHIV-infected animals, the 16 remaining monkeys were re-immunized with MVA/KB9-5 more than three years after the initial immunizations.
Vaccine-induced SHIV antibody responses
Env binding and SHIV/89.6P neutralizing antibody titers were measured after MVA/KB9-5 immunization. All animals developed similar levels of KB9 gp140-binding antibodies (Fig. 2A), with those immunized in the PT having significantly higher values than others only at week 6 (p<0.006 for comparisons with IM and ID). In a previous study (Earl et al., 2002) employing IM needle delivery of an rMVA that expresses the 89.6 env, the binding antibody titers were significantly lower after two immunizations than those reported here, with an average reciprocal titer of 4,500 (p<0.025 for comparison of two IM delivery modes). Neutralizing antibody to SHIV/89.6P was elicited in 2, 4, and 3 of the animals in the PT, ID, and IM groups, respectively with reciprocal titers ranging from 29 to 262 (Fig. 2B). In addition, several of these animals also had low neutralizing activity against the partially heterologous SHIV/89.6, with reciprocal titers ranging from 32 to 71. In contrast, no neutralizing activity was previously found after two IM immunizations using needle delivery (Earl et al., 2002). Because the two delivery systems were not evaluated concurrently, further evaluation of the Biojector for IM delivery of MVA is warranted.
Fig. 2.
Temporal immune responses induced by vaccination with MVA/KB9-5 and challenge with SHIV/89.6P. (A) Serum antibody endpoint titers were measured by ELISA with KB9 gp140 captured via antibody to the C-terminus of gp120. Average titers for each immunization group are shown with standard errors. (B) SHIV/89.6P neutralizing antibody titers were determined in an MT-2 cell-killing assay. Titers are the reciprocal of the dilution giving 50% neutralization; averages for each group are shown with standard errors. (C) The percent of CD8 T-cells that were positive for the gag CM9 peptide was measured by tetramer binding in the MamuA*01+ animals; averages for each group are shown. (D) Frequencies of Gag, Env, Tat and Nef specific cells was measured by IFN-γ ELISpots. Fresh PBMC were stimulated for 36 h with pools of approximately 10 peptides. Averages for each group are shown as follows: PT, white bars; ID, light gray bars; IM, dark gray bars; naïve (unimmunized) black bars. Arrowheads indicate times of MVA immunization; stars indicate day of challenge with SHIV/89.6P.
Vaccine-induced SHIV T cell responses
T cell responses to the immunodominant Gag epitope CM9 were measured by tetramer staining in the MamuA*01+ animals (Altman et al., 1996) (Fig. 2C). All MVA/KB9-5-immunized animals had detectable responses after a single immunization (0.31 to 1.34% tetramer +/CD8+ cells), which were boosted in most animals after the second immunization (0.29 to 2.87% tetramer+/CD8+ cells). The responses appeared sooner in the PT group than in the others with averages at one week after immunization of 0.53, 0.17 and 0.18% tetramer+/CD8+ cells in the PT, ID, and IM groups, respectively. However, by the second week and at every time point thereafter, differences between groups were not observed.
T cell responses to the entire Gag, Env and Tatnef proteins were measured in an ELISpot assay using pools of overlapping peptides (Fig 2D). Gag and Env-specific T cells were generated by a single MVA/KB9-5 immunization and were somewhat lower on average after the second immunization. Gag responses ranged from none to 468 and none to 595 spots per million cells (weeks 1 and 5, respectively) although every animal had a measurable response at a minimum of one time point. At week 5, Gag responses were significantly higher in the PT as compared to the IM group (p=0.034). Env responses were found in every animal at both time points with values ranging from 273 to 2293 and 45 to 1553 spots per million cells at weeks 1 and 5, respectively. Although values were higher in the PT group the differences were not statistically significant. ELISpot responses to the immunodominant Gag epitope CM9 were also measured (not shown). These followed the same pattern found with CM9 tetramer staining, i.e. they were higher after the second immunization than after the first. The frequencies of Tat- and Nef-specific T cells was low with only half of the animals having any response at either time point.
SHIV/89.6P challenge
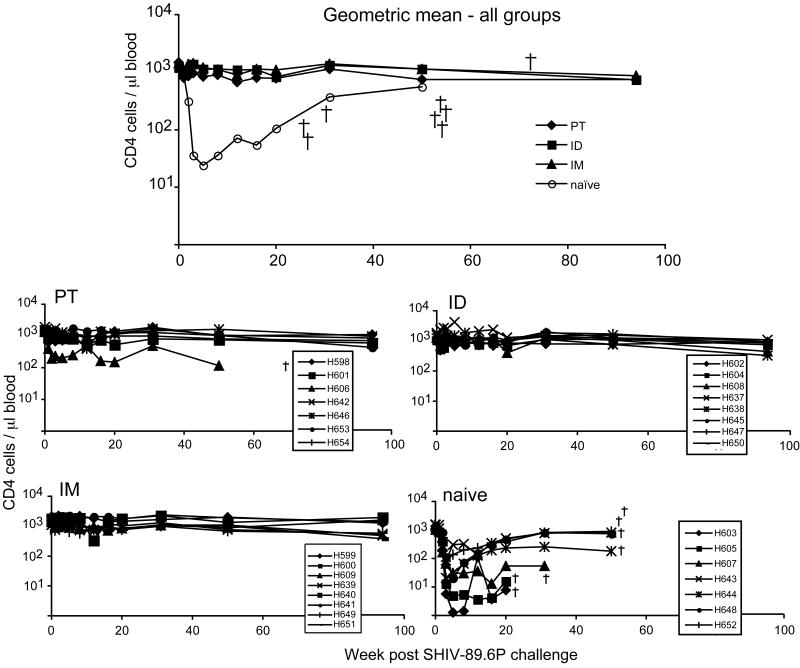
Nine months after the second rMVA immunization, the animals were challenged intrarectally with 20 infectious units of SHIV/89.6P (1.2 × 1010 copies of SHIV/89.6P RNA) as described (Amara et al., 2001). All of the control and all but one of the vaccinated animals (Rh641 in the IM group) became infected as judged by increased humoral and cellular responses to Env and Gag (Fig. 2). Control animals exhibited a rapid and profound loss of CD4 T cells within three weeks after challenge (Fig 3) and died between 8 and 13 months. In contrast, all but one of the vaccinated animals remained healthy for more than two additional years. An outlier Rh606 in the PT group became ill and died at month 17 after challenge. CD4 T cell counts were significantly lower in controls than in vaccinated groups between weeks 3 and 20 (p<0.0019).
Fig. 3.
CD4 T cell counts after challenge with SHIV/89.6P. CD4 cell counts of individual animals are shown in the four lower panels with animal deaths indicated by a cross (all naïve, one P.T.-immunized animal). The geometric mean for all groups is shown in the top panel.
At peak viremia, two weeks after challenge, the geometric mean RNA viral load was greater than one log lower in the immunized groups than in the control group (p=0.0339, 0.0017, and 0.0175 for PT, IM, ID, respectively) (Fig. 4). By eight weeks, the geometric mean titers in the immunized groups were greater than two logs lower than in the control group (p<0.006) but there were no significant difference between any of the vaccine groups.
Fig. 4.
SHIV viral load. The number of copies of SHIV RNA per ml of plasma was determined by quantitative PCR (Cline et al., 2005). Data from individual animals are shown in the four lower panels; geometric means for all groups are shown in the top panel.
SHIV/89.6P-induced immune responses
Rapid and strong anamnestic Env binding and SHIV neutralizing responses occurred in the vaccinated animals after challenge (Fig 2A, B). The average reciprocal Env ELISA titer for each vaccine group was greater than 165,000 and 970,000 at weeks two and three, respectively, and was 1,368,000 at week 196. This long-lasting response was likely due to persistent low level SHIV/89.6P infection. SHIV/89.6P neutralizing antibody titers also rose dramatically after challenge with average reciprocal titers greater than 1,700 and 6,500 at weeks two and three, respectively. In contrast, neither Env binding nor SHIV neutralizing antibodies were detected in any of the control animals until week eight.
T cell responses were also boosted rapidly in vaccinated animals after SHIV challenge (Fig 2C,D). Tetramer binding increased from an average of 0.15% to 3.6% tetramer +/CD8+ cells by two weeks after challenge in vaccinated animals. Average Gag and Env ELISpots increased from 31 to 799 and from 118 to 1004 spots per million cells, respectively, by two weeks after challenge.
At 3.7 years after vaccination, sixteen of the animals were boosted with MVA/KB9-5 by the same route as their initial immunizations. There was no increase in HIV Env binding titers, likely reflecting the persistence of antibody at high levels as noted above. However, there was a 2-fold (p=0.309) increase in tetramer and 3.3-fold (p=0.0025) increase in Gag ELISPOT values after boosting (not shown).
VACV immune responses
Immune responses to both VACV MV and EV were determined in several assays shown in Fig 5. For the VACV binding and neutralizing analyses, the pre-challenge data from animals immunized with MVA and MVA/KB9-5 were combined, as they were similar. Titers for binding and neutralizing antibodies to VACV (Fig 5A, D) were higher in the ID and IM groups than they were in the PT group (p<0.041 for binding and ≤0.0031 for neutralizing antibodies) at week six and thereafter. Antibody titers to the MV protein L1 (Fig 5B) and the EV protein B5 (Fig 5C) were also higher in the IM and ID groups than in the PT group. Antibodies to the EV form of VACV were determined by two methods: a plaque reduction assay that directly measures neutralization of EV (Fig 5E) and a comet reduction assay that measures inhibition of satellite plaque formation (Fig 5F). Strong EV neutralizing activity, comparable to that of VACV immune globulin, was found in all animals at week six. Although this activity declined by week 134, it was still readily demonstrable in all animals. Similarly, comet reduction was highest at 6 weeks.
Fig. 5.
Temporal VACV antibody responses. (A, B, C) Serum antibody titers were determined by ELISA against purified VACV MV particles, purified VACV L1 protein, and purified VACV B5 protein. Average titers with standard errors are shown for animals immunized by each route: PT, diamond; ID, square, IM, triangle. Times of MVA immunization are shown with arrowheads; star indicates day of challenge with SHIV/89.6P. (D) Neutralizing antibody titers were determined using a flow cytometric assay that measures reduction in infectivity of a VACV expressing EGFP (Earl et al., 2003). Symbols as above. (E) Percent neutralization of VACV EV was determined in the presence of excess L1 Mab 7D11. Serum samples from individual animals were assayed and the averages for animals in each group are indicated as: PT, light gray; IM, medium gray; ID, black. Vaccinia virus immune globulin (VIG) was used as a positive control. (F) A comet reduction assay was performed using VACV strain IHD-J. Serum samples from all animals were assayed and one representative example from each immunization group is shown.
To examine the boosting effect of rMVA after a prolonged period of time, the remaining sixteen animals were re-immunized 3.7 years after the initial immunization. VACV MV binding and neutralizing titers which had slowly declined as shown in Fig. 5 were enhanced by this third immunization to levels equivalent to that elicited immediately after the second immunization (not shown).
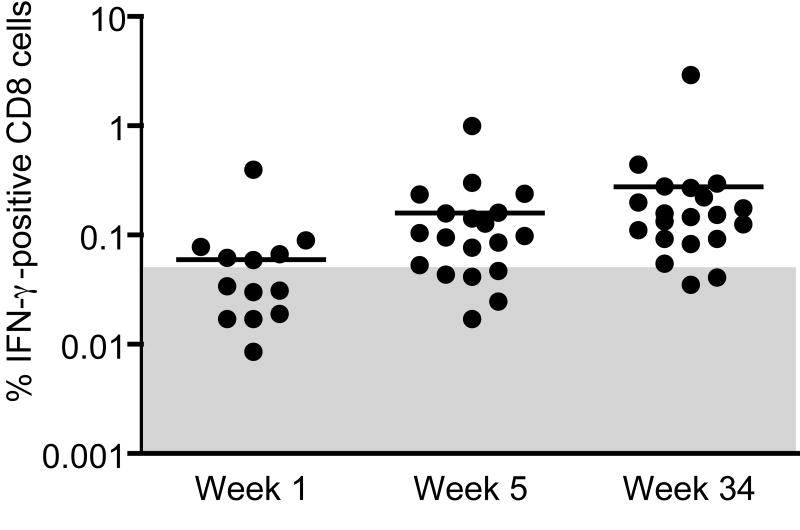
VACV-specific interferon (IFN)-γ producing CD8 cells were enumerated using cryopreserved cells obtained one week after the first (week 1) and second (week 5) rMVA immunization as well as from a later time point (week 34) when responses had receded into memory. Positive responses were detected in 6/15, 13/20, and 18/20 animals at weeks 1, 5, and 34, respectively (Fig 6). No differences were seen between the three routes of immunization. Although higher responses would be expected immediately after immunization, we found the highest percent of IFN-γ producing cells at the later time point. This may be explained by the observation (R. R. A., unpublished) that cells in memory are more efficiently revived after freezing.
Fig. 6.
INF-γ producing MVA-specific CD8 cells. Quantitation of VACV-specific IFN- γ producing cells was performed on cryopreserved PBMC after infection with MVA overnight. Each dot represents one animal with positive values (>2-fold background) located above the shaded area. The average for each time point is denoted by a line.
MPXV challenge
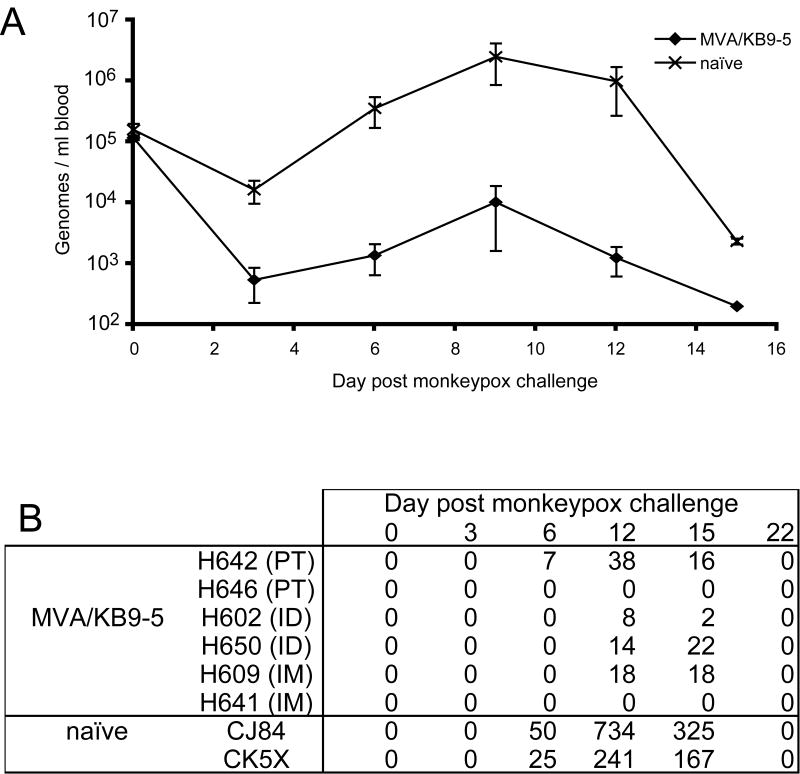
Two and a half years after the second MVA/KB9-5 immunization, six of the monkeys (two from each immunization group) were challenged intravenously with 5 × 107 infectious units of MPXV to determine the long-term protective effect of immunization with rMVA. At this time the VACV MV binding antibody titers had declined to less than 1:6400 and neutralizing titers to less than 1:563. Two naïve rhesus monkeys were added to the study as negative controls. MPXV load and appearance of lesions were monitored every three days and are shown in Fig 7A and B, respectively. The peak viral load was lower by 2.5 logs in the immunized as compared to the naïve animals. The difference between the two groups was statistically significant from day 3 through day 12 (p<0.0054). Also, the naïve monkeys developed large numbers of lesions and became seriously ill. As is typical in rhesus macaques, the lesions resolved by day 22 after infection. In contrast, the immunized monkeys developed few or no lesions that were small and atypical in appearance and resolved quickly. Most importantly, the vaccinated animals showed no clinical signs of illness. In the vaccinated animals, no correlations were found between the magnitude of the pre-challenge immune responses and the post-challenge viral load or numbers of lesions.
Fig. 7.
MPXV challenge. (A) Following challenge with MPXV, the number of MPXV genomes per ml of blood was determined by quantitative TacMan PCR. Average values for MVA-immunized and naïve groups are shown. (B) The number of lesions induced by MPXV challenge was enumerated every 3 days for 2 weeks after challenge. The number of lesions found on each animal is shown.
Discussion
The major conclusions of this study are that only two vaccinations with a rMVA administered by any of three routes provides durable protection against disease caused by a pathogenic SHIV as well as long term immunity to a severe MPXV challenge. Previous studies showed that repeated immunizations with rMVAs expressing HIV and/or SIV proteins, either alone or as a boost to a DNA vaccine, induce immune responses that partially protect against SHIV or SIV challenge (Amara et al., 2001; Amara et al., 2002b; Barouch et al., 2001b; Earl et al., 2002; Ourmanov et al., 2000; Van Rompay et al., 2005). In anticipation of Phase I clinical trials utilizing rMVA viruses developed in our laboratory, we evaluated a simplified vaccine strategy consisting of only two immunizations given within a one month period. In addition, we compared various routes of immunization. Although the SHIV/89.6P model does not precisely mimic the course of HIV in humans, it does allow the use of HIV env and other genes in a pathogenic non-human primate model. Thus our rMVA contained HIV-1 env and tat genes in addition to SIV gag, pol and nef genes. Strong early/late VACV promoters were used to drive high level expression of native Env, Gag and Pol and a chimeric fused form of Tat and Nef.
We determined that two immunizations with rMVA, given one month apart, were sufficient to protect monkeys against disease induced by a lethal dose of SHIV/89.6P, and that similar protection was achieved irrespective of the IM, ID, or PT route of immunization. In contrast, control animals rapidly lost CD4 T cells, succumbed to disease characterized by low CD4 count and were euthanized within 8-13 weeks after challenge. The peak and setpoint RNA loads in the vaccinated animals were significantly lower than that in naïve controls. Additionally, the protected animals maintained normal CD4 T cell levels, good health, and undetectable viral RNA for up to 3.5 years after challenge. A similar level of protection against intrarectal challenge with SHIV/89.6P was reported in several papers by Amara et al. (Amara et al., 2001; Amara et al., 2002b). In those reports, animals received either an rDNA prime/rMVA boost or three rMVA immunizations. Interestingly, although the rDNA/rMVA protocol induced much higher levels of Gag-specific T cells than did the rMVA protocol, the protection was indistinguishable. Also, the third rMVA immunization resulted in only a minor increase in Gag-specific T cells. Here we showed that Gag- as well as Env-specific T cell responses were higher after one than after two rMVA immunizations. Nevertheless, antibody titers as well as CM9 tetramer responses were boosted after the second immunization. It would be interesting to challenge monkeys after a single rMVA immunization to determine if there is protective immunity at that time.
Although the route of immunization did not affect the degree of protection against SHIV/89.6P, we did observe small differences in immune responses. Gag and Env ELISpot and Env binding antibody titers were higher in the PT-immunized group, but the differences were not always significant. Two individual animals were outliers in the study: animal Rh606 in the PT group who succumbed to disease and animal Rh641 in the IM group who resisted infection. However, in comparison to the other animals in the study, neither of the outliers had noticeably different immune responses that could account for their fates. Although a correlation between Gag-specific T cell responses and levels of SHIV RNA in individual animals after challenge has been reported (Barouch et al., 2001b), no direct correlation between the magnitude of any of the immune responses measured and the level of SHIV RNA were found here.
We used the Biojector for IM and ID delivery of MVA because it was reported to generate higher immune responses (Wang et al., 2001), while avoiding the use of needles. Although we did not perform a direct comparison, we have previous experience using a needle for IM delivery of a related rMVA (Earl et al., 2002). In comparing the results of the two studies, there was significantly higher Env binding antibody to the respective matched gp140 proteins and some neutralizing antibody responses to SHIVs after only two immunizations with the Biojector, possibly due to wider distribution of the virus at the site of inoculation. However, a direct comparison of the two inoculation methods is needed to confirm this impression.
Our previous non-human primate studies utilized a rMVA that contained only env and gagpol immunodeficiency virus genes (Earl et al., 2002) (Amara et al., 2002a). To potentially increase immunity to the challenge virus and to examine immune responses to accessory proteins, we included tat and nef sequences in the new rMVA. Both Tat and Nef are expressed early after infection with HIV and induce CTL (Addo et al., 2001; Sriwanthana et al., 2001). In addition, immune responses to vaccines containing tat and nef have been obtained (Cafaro et al., 2001) (Hel et al., 2002) (Richardson et al., 2002) and there is a reported that immunization with tat alone can be protective (Cafaro et al., 2001). However, in the present study only a few animals developed ELISpot responses to Tat and Nef and these were low and sporadic. The fact that responses were high after challenge indicates that the earlier low results were not artifactual false negative results due to the assay. Although we demonstrated good expression of the chimeric protein in tissue culture experiments, evidently it was not very immunogenic.
Development of a smallpox vaccine with an improved safety profile over the licensed Dryvax vaccine is currently a priority (Fulginiti et al., 2003). Because MVA was shown to be safe in immune compromised non-human primates (Stittelaar et al., 2001) and was tested without apparent adverse effects in over 120,000 people (Mayr et al., 1975a) (Stickl et al., 1974), it is being considered as an alternative smallpox vaccine. However, since smallpox has been eradicated, the efficacy of MVA cannot be tested in humans. Instead, protection must be demonstrated in suitable animal models. Toward this end, we and others showed that two immunizations of non-recombinant MVA strongly protected cynomolgous monkeys against a lethal dose of MPXV administered shortly after vaccination (Earl et al., 2004) (Stittelaar et al., 2005). Here we evaluated the long-term immunity induced by a similar vaccination protocol in rhesus macaques. While the VACV antibodies declined in the monkeys during the 2.7-year period between vaccination and challenge, sufficient memory remained to provide solid protection against the MPXV challenge. Additionally, these animals resisted MPXV challenge in the face of SHIV infection that had been controlled for more than two years. That these monkeys had normal CD4 T cell counts was likely crucial, as in another study, SHIV-infected monkeys with low CD4 T cell counts succumbed readily to MPXV challenge (Edghill-Smith et al., 2005). Nigam and coworkers (manuscript submitted) have also found long-lived protective immunity to MPXV in rhesus macaques that were vaccinated twice with another rMVA but not challenged with SHIV.
In addition, we re-immunized 16 animals with MVA/KB9-5 at three years after their initial immunization to determine the efficiency of boosting after a prolonged period of rest. The HIV Env antibody titers were high at the time of re-immunization, probably due to persistent low level infection with SHIV/89.6P, and no increase in titer was observed. Nevertheless, there was more than a 2-fold increase in Gag ELISpot and tetramer values after boosting. In contrast, VACV does not cause a persistent infection and the VACV MV ELISA and neutralizing titers increased by more than one log within one week of boosting, showing that a rapid anamnestic response could be elicited.
In conclusion, we described a simplified vaccination schedule in which a rMVA protected against disease following challenge with a pathogenic SHIV 9 months later and a MPXV challenge 2.7 years later. These results indicate that immunity to orthopoxviruses may be a collateral benefit of an MVA HIV vaccine.
Materials and methods
Cells and viruses
BS-C-1 cells (ATCC CCL-26) and RK13 cells (ATCC CCL-37) were maintained at 37°C in modified Eagle’s minimal essential medium (EMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Hyclone, Logan, UT), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 10 U/ml penincillin and 10 μg/ml streptomycin (Invitrogen). HeLa S3 cells (ATCC-CCL-2.2) were maintained in suspension in MEM for spinner cells (Quality Biologicals, Inc., Gaithersburg, MD) containing 5% heat-inactivated equine serum (Hyclone).
VACV strain Western Reserve (WR) (ATCC VR-1354), VV-NP-S-EGFP (Anton et al., 1999; Norbury et al., 2002) and IHD-J (S. Dales, Rockefeller University, NY), were grown in HeLa S3 cells and purified by sucrose density centrifugation. MPXV strain Zaire 79 (V-79-I-005) isolated from the scab of an infected human by incubation in LLC-MK2 cells and then passaged twice in BS-C-40 cells was obtained from J. Esposito (Centers for Disease Control, Atlanta) and propagated in MA-104 cells. A titered clarified lysate was used for the MPXV challenge.
Construction of MVA/KB9-5
Recombinant virus, MVA/KB9-5, expressing 5 genes corresponding to those in SHIV/89.6P was constructed. An MVA shuttle plasmid containing env, and a chimeric tatnef was contructed in 3 steps. Step 1: The env gene, originating from pSHIV/KB9 3’end (J. Sodroski, AIDS Repository) (Karlsson et al., 1997) was cloned into pLW17 and two modifications were made. First, the cytoplasmic tail was truncated by 393 nucleotides, generating the amino acid sequence EEGERD at the C-terminus. Second, a silent mutation was made at nucleotide 1149 (T to C) (Quik Change kit, Stratagene) to eliminate a naturally occurring VACV early transcription termination signal (Earl et al., 1990). The resulting plasmid was called pLW-KB9env. Step 2: A chimeric tatnef gene was constructed as follows: the tat gene was generated by ligation of 12 overlapping synthetic oligonucleotides followed by PCR amplification. The sequence used was from pSHIV/KB9 3’end with the following changes: The nuclear localization signal, RKKRR, was not included and codons overlapping with the env gene were changed to prevent potential recombination between env and tat genes in the recombinant MVA. The SIVmac239 nef gene was amplified from pSHIV KB9 3’end such that the newly generated 5’ end overlapped with the 3’ end of the tat gene. The tat and nef DNA products were mixed and amplified using oligonucleotides designed to generate a chimeric tatnef gene with BamHI and KpnI at the 5’ and 3’ ends, respectively. Step 3: A DNA fragment containing the modified p7.5 promoter was amplified from pLW40 (L.S.W. and B. M., unpublished) to generate a fragment containing KpnI and BamHI at the 5’ and 3’ ends, respectively. Finally, tatnef and promoter fragments were ligated simultaneously into pLW17-KB9env that had been linearized with KpnI (5’end) and BamHI (3’end) to generate pLW-KB9env/tatnef. pLW17-KB9env/tatnef was inserted into deletion II of vJH4, a virus that contains the SIVmac239 gagpol gene in deletion III, by homologous recombination using standard techniques. Sequences of the chimeric tatnef gene and of oligonucleotides used in cloning will be provided upon request.
Immunoprecipitation of viral antigens
Infection, metabolic labeling, and immunoprecipitation were performed as previously described (Sugiura et al., 1999). Briefly, BS-C-1 cells were infected with 5 pfu/cell of rMVA. Five hours post-infection, cells were washed and overlaid with methionine-free EMEM containing 5% dialyzed FBS and 100 μCi of [35S]methionine (New England Nuclear, Boston, MA) per ml. After 20 h, cells were lysed in buffer containing 100 mM Tris-HCl, pH 8.0, 100 mM NaCl, and 0.5% Triton X-100. Lysates were clarified by centrifugation for 10 min at top speed in a microcentrifuge and subjected to immunoprecipitation. Antibodies used were: T8 monoclonal antibody to gp120 (P.L.E. and B.M., unpublished), serum E544 from a SIV-infected monkey (gift of V. Hirsch) or HIV-1 BH10 Tat antiserum (AIDS Repository, cat#705). Immunoprecipitated proteins were separated by 10% polyacrylamide SDS electrophoresis and visualized by autoradiography.
Immunization and challenge of rhesus macaques
The study utilized a total of 30 monkeys (Macaca mulatta) from Morgan Island, SC, fifteen of which were MamuA*01, as determined by the Wisconsin Regional Primate Research Center MHC Typing Core. Immunization and SHIV challenge were conducted at Bioqual, Inc., Rockville, MD, an AAALAC-International accredited facility, in accordance with NIAID animal care and use protocols. Three needle-free routes of immunization were employed for MVA administration. Each dose was administered at two sites for a total amount of 2 × 108 infectious units. A Syrijet Mark II apparatus (Mizzy, Inc., Cherry Hill, NJ) was used for immunization directly into the PT in a volume of 50 μl per site. IM and ID immunizations were performed using a Biojector (Bioject Medical Technologies, Inc., Portland, OR), delivering 0.5 ml and 0.1 ml per site, respectively. Control animals received non-recombinant MVA via the same routes (3 animals ID, 2 animals IM, 2 animals in PT). Immunizations were performed at weeks 0 and 4 in all animals. The IM and ID groups contained 8 animals each; the PT and control groups contained 7 animals each. Vaccine and control groups were assigned four or three MamuA*01 animals, respectively. All monkeys were challenged with 20 intrarectal infectious units of the pathogenic SHIV/89.6P by the intrarectal route at week 41.
KB9 env ELISA
Immulon-2-HB 96-well microtiter plates (Thermo Labsystems, Franklin, MA) were coated overnight at 4°C with sheep antibody to the C-terminus of gp120 (Cliniqa Corporation, Fallbrook, CA) at 2.2μg/ml in CB1 bicarbonate buffer (Immunochemistry Technologies, Bloomington, MN). KB9gp140, purified from the medium of BS-C-1 cells infected with MVA/KB9gp140 (P.L.E. and B.M., unpublished), was diluted in blocking buffer (PBS containing 5% non-fat dry milk and 0.2%Tween-20) to a concentration of 0.6 μg/ml and captured for 2 h at room temperature. Plates were incubated with blocking buffer for 1 h at room temperature. Two-fold serial dilutions of monkey sera were incubated for 2 h at room temperature followed by horseradish peroxidase-conjugated anti-monkey IgG (Accurate Chemical and Scientific Corp, Westbury, NY) for 1 h at 37°C and then 3,3’,5,5’-tetramethylbenzidine (BM Blue, POD substrate Roche Molecular Biochemicals) for 30 min at room temperature. Absorbency was measured at 370 and 492 nm.
Neutralization of SHIV/89.6P
Neutralization was measured in an MT-2 cell assay as described previously (Crawford et al., 1999). Virus stocks were produced in human peripheral blood mononuclear cells (PBMC). Titers of neutralizing antibodies are presented as the reciprocal of the serum dilution that reduced virus-induced cell killing by 50% as measured by neutral red dye uptake.
Tetramer staining
Approximately 106 fresh PBMC were surface stained with PE-conjugated CM9-MamuA*01 tetramer (AIDS Tetramer Facility) (Altman et al., 1996) for 30 min at 4°C followed by anti-CD3 FITC (Becton Dickinson, San Jose, CA) and anti-CD8 APC (Becton Dickinson) for 30 min at 4°C. After washing with PBS, cells were fixed with 2% paraformaldehyde and analyzed with a FACSCalibur (Becton Dickinson) using Cell Quest and FlowJo software (Treestar, Inc., Ashland, OR).
IFN-γ ELISpot
96-well Multiscreen IP plates (Millipore Inc., Bedford MA) were briefly soaked with 100 μl of 70% ethanol and then rinsed 3X with deionized water. Plates were coated with 50 μl of GZ-4 anti-human IFN-γ (Mabtech, Mariemont, OH) at 5 μg/ml in 1X Dulbecco’s phosphate buffered saline (DPBS) without Ca+ or Mg+ for 2 h at 37°C. Plates were washed 3X with DPBS and blocked with 150 μl Roswell Park Memorial Institute (RPMI) buffer containing 10% FBS for at least 30 min at 37°C. After incubation, RPMI was removed and 50 μl peptide pools at a concentration of 4 μg/ml were added to each well. Plates were then seeded with 2×105 fresh PBMC/well. Final concentration of peptide was 0.2 μg in 100 μl. Peptides used were as follows: Fifty SIVmac239 Gag peptides (20-mers overlapping by 10 amino acids, AIDS Repository, cat #4495-4544) were dissolved at 1mg/ml in either water or dimethylsulfoxide according to the Repository recommendations. Five pools containing 10 peptides each were prepared and stored in aliquots. Twenty-one pools, each containing 10 peptides, spanning the entire 89.6 Env coding sequence (15-mers overlapping by 11 amino acids). Peptides in pool #21 were from the C-terminus of gp160, a region not included in the protein expressed in MVA/KB9-5, and served as a background control. Peptides spanning the Tatnef chimeric protein (15-mers overlapping by 11) were synthesized by Emory University Microchemical Facility, Winship Cancer Center. These were dissolved in dimethylsulfoxide at a concentration of 100 mg/ml and subsequently diluted and pooled (10 per pool) for use in the assay. Cells were stimulated with peptides for approximately 36 h in a CO2 incubator at 37°C. The plates were washed six times with DPBS containing 0.05% Tween-20. Biotinylated 7B6-1 anti-human IFN-γ antibody (Mabtech 7B6-1) at 2 μg/ml was added for 2 h at 37°C followed by washing and incubation with avidin peroxidase (Vectastatin ABC Elite Kit, Vector Laboratories, Burlingame, CA) according to the manufacturer’s directions. Spots were developed with peroxidase substrate (Vectastatin AEC Substrate) and enumerated with an automated ELISpot reader (ZellNet Consulting, Inc., Fort Lee, NJ). The number of background spots ranged from 0 to 95/106 but was typically under 15/106 cells. Only counts of greater than 20/106 cells after subtraction of background were used.
CD4+ T cell counts
CD4+ T cells were enumerated by flow cytometry at Advanced Biosciences Laboratories, Inc. (Kensington, MD).
Plasma SHIV RNA copy number
SHIV RNA copy number was enumerated by quantitative PCR using plasma collected after SHIV infection (Cline et al., 2005)
VACV MV ELISA
VACV strain WR mature virions were purified by sucrose gradient sedimentation from lysates of infected HeLa cells and used to coat 96-well microtiter plates (Thermo Labsystems) at 107pfu/ml in CB1 bicarbonate buffer (Immunochemistry Technologies) at 37°C overnight. Liquid was removed from the plates and virus was inactivated by incubation with 2% paraformaldehyde for 10 min at 4°C. Plates were washed with buffer (0.15M NaCl, 0.5% Tween-20), blocked for 1 h with blocking buffer (PBS containing 5% non-fat dry milk and 0.2% Tween-20) and subsequently incubated with 2-fold dilutions of sera for 1 h at 37°C. Peroxidase conjugated anti-monkey IgG (Accurate Chemical and Scientific Corp) was added at a dilution of 1:4000 and incubated for 1 h at 37°C followed by BM Blue substrate (Roche Molecular Biochemicals) for 30 min at room temperature. Absorbency was measured at 370 and 492 nm.
VACV L1 and B5 protein ELISA
96-well microtiter plates were coated overnight at 4°C with either soluble recombinant L1 (0.4 μg/ml) or B5 (1 μg/ml) protein diluted in PBS. Both proteins were produced in insect cells infected by a recombinant baculovirus and purified from the medium by nickel affinity (Aldaz-Carroll et al., 2005a; Aldaz-Carroll et al., 2005b). Plates were washed, blocked, and endpoint titers for individual serum samples determined as described above for MV ELISA.
VACV mature virus neutralization assay
Neutralization of VACV WR was performed as previously described using a flow cytometric assay employing a recombinant virus expressing EGFP (Earl et al., 2003).
Comet reduction assay
BSC-1 cells were infected with the VACV strain IHD-J, which produces comet-like satellite plaques due to the release of large amounts of extracellular virus. After 2 h, monolayers were washed three times and overlaid with medium containing serum samples diluted 1:66. After 40-44 h, cells were stained with crystal violet and photographed.
VACV EV neutralization assay
For production of extracellular virus (EV), RK-13 cells were infected with VACV strain IHD-J at a multiplicity of infection of 0.1 plaque forming units for 1.5 h after which the monolayer was washed twice and overlaid with EMEM containing 2.5% FBS. Approximately 20 h after infection, the EV-containing medium was transferred to a conical tube and debris was removed by low speed centrifugation. Serum samples (1:5 final dilution) were incubated with 10 μl of EV in PBS containing 3% bovine serum albumin and 6.9 μg/ml VACV L1 monoclonal antibody 7D11 in 96-well round bottom plates. The amount of 7D11 used was determined to be more than sufficient to neutralize the contaminating MV virus in the EV preparation. After 1.5 h at 37°C, samples were diluted and used to infect monolayers of BSC-1 cells in 6-well plates. The volume used for infection was determined empirically to give 90-150 plaques/well. Samples were plated in quadruplicate. After 48 h, cells were stained with crystal violet and plaques were enumerated. The percent neutralization was determined by the formula: 100 − number of plaques with test sample and 7D11 MAb × 100/ number of plaques with 7D11 MAb only. VACV Immune Globulin (Cangene Corp., Winnipeg, Canada) was included as a positive control in all experiments.
VACV-specific IFN-γ producing PBMC
Quantitation of VACV-specific IFN-γ producing cells was performed on cryopreserved cells as follows. Cells were thawed at 37°C and transferred to a 50 ml conical tube. Ten ml of warm RPMI containing 10% FBS (RPMI-10) and 50 U/ml Benzonase nuclease (Novagen, CN Biosciences, Inc., Madison, Wisconsin) were slowly added to the tube. Cells were centrifuged for 5 min at 1200 rpm, washed once in RPMI-10 containing Benzonase, and suspended in 1 ml of RPMI-10. After overnight incubation at 37°C, they were centrifuged at 1200 rpm for 5 min and suspended in RPMI-10 containing CD28 and CD49d (BD Biosciences, San Jose, CA) each at a final concentration of 1 μg/ml. Cells were either infected with MVA at a multiplicity of infection of 6, stimulated with PMA (50 ng/ml)/ionomycin (2 μg/ml) or unstimulated. After 7 h, brefeldin A was added to a final concentration of 10 μg/ml and incubation was continued overnight. Cells were then washed with 3 ml RPMI-10, stained for 30 min at 4°C with CD8 fluorescein isothiocyantate and CD3 phycoerythrin (BD Biosciences), treated with Cytofix/cytoperm (BD Biosciences), and stained for 30 min at 4°C with IFN-γ allophycocyanin (BD Biosciences). After washing, cells were suspended in 2% paraformaldehyde and acquired on a FACSCaliber using CellQuest software. Analysis was performed using FlowJo software (TreeStar, Inc., Ashland, OR).
MPXV challenge
Inmunized and control monkeys were delivered to USAMRIID, Ft. Detrick, MD where they were challenged with 5 × 107 infectious units of MPXV by the intravenous route. The number of monkeypox genomes per ml of blood was determined by extraction of DNA with the QIAGEN QIAmp DNA mini Kit and quantitative TaqMan-MGB PCR as previously described (Earl et al., 2004). Monkeypox lesions were enumerated every 3 days after challenge.
Statistical analysis
Statistical differences between vaccine groups were assessed by ANOVA using Statview software (SAS Institute Inc., Cary, NC)
Acknowledgments
We thank Norman Cooper for cells and VACV. Vanessa Hirsch kindly provided E544 monkey serum and Gary Cohen and Roselyn Eisenberg provided VACV recombinant proteins. Additional materials were obtained from the NIAID AIDS Repository, the NIAID AIDS Tetramer Facility, and BEI Resources. MamuA*01 typing was performed by the Wisconsin Regional Primate Research Center MHC Typing Core. Richard Stout provided assistance with the Biojector. This work was supported in part by funds from the intramural program of NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addo MM, Altfeld M, Rosenberg ES, Eldridge RL, Philips MN, Habeeb K, Khatri A, Brander C, Robbins GK, Mazzara GP, Goulder PJ, Walker BD. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc Natl Acad Sci U S A. 2001;98:1781–6. doi: 10.1073/pnas.98.4.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Hirao L, Isaacs SN, Moss B, Eisenberg RJ, Cohen GH. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J Virol. 2005a;79:6260–71. doi: 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Pannell LK, Lebowitz J, Fogg C, White CL, Moss B, Cohen GH, Eisenberg RJ. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005b;341:59–71. doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Amara RR, Smith JM, Staprans SI, Montefiori DC, Villinger F, Altman JD, O’Neil SP, Kozyr NL, Xu Y, Wyatt LS, Earl PL, Herndon JG, McNicholl JM, McClure HM, Moss B, Robinson HL. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J Virol. 2002a;76:6138–46. doi: 10.1128/JVI.76.12.6138-6146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, Montefiori DC, Xu Y, Herndon JG, Wyatt LS, Candido MA, Kozyr NL, Earl PL, Smith JM, Ma H-L, Grimm BD, Hulsey ML, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS in rhesus macaques by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- Amara RR, Villinger F, Staprans SI, Altman JD, Montefiori DC, Kozyr NL, Xu Y, Wyatt LS, Earl PL, Herndon JG, McClure HM, Moss B, Robinson HL. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J Virol. 2002b;76:7625–31. doi: 10.1128/JVI.76.15.7625-7631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–96. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- Anton LC, Schubert U, Bacik I, Princiotta MF, Wearsch PA, Gibbs J, Day PM, Realini C, Rechsteiner MC, Bennink JR, Yewdell JW. Intracellular localization of proteasomal degradation of a viral antigen. J Cell Biol. 1999;146:113–24. doi: 10.1083/jcb.146.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Craiu A, Santra S, Egan MA, Schmitz JE, Kuroda MJ, Fu TM, Nam JH, Wyatt LS, Lifton MA, Krivulka GR, Nickerson CE, Lord CI, Moss B, Lewis MG, Hirsch VM, Shiver JW, Letvin NL. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J Virol. 2001a;75:2462–7. doi: 10.1128/JVI.75.5.2462-2467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–7. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Santra S, Kuroda MJ, Schmitz JE, Plishka R, Buckler-White A, Gaitan AE, Zin R, Nam J-H, Wyatt L, Lifton MA, Nicherson CE, Moss B, Montefiori DC, Hirsch VM, Letvin NL. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J Virol. 2001b;75:5151–5158. doi: 10.1128/JVI.75.11.5151-5158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard TJ, Alcami A, Andrea P, Smith GL. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–67. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- Cafaro A, Titti F, Fracasso C, Maggiorella MT, Baroncelli S, Caputo A, Goletti D, Borsetti A, Pace M, Fanales-Belasio E, Ridolfi B, Negri DR, Sernicola L, Belli R, Corrias F, Macchia I, Leone P, Michelini Z, ten Haaft P, Butto S, Verani P, Ensoli B. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P) Vaccine. 2001;19:2862–77. doi: 10.1016/s0264-410x(01)00002-0. [DOI] [PubMed] [Google Scholar]
- Carroll M, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: Propagation and generation of recombinant viruses in nonhuman mammalian cell line. Virology. 1997;244:365–369. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Crawford JM, Earl PL, Moss B, Reimann KA, Wyand MS, Manson KH, Bilska M, Zhou JT, Pauza CD, Parren PW, Burton DR, Sodroski JG, Letvin NL, Montefiori DC. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73:10199–10207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek DC, Kwong PD, Nabel GJ. The rational design of an AIDS vaccine. Cell. 2006;124:677–81. doi: 10.1016/j.cell.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Drexler I, Heller K, Wahren B, Erfle V, Sutter G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J Gen Virol. 1998;79(Pt 2):347–52. doi: 10.1099/0022-1317-79-2-347. [DOI] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Moss B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J Virol. 2003;77:10684–8. doi: 10.1128/JVI.77.19.10684-10688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, Eisenberg RJ, Hartmann CJ, Jackson DL, Kulesh DA, Martinez MJ, Miller DM, Mucker EM, Shamblin JD, Zwiers SH, Huggins JW, Jahrling PB, Moss B. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Earl PL, Hugin AW, Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodediciency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Sugiura W, Montefiori DC, Broder CC, Lee SA, Wild C, Lifson J, Moss B. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virustType 1 gp140. J Virol. 2001;75:645–653. doi: 10.1128/JVI.75.2.645-653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl PL, Wyatt LS, Montefiori DC, Bilska M, Woodward R, Markham PD, Malley JD, Vogel TU, Allen TM, Watkins DI, Miller N, Moss B. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology. 2002;294:270–81. doi: 10.1006/viro.2001.1345. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y, Bray M, Whitehouse CA, Miller D, Mucker E, Manischewitz J, King LR, Robert-Guroff M, Hryniewicz A, Venzon D, Meseda C, Weir J, Nalca A, Livingston V, Wells J, Lewis MG, Huggins J, Zwiers SH, Golding H, Franchini G. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191:372–81. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- Egan MA, Chong SY, Rose NF, Megati S, Lopez KJ, Schadeck EB, Johnson JE, Masood A, Piacente P, Druilhet RE, Barras PW, Hasselschwert DL, Reilly P, Mishkin EM, Montefiori DC, Lewis MG, Clarke DK, Hendry RM, Marx PA, Eldridge JH, Udem SA, Israel ZR, Rose JK. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res Hum Retroviruses. 2004;20:989–1004. doi: 10.1089/aid.2004.20.989. [DOI] [PubMed] [Google Scholar]
- Enserink M. Bioterrorism. In search of a kinder, gentler vaccine. Science. 2002;296:1594. doi: 10.1126/science.296.5573.1594. [DOI] [PubMed] [Google Scholar]
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. World Health Organization; 1988. [Google Scholar]
- Franchini G, Gurunathan S, Baglyos L, Plotkin S, Tartaglia J. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Expert Rev Vaccines. 2004;3:S75–88. doi: 10.1586/14760584.3.4.s75. [DOI] [PubMed] [Google Scholar]
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37:251–71. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- Hel Z, Johnson JM, Tryniszewska E, Tsai WP, Harrod R, Fullen J, Tartaglia J, Franchini G. A novel chimeric Rev, Tat, and Nef (Retanef) antigen as a component of an SIV/HIV vaccine. Vaccine. 2002;20:3171–86. doi: 10.1016/s0264-410x(02)00258-x. [DOI] [PubMed] [Google Scholar]
- Henderson DA. The looming threat of bioterrorism. Science. 1999;283:1297–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- Hu S-L. Non-human primate models for AIDS vaccine research. Curr Drug Targets - Infec Dis. 2005;5:193–201. doi: 10.2174/1568005054201508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson GB, Halloran M, Li J, Park IW, Gomila R, Reimann KA, Axthelm MK, Iliff SA, Letvin NL, Sodroski J. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J Virol. 1997;71:4218–4225. doi: 10.1128/jvi.71.6.4218-4225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDuc JW, Damon I, Relman DA, Huggins J, Jahrling PB. Smallpox research activities: U.S. interagency collaboration, 2001. Emerg Infect Dis. 2002;8:743–5. doi: 10.3201/eid0807.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Erdmann I, Basch V, S S, Kramps TA, Zinkernagel RM, Oehen S, Kuendig TM. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci. 2001;98:3299–3203. doi: 10.1073/pnas.051630798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR. Defining the protective antibody response for HIV-1. Curr Mol Med. 2003;3:209–216. doi: 10.2174/1566524033479799. [DOI] [PubMed] [Google Scholar]
- Mayr A, Hochstein-Mintzel, Stickl H. Abstammung, eigenschaften und verwendung des attenuieten vaccinia-stammes MVA. Infection. 1975a;3:6–14. [Google Scholar]
- Mayr A, Hochstein-Mintzel V, Stickl H. Passage history, properties, and applicability of the Attenuated Vaccinia Virus Strain MVA. Infection. 1975b;3:6–14. [Google Scholar]
- Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991;72(Pt 5):1031–8. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A. 1996;93:11341–8. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat Immunol. 2002;3:265–71. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- Otten GR, Schaefer M, Doe B, Liu H, Megede JZ, Donnelly J, Rabussay D, Barnett S, Ulmer JB. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine. 2005 doi: 10.1016/j.vaccine.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Ourmanov I, Brown CR, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch VM. Comparative efficacy of recombinant modified vaccinia virus ankara expressing simian immunodeficiency virus (SIV) gag-Pol and/or env in macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Wang LR, Gomez-Roman VR, Davis-Warren A, Montefiori DC, Kalyanaraman VS, Venzon D, Zhao J, Kan E, Rowell TJ, Murthy KK, Srivastava I, Barnett SW, Robert-Guroff M. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J Virol. 2005;79:10200–9. doi: 10.1128/JVI.79.16.10200-10209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Gomez P, Mascola JR, Dang V, Krivulka GR, Yu F, Lord CI, Shen L, Bailer R, Nabel GJ, Letvin NL. Comparative evaluation of three different intramuscular delivery methods for DNA immunization in a nonhuman primate animal model. Vaccine. 2006;24:367–73. doi: 10.1016/j.vaccine.2005.07.072. [DOI] [PubMed] [Google Scholar]
- Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MW, Mirchandani J, Silvera P, Regulier EG, Capini C, Bojczuk PM, Hu J, Gracely EJ, Boyer JD, Khalili K, Zagury JF, Lewis MG, Rappaport J. Immunogenicity of HIV-1 IIIB and SHIV 89.6P Tat and Tat toxoids in rhesus macaques: induction of humoral and cellular immune responses. DNA Cell Biol. 2002;21:637–51. doi: 10.1089/104454902760330174. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Sriwanthana B, Hodge T, Mastro TD, Dezzutti CS, Bond K, Stephens HA, Kostrikis LG, Limpakarnjanarat K, Young NL, Qari SH, Lal RB, Chandanayingyong D, McNicholl JM. HIV-specific cytotoxic T lymphocytes, HLA-A11, and chemokine-related factors may act synergistically to determine HIV resistance in CCR5 delta32-negative female sex workers in Chiang Rai, northern Thailand. AIDS Res Hum Retroviruses. 2001;17:719–34. doi: 10.1089/088922201750236997. [DOI] [PubMed] [Google Scholar]
- Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author’s transl) Dtsch Med Wochenschr. 1974;99:2386–92. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- Stittelaar KJ, Kuiken T, de Swart RL, van Amerongen G, Vos HW, Niesters HGM, van Schalkwijk P, van der Kwast T, Wyatt LW, Moss B, Osterhaus ADME. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine. 2001;19:3700–3709. doi: 10.1016/s0264-410x(01)00075-5. [DOI] [PubMed] [Google Scholar]
- Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, Pistoor FH, Niesters HG, van Doornum G, van der Zeijst BA, Mateo L, Chaplin PJ, Osterhaus AD. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79:7845–51. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura W, Broder CC, Moss B, Earl PL. Characterization of conformation-dependent anti-gp120 murine monoclonal antibodies produced by immunization with monomeric and oligomeric human immunodeficiency virus type 1 envelope proteins. Virology. 1999;254:257–267. doi: 10.1006/viro.1998.9549. [DOI] [PubMed] [Google Scholar]
- Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter G, Wyatt LS, Foley PL, Bennink JR, Moss B. A recombinant vector derived from the host-range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Van Rompay KK, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, Earl P, Harvey D, Franchini G, Tartaglia J, Montefiori D, Hattangadi S, Moss B, Marthas ML. Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr. 2005;38:124–34. doi: 10.1097/00126334-200502010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Epstein J, Baraceros FM, Gorak EJ, Charoenvit Y, Carucci DJ, Hedstrom RC, Rahardjo N, Gay T, Hobart P, Stout R, Jones TR, Richie TL, Parker SE, Doolan DL, Norman J, Hoffman SL. Induction of CD4(+) T cell-dependent CD8(+) type 1 responses in humans by a malaria DNA vaccine. Proc Natl Acad Sci U S A. 2001;98:10817–22. doi: 10.1073/pnas.181123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101:4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Srivastava IK, Kuller L, Zarkikh I, Kraft Z, Fagrouch Z, Letvin NL, Heeney JL, Barnett SW, Stamatatos L. Immunization with HIV-1 SF162-derived Envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology. 2006 doi: 10.1016/j.virol.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81:1581–600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]