Abstract
Regulated adhesion of T cells by the integrins LFA-1 (lymphocyte function-associated antigen-1) and VLA-4 (very late antigen-4) is essential for T-cell trafficking. The small GTPase Rap1 is a critical activator of both integrins in murine lymphocytes and T-cell lines. Here we examined the contribution of the Rap1 regulatory pathway in integrin activation in primary CD3+ human T cells. We demonstrate that inactivation of Rap1 GTPase in human T cells by expression of SPA1 or Rap1GAP blocked stromal cell-derived factor-1α (SDF-1α)–stimulated LFA-1–ICAM-1 (intercellular adhesion molecule-1) interactions and LFA-1 affinity modulation but unexpectedly did not significantly affect binding of VLA-4 to its ligand VCAM-1 (vascular cell adhesion molecule 1). Importantly, silencing of the Rap1 guanine exchange factor CalDAG-GEFI inhibited SDF-1α- and phorbol 12-myristate 13-acetate (PMA)–induced adhesion to ICAM-1 while having no effect on adhesion to VCAM-1. Pharmacologic inhibition of Phospholipase C (PLC) blocked Rap1 activation and inhibited cell adhesion and polarization on ICAM-1 and VCAM-1. Protein kinase C (PKC) inhibition led to enhanced levels of active Rap1 concomitantly with increased T-cell binding to ICAM-1, whereas adhesion to VCAM-1 was reduced. Thus, PLC/CalDAG-GEFI regulation of Rap1 is selectively required for chemokine- and PMA-induced LFA-1 activation in human T cells, whereas alternate PLC- and PKC-dependent mechanisms are involved in the regulation of VLA-4.
Introduction
T-cell adhesion is critical for adaptive immune responses such as T-cell trafficking to lymphoid organs and sites of inflammation. These adhesive events are largely mediated by the β2 integrin LFA-1 (lymphocyte function-associated antigen-1) and the β1 integrin VLA-4 (very late antigen-4), which recognize intercellular adhesion molecule (ICAM)–1 and -2 and vascular cell adhesion molecule 1 (VCAM-1), respectively. VLA-4 and LFA-1 also promote trafficking to the bone marrow,1,2 and VLA-4 interaction with extracellular matrix component fibronectin allows T-cell interstitial migration.3 T-cell trafficking is orchestrated by chemokines such as stromal cell-derived factor-1α (SDF-1α) (CXCL12). SDF-1α engagement of its G-protein-coupled receptor, CXCR4, promotes cell adhesion, polarization, and chemotaxis.4 Both LFA-1 and VLA-4 play important roles in human immune diseases, and therapeutics targeting these 2 integrins are in clinical trials to treat autoimmune diseases.5,6 Thus understanding the mechanisms of LFA-1 and VLA-4 functions in the human system will provide important insights into their physiologic role in the adaptive immune response and may aid in the design of more selective therapeutic strategies
The dynamic adhesive interactions of T cells are regulated by in situ integrin activation. Chemokines trigger changes in integrin ligand-binding affinity and/or avidity/receptor clustering through a process known as inside-out signaling. These events are coupled to outside-in integrin signaling as a result of multivalent ligand binding.7 This bidirectional signaling is responsible for the strength of integrin-mediated cell adhesion. The analyses of cellular mechanisms that contribute to integrin activation have led to the identification of the small GTPase Rap1 as a key regulator of this process.8 In particular, studies in murine lymphocytes and cell lines have shown a requirement for Rap1 in chemokine-induced9 and phorbol ester-stimulated10,11 LFA-1- and VLA-4-mediated adhesion. Direct outside-in activation of integrin-mediated adhesion by Mn2+ in Jurkat cell lines also required Rap1, although Mn2+ did not enhance accumulation of active Rap1.12 The downstream targets of active Rap1 include RapL, the kinase Mst1,13,14 and talin.15 RapL complexes with LFA-1 and Mst1 to localize LFA-1 to the leading edge, which is important for T-cell polarization and adhesion.16 Rap1 activation also induces the binding of the cytoskeletal protein talin to the integrin cytoplasmic tail, which promotes integrin activation15 and the localization of high-affinity clustered LFA-1 that is required for cell migration.17
Rap1 cycles between an active GTP-bound and inactive GDP-bound form that is regulated by a diverse family of guanine exchange factors (GEFs) and GTPase-activating proteins (GAPs). Rap GEFs are activated by classical second messengers including adenosine 3′,5′-cyclic monophosphate (cAMP), calcium, and diacylglycerol (DAG).18 CalDAG-GEFs (calcium and diacylglycerol guanine nucleotide exchange factor; Ras guanyl nucleotide-releasing protein, RasGRPs) are a family of calcium and DAG responsive GEFs that contain a Ras exchange motif, a DAG-binding C1 domain, 2 EF hands that bind calcium, and a GEF domain.19 There are currently 4 members in this family. CalDAG-GEFI (RasGRP2) is a Rap GEF expressed in the brain and hematopoietic cells, CalDAG-GEFIII (RasGRP3) is expressed predominantly in B cells, CalDAG-GEFII (RasGRP1) is a Ras regulator preferentially expressed in T cells, and RasGRP4, a Ras activator, is highly expressed in mast cells.19 Compelling in vivo evidence for the role of Rap1 in integrin function came from mice deficient in CalDAG-GEFI. These mice exhibited impaired Rap1 activation and αIIbβ3-mediated adhesive functions in platelets that was associated with defective thrombus formation in vivo.20
Characterization of the intracellular signaling pathways that regulate chemokine-mediated human T-cell integrin function is of key importance to understanding the molecular mechanisms of T-cell trafficking. Here, we addressed the role of Rap1 GTPases and CalDAG-GEFI in SDF-1α- and phorbol 12-myristate 13-acetate (PMA)–mediated LFA-1 and VLA-4 regulation in primary human T cells. This was accomplished by expressing GAPs, signal-induced proliferation associated gene-1 (SPA1), or Rap1GAP to inactivate Rap GTPases or by silencing CalDAG-GEFI using siRNA approaches in primary human CD3+ T cells. Pharmacologic inhibitors of phospholipase C (PLC) and protein kinase C (PKC) were also exploited to examine their contribution to Rap1 activation and SDF-1α-stimulated adhesion under flow conditions. Our studies demonstrate that Rap1 and CalDAG-GEFI are required for LFA-1-mediated adhesion of human T cells, whereas alternative PKC-dependent signaling pathways regulate VLA-4 function. The selective role for the Rap1 regulatory pathways in LFA-1-mediated adhesion is unanticipated from previous work conducted in murine lymphocytes and T-cell lines that reported a role for Rap in both the bidirectional signaling in LFA-1 as well as VLA-4-mediated T-cell adhesion. Furthermore, our studies show that CalDAG-GEFI is a critical link between a G-protein-coupled receptor and β2 integrin activation in primary human T cells.1
Patients, materials, and methods
Approval for blood donations was obtained from the Brigham and Women's Hospital Institutional Review Board with informed consent in accordance with the Declaration of Helsinki.
Cell isolation
T cells were isolated from sodium-citrated anticoagulated whole blood from healthy donors by negative selection using the human T-cell enrichment cocktail RossetteSep (StemCell Technologies, Vancouver, BC). Isolated T cells consisted of more than 95% CD3+ T lymphocytes. Cells were transfected within 1 hour from isolation and maintained in culture media (RPMI-1640 medium with 10% fetal calf serum [FCS], 2 mM glutamine, and antibiotics). Twenty-four hours after transfection, cultures were supplemented with interleukin 2 (24 U/mL; R&D Systems, Minneapolis, MN).
siRNA
Silencing RasGRP2 (CalDAG-GEFI) was attained by small RNA interference (siRNA) technology. A siRNA duplex to target the sequence 5′-AATTCTCCGAACGTGTCACGT-3′ corresponding to set off position 784 of RasGRP2 was purchased from Qiagen (Valencia, CA). The sequences were as follows: 5′-AGCGCAAGAUGUCCCUGUU-3′ sense and 3′-AACAGGGACAUCUUGCGCU-5′ antisense. A nonspecific RNA duplex (Control-siRNA) was also obtained and used as negative control.
Plasmids
SPA1 cDNA, cloned into the vector pLEGFP-C1 (BD Biosciences, Palo Alto, CA), is expressed as a green fluorescence protein (GFP)–tagged fusion protein.21 Glutathione S-transferase (GST)–RBD (a GST fusion protein containing the Rap1-binding domain of Ral-GDS) cDNA was used for Rap1 activation assays. cDNA encoding Rap1GAP (gift from P. Casey, Duke University) was cloned into pTRE-Tight vector (Clontech, Palo Alto, CA) and transfected into T cells together with pUHD 15.1. This plasmid encodes tetR-VP16 fusion protein that transactivates the promoter from pTRE-Tight allowing expression of Rap1GAP. Control transfections for GFP used pmaxGFP plasmid (Amaxa Biosystems, Gaithersburg, MD).
Cell transfection
Human T cells (5 × 106) were transfected with siRNA (1200 nM) by electroporation using the Nucleofection system (Amaxa Biosystems), according to the manufacturer's protocols. Expression of CalDAG-GEFI, monitored by immunoblot using a rabbit polyclonal antibody (Ab) (a gift from Dr A. M. Graybiel, MIT, Cambridge, MA), was maximally suppressed 96 hours after transfection, the time point chosen for all siRNA assays. Plasmids transfections were also achieved using Amaxa Nucleofection system, and cells were maintained for 24 hours in culture media until assay. Transfection efficiency was evaluated by measurement of GFP fluorescence and protein expression by immunoblot using monoclonal Ab (mAb) against SPA1 (BD Transduction Laboratories, Lexington, KY) and Rap1GAP (Santa Cruz Biotechnology, Santa Cruz, CA).
Rap1 activation assay
Levels of activated Rap1 were determined as previously described,22 with minor modifications: Transfected T cells were incubated in 0.1% bovine serum albumin (BSA) for 30 minutes at 37°C and stimulated with 1 μg/mL PMA (Sigma, St. Louis, MO) for 5 minutes, 100 nM SDF-1α (PeproTech, Rocky Hill, NJ) for 10 seconds, or vehicle control. After extraction with lysis buffer [25 mM tris(hydroxymethyl)aminomethane (Tris)-HCl at pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Nonidet P-40, 1 mM DTT (dithiothreitol), 5% glycerol, 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mM phenyl methylsulphonyl fluoride (PMSF)] on ice for 10 minutes, cells were centrifuged at 16 000g for 10 minutes at 4°C. Aliquots of the lysate were used to detect total Rap1 levels. The remaining supernatants were incubated with 50 μg of GST fusion protein containing the Rap1-binding domain (RBD) of Ral-GDS coupled to glutathione-sepharose beads for 45 minutes at 4°C. Proteins were separated on a 4 to 20% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to nitrocellulose membranes (BioRad, Hercules, CA). Rap1 levels were detected with rabbit polyclonal antibodies (121; Santa Cruz Biotechnology) followed by horseradish peroxidase (HRP)–conjugated goat anti-rabbit antibodies (Zymed, South San Francisco, CA). Immunoreactivity was detected by chemiluminescence (SuperSignal; Pierce, Rockford, IL).
Pharmacologic treatments
Where indicated, preincubations were performed for 10 minutes at 37°C with the following pharmacologic inhibitors: PKC inhibitor, BisIndolylmaleimide I Gö 6850 (Calbiochem, San Diego, CA; 10 μM) or Gö 6976 (Biomol, Plymouth Meeting, PA; 20 nM); calcium chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-AM (BAPTA-AM) (Calbiochem; 10 μM); PLC inhibitor, U73122 (Biomol; 10 μM), or activators (Ca2+ ionophore, A23187 [10 μM, Sigma]; Mn2+ [50 μM]).
Cell adhesion assay
Recombinant human Fc fusion proteins ICAM-1 (2.5 μg/mL) or VCAM-1 (2 μg/mL) (R&D Systems) were applied to glass coverslips (12-mm; Fisher, Pittsburgh, PA) precoated with protein A (1 μg/mL). Transfected T cells were washed and resuspended in medium (cation-free phosphate-buffered saline [PBS] [pH 7.4] supplemented with 1 mM CaCl2, 1 mM MgCl2, and 2 mg/mL BSA), and subsequently 100 μL cell suspension was added per coverslip with or without the indicated stimuli. Cells were allowed to adhere for 30 minutes at 37°C, and nonadherent cells were removed by washing with PBS. Adherent cells were fixed with 10% buffered formalin, and differential interference contrast (DIC) images were taken with a Nikon Eclipse TE2000-U microscope coupled to the MetaMorph system (Molecular Devices, Downingtown, PA). Cells bound were calculated from 5 fields of view and expressed as number of adherent cells per mm2. For GFP transfection assays, adherence was calculated from 3 fields of view after correcting for transfection efficiency (number of GFP positive adherent cells per number of GFP positive in total input cells) ×100.
Laminar shear flow assays
Shear flow assays used a parallel plate flow chamber with human Fc-ICAM-1 (2.5 μg/mL) or VCAM-1 (2 μg/mL) coimmobilized with SDF-1α (2 μg/mL) on glass coverslips (25-mm; Carolina Biological Supply, Burlington, NC) as described.23 T cells were perfused through the chamber at 37°C in flow buffer (Dulbecco PBS and 0.1% human serum albumin) at shear stress rate of 0.75 dyne/mm2 for 10 minutes, and the entire period of perfusion was recorded using a digital imaging system (Meta Morph System; Molecular Devices) coupled to the Nikon Eclipse TE2000-U inverted fluorescence microscope equipped with DIC (20× phase objective). Alternatively, DIC or fluorescence sequential images were taken every 10 seconds in representative fields, and the total number of accumulated T cells was determined by counting the total adherent cells in 5 fields of view. Number of adherent cells was expressed per mm2. For GFP transfection assays, adherence was calculated as described in the cell adhesion assay.
Flow cytometry
For flow cytometric detection of the LFA-1 activation epitope KIM127, T cells were resuspended in 5% fetal calf serum and stimulated in the presence of 2 μg/mL KIM127 mAb (a gift from N. Hogg, Cancer Research UK London Research Institute, London, United Kingdom) for 10 minutes at 37°C. After washing, cells were incubated for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)–conjugated goat antimouse antibody or allophycocyanin (APC) crosslinked goat antimouse (Molecular Probes, Eugene, OR) for GFP-transfected cells.
For soluble VCAM-1/Fc (sVCAM-1/Fc) binding assays, cells were suspended in 100 μL of buffer (Hank balanced salt solution [HBSS] containing 1 mM Mg2+/Ca2+, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [Hepes], and 0.5% fetal bovine serum [FBS]) and incubated at 37°C with 20 μg/mL sVCAM-1/Fc (R&D Systems) or 20 μg/mL human immunoglobulin G (IgG) (isotype control; Alexis Biochemicals, San Diego, CA). Cells were stimulated with PMA, SDF-1α, or Mn2+ for 30 s and immediately diluted with HBSS and fixed with 4% paraformaldehyde. Binding of sVCAM/Fc was detected by APC-conjugated goat anti-human IgG (1:300, 30 minutes at 4°C; Jackson ImmunoResearch, West Grove, PA), and flow cytometry was carried out using a BD FACS Calibur.
Statistical analysis
Comparisons were made with an unpaired, 2-tailed Student t test, and significance was considered when P was less than .05.
Results
Role of Rap1 GTPase in LFA-1 and VLA-4 integrin-mediated adhesion
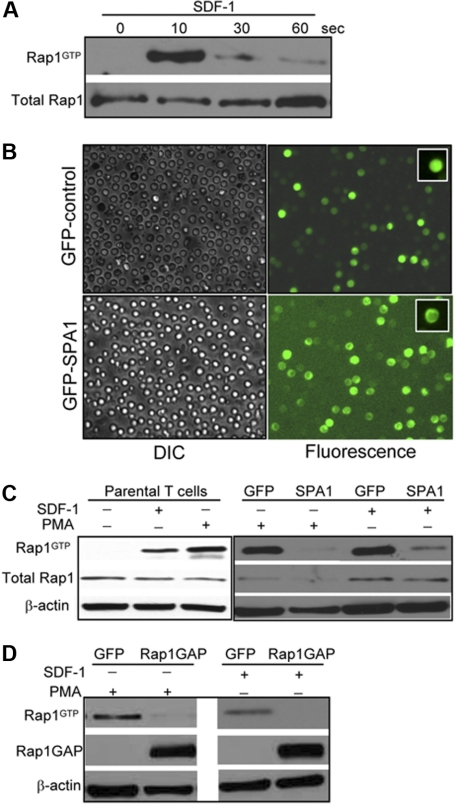
Time-course analysis of SDF-1α-induced Rap1 activation demonstrated strong GTP loading of Rap1 that peaked within 10 seconds and diminished rapidly thereafter (Figure 1A). The kinetics of Rap1 activation by SDF-1α parallel those reported using Jurkat cell lines.24 To examine the role of Rap1 GTPase in agonist-induced adhesion, human CD3+ T cells were transfected with GFP or GFP-SPA1, a well-described GAP for Rap expressed predominantly in lymphoid tissues.25 Transfection efficiencies ranged from 75 to 85% as assessed by GFP fluorescence (Figure 1B), and full-length GFP-SPA1 fusion protein was detected by Western blot (data not shown). SPA1 was observed in the cortical regions of the cell as previously described in other cell types,26 whereas GFP was distributed diffusely throughout the cell. Analysis of Rap1 activation in untransfected T cells revealed efficient GTP loading of Rap1 after stimulation with 100 nM SDF-1α or 1 μg/mL of PMA (Figure 1C). Rap1-GTP levels in GFP control cells were comparable with those observed in parental untransfected cells. GFP-SPA1 expression dramatically reduced Rap1 activation in response to SDF-1α or PMA, indicating efficient inactivation of Rap1 by SPA1 (Figure 1C). Similar results were obtained by transient transfection of another Rap GAP, Rap1GAP, which efficiently inactivated Rap1 after stimulation with PMA or SDF-1α (Figure 1D).
Figure 1.
Rap1 activation in human primary T cells. (A) Primary human T cells were stimulated with 100 nM SDF-1α for the indicated times in seconds, and Rap1 (Rap1GTP) was detected by pull-down assay. Total Rap1 is shown as loading control. (B) Transient transfection of T cells with GFP-control (upper) and GFP-SPA1 vectors (lower). Twenty-four hours after transfection, cells were visualized by DIC (left) or fluorescence (right) to evaluate GFP expression and transfection efficiency. Insets show peripheral localization of GFP-SPA1 compared with GFP-control. See “Cell adhesion assay” and “Laminar shear flow assays” for image acquisition information. (C) Analysis of Rap1 activation in parental T cells (left) and GFP-control– or GFP-SPA1–expressing cells (right). Cells were stimulated with 100 nM SDF-1α for 10 seconds or 1μg/mL PMA for 5 minutes, and active Rap1 (Rap1GTP) was determined as in panel A. Cells expressing GFP-SPA1 showed significant reduction in levels of active Rap1 compared with GFP-control-expressing cells. Total Rap1 and β-actin are shown as loading controls. (D) Rap1 activation in T cells expressing GFP-control or Rap1GAP. Cells were stimulated with PMA or SDF-1α as described in panel C, and active Rap1 (Rap1GTP), Rap1GAP levels, and β-actin (loading control) were evaluated. Overexpression of Rap1GAP efficiently reduced levels of active Rap1. Three to 5 independent experiments were done for each panel.
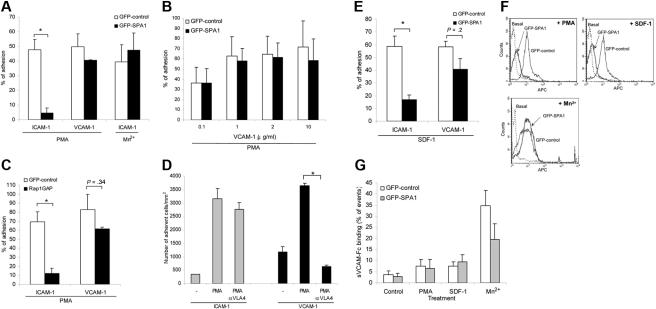
SPA1 overexpression significantly reduced T-cell adhesion to ICAM-1 in response to PMA treatment in static assays (Figure 2A). Mn2+ interaction with the divalent cation binding sites in the extracellular I domain of LFA-1 induces outside-in activation of the integrin,27 circumventing the need for inside-out cell signaling. Mn2+ treatment of SPA1 cells triggered cell adhesion on ICAM-1 that was indistinguishable from GFP control cells (Figure 2A), indicating that outside-in activation of LFA-1 is independent of active Rap1 in these cells. Unlike LFA-1-mediated adhesion on ICAM-1, overexpression of SPA1 did not prevent adhesion to VCAM-1 (Figure 2A) or another VLA-4 ligand fibronectin (data not shown) after PMA stimulation, indicating that Rap1 is not essential for VLA-4–mediated adhesion in this system. Similar results were obtained for a range of VCAM-1 concentrations tested (0.1, 1.0, 2.0, and 10.0 μg/mL; Figure 2B). PMA-induced adhesion to ICAM-1 was also significantly reduced in T cells overexpressing Rap1GAP, whereas adhesion to VCAM-1 remained largely unaffected (Figure 2C). Incubation of T cells with a functional blocking antibody to VLA-4 had no effect on ICAM-1 binding but completely abolished adhesion to VCAM-1 (Figure 2D) and FN (data not shown), demonstrating receptor-ligand specificity in these assays. Together these data demonstrate that Rap activation is essential for primary human T-cell adhesion to ICAM-1 but not VCAM-1. To address the role of Rap1 in T-cell adhesion under conditions that mimic physiologic blood flow, we examined T-cell adhesion to ICAM-1 or VCAM-1 coimmobilized with SDF-1α under defined flow conditions. Adhesion and polarization of T cells on these ligands was SDF-1α–dependent under shear stress rate of 0.75 dyne/mm2 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Under these conditions, GFP-SPA1 cells showed substantially reduced adhesion to ICAM-1-coated surfaces, whereas adhesion to VCAM-1 was similar to GFP-control cells (Figure 2E).
Figure 2.
Role of Rap1 GTPase in LFA-1– and VLA-4–mediated T-cell adhesion. (A) Adhesion of GFP-control– or GFP-SPA1–transfected T cells to immobilized ICAM-1 (2.5 μg/mL) or VCAM-1 (2 μg/mL) after treatment with 1 μg/mL PMA or 50 nM Mn2+. Percentage of adherent GFP-positive cells from initial GFP-positive cell input is expressed as mean plus or minus standard error of the mean (SEM). SPA1-GFP-positive cells failed to adhere to ICAM-1 after stimulation with PMA, whereas adhesion to VCAM-1 and Mn2+-induced adhesion to ICAM-1 were unaffected. *P < .001. (B) Effect of VCAM-1 concentration on cell adhesion. T cells were transfected as in panel A and assayed for PMA-induced adhesion to the indicated concentrations of immobilized VCAM-1. Adhesion of cells overexpressing GFP-SPA1 was comparable with GFP-control cells at all doses of VCAM-1 analyzed. (C) T cells transfected with Rap1GAP failed to adhere to immobilized ICAM-1 after PMA stimulation compared with cells transfected with GFP-control alone but were capable of adhering to immobilized VCAM-1 after similar PMA stimulation. *P < .05. (D) Adhesion to VCAM-1 is α4-dependent. T cells were incubated with a functional blocking antibody to VLA-4 (mAb HP2/1), and PMA-induced adhesion to ICAM-1 and VCAM-1 was performed as described in panel A. Blocking α4 had no effect on ICAM-1 binding but completely abolished adhesion to VCAM-1. *P < .001. (E) Effects of inactivation of Rap1 by SPA1 on SDF1-stimulated adhesion to ICAM-1 or VCAM-1 under shear flow conditions. GFP-control or GFP-SPA1-positive T cells were perfused over ICAM-1 or VCAM-1 coimmobilized with SDF-1α at a shear stress rate of 0.75 dyne/cm2. Percentage of adherent GFP-positive cells from total GFP-positive cell input is expressed as the mean plus or minus SEM; *P < .01. T cells overexpressing GFP-SPA1 failed to adhere to ICAM-1 but were able to adhere to VCAM-1 in response to SDF-1α compared with GFP-control cells. (F) Flow cytometry of GFP-control or GFP-SPA1-positive T cells stained with the high-affinity extension reporter mAb KIM127 in the absence (Basal) or presence (top left) of PMA or SDF-1α (top right) or Mn2+ (bottom). GFP-SPA1 cells failed to expose the neoepitope KIM127 in response to both PMA and SDF-1α compared with a substantial induction in GFP-control cells. In contrast, SPA1 cells expressed the KIM127 neoepitope in response to Mn2+. (G) sVCAM-1/Fc binding was determined by flow cytometry after 30 s stimulation with vehicle control, PMA, SDF-1α, or Mn2+. Data are expressed as percentage of events of GFP-positive cells that were positive for APC. Agonist-induced up-regulation of α4 integrin affinity as measured by sVCAM-1/Fc binding was similar in SPA-1 and GFP-control cells. Three to 5 independent experiments were done for each panel.
To examine the role of Rap1 in LFA-1 affinity modulation upon inside-out stimulation, we assessed the ability of cells to bind the conformation sensitive antibody to LFA-1, KIM127.28 SPA1 cells failed to expose the LFA-1 activation neoepitope recognized by KIM127 antibody upon SDF-1α or PMA stimulation (Figure 2F), suggesting that Rap1 is required for LFA-1 affinity modulation. On the other hand, Mn2+ treatment resulted in LFA-1 neoepitope exposure in SPA1-expressing cells (Figure 2F). To examine VLA-4 integrin affinity up-regulation, we used a flow cytometry assay to quantify sVCAM/Fc binding to GFP- and SPA1-GFP transfected T cells after stimulation with vehicle control, SDF-1α, PMA, or Mn2+. Comparable sVCAM/Fc binding was observed in GFP- and SPA1-GFP cells (Figure 2G). The small increase in the number of cells binding sVCAM/Fc after SDF-1α and PMA stimulation is consistent with previous reports. sVCAM/Fc binding to primary T cells after SDF-1α stimulation was found to be only 10% of that observed after Mn2+ treatment,29 and just a small subgroup of PMA-stimulated CD3+ T cells bound sVCAM-1.30
Together our data show that Rap1 plays a major role in chemokine-dependent LFA-1-mediated adhesion in human T cells through effects on inside-out integrin activation while alternative Rap1-independent mechanisms are used for VLA-4–mediated adhesion.
Role of CalDAG-GEFI in LFA-1 and VLA-4 integrin-mediated adhesion
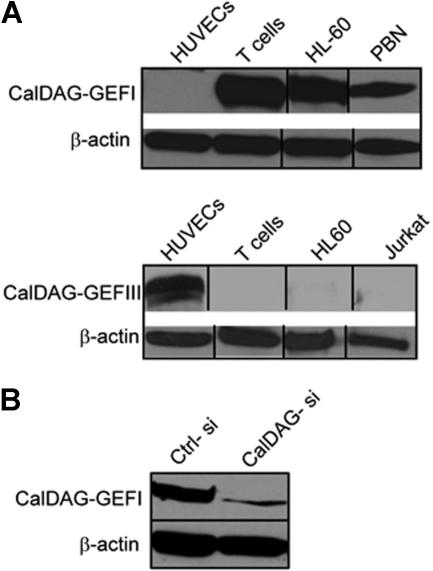
Members of the CalDAG-GEFI protein family are differentially expressed in several hematopoietic cell types.17 Western blot analysis demonstrated expression of CalDAG-GEFI in human primary T cells as well as peripheral blood neutrophils and the myeloid cell line HL-60 but not in human umbilical vein endothelial cells (HUVEC). In contrast, CalDAG-GEFIII was not detected in these lymphoid/myeloid lineage cells but was highly expressed in endothelial cells (Figure 3A).
Figure 3.
CalDAG-GEFs protein expression. (A) Western blot analysis of human T cells, peripheral blood neutrophils (PBN), human umbilical vain endothelial cells (HUVECs), HL60 promyelocytic cell line, and Jurkat T cells using specific mAb for CalDAG-GEFI (top) and CalDAG-GEFIII (bottom). (B) T cells were transfected with CalDAG-GEFI-specific (CalDAG-si) or control nonsilencing (Ctrl-si) siRNA, and total lysates were immunoblotted with mAb to CalDAG-GEFI. β-actin is shown as loading control. Representative blots are shown. Two to 5 independent experiments were done for each panel. Vertical lines have been inserted to indicate a repositioned gel lane.
To assess the contribution of CalDAG-GEFI in Rap1 activation and integrin-mediated adhesion, we used small interference RNA technology (siRNA) in human CD3+ primary T cells to knock-down CalDAG-GEFI protein. A 21-mer RNA dimer was designed to target human CalDAG-GEFI (GenBank accession number NM_005825) and was transiently transfected into human T cells. CalDAG-GEFI protein levels were reduced by 70% to 80% compared with control siRNA as analyzed by Western blot (Figure 3B).
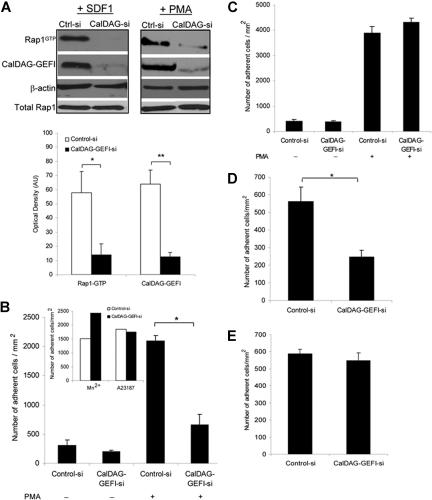
The role of CalDAG-GEFI in both SDF-1α- and PMA-dependent Rap1 activation was assessed in pull-down assays. Optical density analysis of representative Western blots demonstrates a significant correlation between CalDAG-GEFI knock-down and reduction in Rap1 activation after T-cell stimulation with SDF-1α (Figure 4A) and PMA (data not shown). Under static conditions, silencing CalDAG-GEFI resulted in a significant reduction in the number of adherent cells to immobilized ICAM-1 after PMA treatment (Figure 4B). In contrast, treatment of CalDAG-GEFI -siRNA cells with Mn2+ or the calcium ionophore A23187 resulted in normal adhesion to ICAM-1 (Figure 4B inset). This suggests that activation of LFA-1 from outside-in divalent cation binding or through alternative pathways that lead to intracellular calcium mobilization operates in the absence of this GEF. Adhesion to VCAM-1 was unaffected in CalDAG-GEFI siRNA cells after PMA stimulation (Figure 4C), confirming our data that VLA-4 signaling is independent of Rap1.
Figure 4.
Role of CalDAG-GEFI in LFA-1– and VLA-4–mediated T-cell adhesion. (A) Representative Western blots of Rap1-GTP and CalDAG-GEFI levels in T cells transfected with control or CalDAG-GEFI siRNA and stimulated with 100 nM SDF-1α for 10 seconds (left) or 1μg/mL PMA for 5 minutes (right). β-actin is shown as a loading control. Severely diminished activation of Rap1 was observed in CalDAG-GEFI knock-down cells. Densitometric analysis evaluated the optical density, in arbitrary units, of Rap1-GTP levels after stimulation with SDF-1α in control siRNA and CalDAG-GEFI siRNA cells. CalDAG-GEFI levels in the same samples were also quantified by densitometry. Extent of Rap1 inactivation correlated with CalDAG-GEFI knock-down. *P < .04, **P < .005. (B) PMA-stimulated cell adhesion to immobilized ICAM-1 (2.5 μg/mL) by control or CalDAG-GEFI siRNA-transfected cells shows impaired adhesion in cells deficient in CalDAG-GEFI. *P < .001. Inset, Mn2+ or Ca2+ ionophore (A23187) treatments of T cells transfected with CalDAG-GEFI siRNA resulted in normal adhesion to ICAM-1. (C) Silencing CalDAG-GEFI does not prevent PMA-stimulated T-cell adhesion to immobilized VCAM-1 (2 μg/mL), compared with cells transfected with control siRNA. (D) Effects of CalDAG-GEFI knock-down on chemokine-stimulated LFA-1 adhesion to ICAM-1 under shear flow. T cells transfected with control siRNA or CalDAG-GEFI siRNA were perfused over ICAM-1 coimmobilized with SDF-1α at shear stress rate of 0.75 dyne/cm2. The number of adherent cells is expressed as the mean plus or minus SEM. T cells lacking CalDAG-GEFI show impaired SDF-1α-mediated adhesion to ICAM-1 compared with control cells. *P < .05. (E) T cells, treated as described in panel D, were perfused over VCAM-1 coimmobilized with SDF-1α. Both control siRNA cells and CalDAG-GEFI siRNA cells adhered to VCAM-1. Three to 5 independent experiments were done for each panel.
CalDAG-GEFI knock-down significantly impaired the ability of T cells to adhere to ICAM-1 under shear flow conditions in response to SDF-1α (Figure 4D), whereas adhesion to VCAM-1 remained unaffected compared with control-siRNA cells (Figure 4E). Together these findings indicate that CalDAG-GEFI signaling is critical for SDF-1α- and PMA-induced LFA-1 binding to its ligand ICAM-1 but plays no role in VLA-4–VCAM-1 interaction.
Modulation of Rap1 activation and integrin-dependent adhesion by PLC and PKC
Chemokine-dependent activation of Rap1 in murine lymphocytes and Jurkat T cells is PLC dependent.24 The PLC products, DAG and Ca2+, are known to activate members of the CalDAG-GEF family.31 Using the PLC inhibitor, U73122, we observed that both SDF-1α- and PMA-induced Rap1 activation in human T cells is dependent on PLC activity (Figure 5A). The intracellular calcium chelator BAPTA-AM did not affect Rap1 activation, as previously shown in Jurkat cells,24 indicating that Ca2+ is not a major determinant of SDF-1α-induced Rap1 activation. However BAPTA-AM treatment inhibited PMA-induced activation of Rap1 (Figure 5A), indicating that Ca2+ depletion affects only PMA-dependent signaling to Rap1. PMA-induced PKC activation is known to activate integrins in many cell types and leads to affinity regulation of VLA-4.30,32 Interestingly, we observed that inhibition of classical and novel PKC isoforms by Gö 6850 further increased active Rap1 levels after SDF-1α stimulation, uncovering a negative role for PKC in Rap1 activation (Figure 5A), whereas the same treatment inhibited Rap1 activation by PMA as expected (Figure 5A). Similar results were observed with another inhibitor of classical PKCs (Gö 6976), which increased SDF-1α-induced Rap1 activation (supplemental S2). Further studies exploring the role of specific PKC isoforms in modulating Rap activity will be an interesting area for future investigation.
Figure 5.
Role of PLC and PKC in Rap1 activation and stimulated T-cell adhesion. (A) Western blots of Rap1-GTP in T cells pretreated with a PLC inhibitor (10 μM U73122), a calcium chelator (1 μM BAPTA-AM), or a PKC inhibitor (10 μM Gö 6850) for 10 minutes, and then treated with 100 nM SDF-1α for 10 seconds (left) or 1μg/mL PMA for 5 minutes (right). Active Rap1 was detected by pull-down assay. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Inhibition of PLC or PKC impairs SDF1-stimulated adhesion to ICAM-1 or VCAM-1 under shear flow. T cells were pretreated with 10 μM U73211 or 10 μM Gö 6850 for 10 minutes and perfused over ICAM-1 or VCAM-1 coimmobilized with SDF-1α at shear stress rate of 0.75 dyne/cm2. The number of adherent cells is expressed as the mean plus or minus SEM, *indicates P less than .02. (C) The percentage of adherent cells with polarized morphology was determined. Cell polarization was inhibited by the PLC inhibitor (U73122), whereas PKC inhibition (Gö 6850) had no observable effect. Data are the mean plus or minus SEM, *indicates P less than .02. (D) PLC-dependent activation of LFA-1. Flow cytometry of T cells incubated with 10 μM U73211 (left) or 10 μM Gö 6850 (right) for 10 minutes and stained with the high-affinity extension reporter mAb KIM127 in the absence (Basal) or presence of SDF-1α. Inhibition of PLC prevented the exposure of the KIM127 neoepitope in response to SDF-1α, whereas PKC inhibition had no effect. Three to 5 independent experiments were done for each panel.
The role of PLC and PKC in SDF-1α-induced cell adhesion was analyzed under shear flow conditions. Pharmacologic inhibition of PLC resulted in a significant decrease in SDF-1α-dependent adhesion to both VCAM-1 and ICAM-1, demonstrating a prominent role for this lipase in chemokine-induced activation of both LFA-1 and VLA-4. Importantly, PKC inhibition with Gö 6850 resulted in enhanced adhesion to ICAM-1 whereas the same treatment significantly decreased adhesion to VCAM-1 (Figure 5B). The opposing effects of PKC inhibition on cell adhesion to ICAM-1 or VCAM-1 correlated with its effects on Rap1 activation. That is, PKC inhibition resulted in an increase in Rap1 activation as well as in the number of adherent cells to ICAM-1, whereas it reduced adhesion to VCAM-1 despite high Rap1-GTP levels. This is consistent with our findings in Figure 2 that Rap1 regulates LFA-1 function but does not significantly modulate VLA-4–mediated adhesion. In addition, SDF-1α also induces cell polarization of adherent T cells on both ICAM-1 and VCAM-1 under shear flow conditions. In the presence of the PLC inhibitor, significantly fewer of the remaining adherent T cells were polarized. On the other hand, T-cell polarization on both ligands was not affected by PKC inhibition (Figure 5C). In addition, PLC inhibition abolished exposure of the LFA-1 neoepitope recognized by KIM127, suggesting a requirement for this enzyme in chemokine-dependent LFA-1 affinity modulation (Figure 5D). In contrast, inhibition of PKC did not prevent the exposure of the KIM127 neoepitope (Figure 5D), which further supports our result that PKC inhibition does not inhibit Rap1 activation and β2 integrin-mediated cell adhesion.
Discussion
Our data clearly demonstrate a critical role for Rap1 in LFA-1 inside-out activation in human CD3+ T cells, whereas no major role for this GTPase was found in VLA-4–mediated adhesion. Rap1 was required for LFA-1 affinity modulation leading to cell adhesion to ICAM-1 but not for Mn2+-induced outside-in LFA-1 activation, demonstrating a selective role for Rap1 in signaling integrin activation from within the cell. Interestingly, talin knock-down in both Jurkat and peripheral blood T cells abolishes integrin activation after agonist stimulation.33 However, similar to our results, Mn2+ treatment alone induced up-regulation of LFA-1 affinity and adhesion to ICAM-1 that was comparable in control and talin-deficient cells. Together this supports the model that Mn2+-induced changes in integrin high-affinity conformation can be induced in the absence of inside-out signaling. Previous studies have shown that inhibition of Rap1 activity by RapGAPs abolishes Mn2+-induced LFA-1- and VLA-4–mediated Jurkat T-cell adhesion.12 However, effects on integrin affinity changes were not addressed. Differences could be attributed to the potential generation of Rap-dependent signaling pathways in Jurkat T cells after Mn2+ treatment that result in integrin avidity changes which, in turn, may impact the overall strength of adhesion.
Our studies demonstrate for the first time that CalDAG-GEFI is a critical regulator of chemokine-induced LFA-1-mediated adhesion in human T cells. The absence of CalDAG-GEFIII expression in human T cells and the strong reduction in cell adhesion to ICAM-1 by CalDAG-GEFI-deficient T cells suggest that CalDAG-GEFI is the primary Ca2+ and DAG responsive Rap GEF in human T cells responsible for β2 integrin regulation. As shown for Rap1 GTPase, CalDAG-GEFI was not required for VLA-4–mediated adhesion, which suggests that the CalDAG-GEFI/Rap1 regulatory pathway has a selective role in LFA-1- but not VLA-4–mediated function. The contribution of CalDAG-GEFI in chemokine-induced adhesion in T cells cannot be appreciated in murine models because although CalDAG-GEFI is expressed in both mouse platelets and neutrophils, its expression in murine lymphocytes is below detectable levels by immunoreactivity.20 Thus, alternative mechanisms for Rap1 activation, which may include other CalDAG-GEFs isoforms, may be operative in murine lymphocytes. A group of LAD patients with a syndrome referred to as Leukocyte Adhesion Deficiency-III have been recently described with severe defects in platelet and leukocyte functions associated with defects in integrin activation that have been attributed to a mutation in CalDAG-GEFI,34 and CalDAG-GEFI–deficient mice may represent a murine model of this disorder.35 However, LAD patients identified by other groups with similar clinical symptoms appear to exhibit normal chemokine-induced Rap activation in leukocytes and platelets, suggesting that the underlying molecular basis of these LAD variants may be different.36 The pathways that regulate CalDAG-GEFI function are not well-characterized.19 Our studies suggest that PLC, through its products cytosolic Ca2+ and DAG, is probably an upstream regulator of CalDAG-GEFI19,37 (Figure 6). PLC inhibition resulted in decreased Rap1 activation, LFA-1 affinity modulation and adhesion to ICAM-1. Because intracellular Ca2+ chelation did not affect Rap1 activation and the Ca2+ ionophore A23187 induced adhesion to ICAM-1 in CalDAG-GEFI–silenced cells, it is intriguing to speculate that DAG is the relevant SDF-1α–induced second messenger responsible for linking CXCR4 to CalDAG-GEFI activation in human T cells, and needs further investigation.
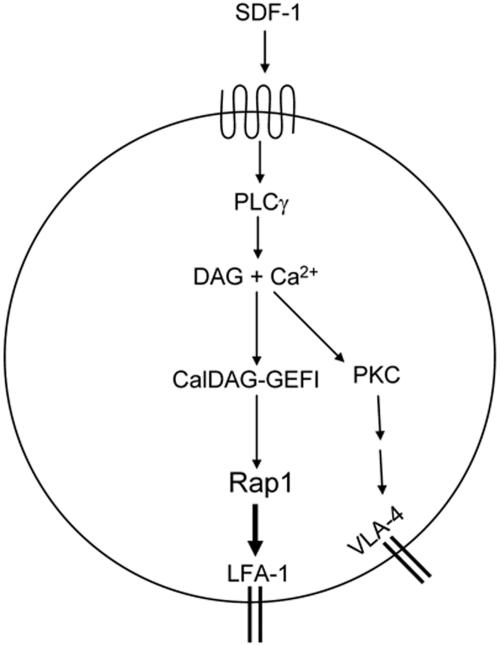
Figure 6.
Model of chemokine induced T-cell adhesion through LFA-1 and VLA-4. Chemokine-dependent G-protein-coupled-receptor signaling leads to activation of PLCγ. PLCγ products Ca2+ and DAG activate CalDAG-GEFI. This leads to Rap1 activation, which triggers inside-out activation of LFA-1 to promote T-cell adhesion. SDF-1α activates VLA-4 through PLCγ. PLCγ-generated products may promote PKC activation and subsequent integrin activation through unknown intermediates.
Others have reported that Rap1 plays a role in VLA-4–mediated adhesion, in addition to LFA-1, in murine lymphocytes.9,11 We observed no significant role for Rap in PMA-induced adhesion to a range of VCAM-1 densities (0.1-10 μg/mL). The differences between their and our results may be attributed to species-specific expression of signaling elements38 that contribute to VLA-4–mediated adhesion or may involve mechanisms of VLA-4 affinity modulation that are cell context dependent. In this regard, SDF-1α-induced integrin affinity changes, as detected by soluble ligand binding, was markedly weaker in human T cells in comparison to monocytes,29 and PMA-induced soluble VCAM-1 binding was observed only in a subset of CD3+ T cells,30 whereas Jurkat T cells constitutively expressed predominantly high-affinity VLA-4 integrins.39 It is noteworthy that overexpression of constitutively active Rap1 (Rap63E) in human T cells enhanced adhesion to both ICAM-1 and VCAM-1 (data not shown), which is consistent with previous reports.9,40 This suggests that although artificially activated Rap1 can couple to pathways that lead to VLA-4–mediated adhesion, the physiologic activation of this GTPase through SDF-1α does not signal to VLA-4 activation.
Our knowledge of mechanisms leading to VLA-4 activation is still limited. Studies suggest that VLA-4 can spontaneously support T-cell adhesion to high-density ligand, indicating that VLA-4 may exist in an overall extended conformation accessible to ligands.41 In our analyses, T-cell adhesion to VCAM-1 was observed under unstimulated conditions but was clearly further induced by SDF-1α or PMA treatment, suggesting a change in VLA-4 affinity/avidity for its ligand. The agonist-induced increase in adhesion may not correlate with exposure of activation epitopes that report high affinity for ligands,42,43 a parameter classically associated with LFA-1–mediated activation. Instead, it may be mediated primarily by postreceptor events such as VLA-4 diffusion/clustering and/or changes in cytoskeletal interactions.42 Such a model would be consistent with our data demonstrating that Rap1 GTPase, shown here as a regulator specifically of inside-out integrin signaling, is not required for VLA-4–mediated adhesion. Other GTPases shown to control VLA-4–dependent T-cell adhesion include Rac and its GEF Vav44 and Rho GTPase.45 Chemokine-induced changes in VLA-4 avidity may also rely on lipid raft-associated signaling molecules such as integrin-associated tetraspanins and Src kinase, which are potential modulators of dynamic integrin adhesive contacts.46 Our studies present evidence that PLC and PKC activation are important components in pathways leading to VLA-4–mediated adhesion. It is possible that the pathway that links PLC to VLA-4 activation is mediated by the effect of the PLC-generated products DAG and Ca2+ on PKC (Figure 6). Interestingly, PKC inhibition led to an increase in Rap1 activation after chemokine stimulation, which correlated with an increase in LFA-1–mediated adhesion to ICAM-1 while significantly impairing VLA-4 mediated T-cell adhesion. The latter result further supports our model that Rap1 activation does not substantially modulate VLA-4 function. The mechanism by which PKC differently regulate VLA-4– and LFA-1–mediated adhesion is an obvious area for future investigation.
T cells acquire a polarized morphology after chemokine stimulation, which plays a role in directional movement and facilitates their migration into tissues.47 Shimonaka et al9 have shown that SPA1-transduced Jurkat T cells failed to polarize on activated endothelium. We demonstrate that PLC, the upstream regulator of Rap1 activation and VLA-4– and LFA-1–mediated adhesion after chemokine stimulation, is also critical for human T-cell polarization.
In summary our data provide compelling evidence that chemokine-induced signaling to both major integrins expressed by T cells, LFA-1, and VLA-4 can be distinguished at the levels of Rap and its regulator CalDAG-GEFI (Figure 6). A better understanding of the signaling pathways that regulate LFA-1 versus VLA-4 will provide important insights into both the physiologic role of these molecules in the adaptive immune response and aid in the design of targeted therapeutic strategies. Therapeutic inhibition of VLA-4 and LFA-1 is in clinical trials to treat autoimmune diseases.5,6 The differences in regulation of integrins in primary human T cells versus murine lymphocytes and T-cell lines highlights the importance of examining integrin regulation in primary human T cells to develop targeted therapies that are efficacious in human disease.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant PO1 HL036028.
We would like to thank Deanna Lamont for technical assistance. We would like to thank Dr Peter Casey for kindly providing RapGAP1. CalDAG-GEFI/RasGRP2 antibody and CalDAG-GEFIII/RasGRP3 antibody were generously provided by Dr Anne M. Graybiel (Department of Brain and Cognitive Sciences and McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, MA) and Dr James C. Stone (Department of Biochemistry, University of Alberta, Edmonton, AB), respectively. KIM127 antibody was kindly provided by Dr M. K. Robinson (Celltech R&D, Cambridge, United Kingdom). We also thank Dr Masanori Aikawa (Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA) for providing access to the Amaxa Nucleofector System.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.G. and X.C. designed and performed the research, analyzed data, and wrote the paper; A.A. helped perform the research; F.W.L. helped design the research; and T.N.M. designed the research and wrote the paper.
H.G. and X.C. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tanya N. Mayadas, Brigham and Women's Hospital, 77 Ave Louis Pasteur, NRB-752O, Boston, MA 02115; e-mail: tmayadas@rics.bwh.harvard.edu.
References
- 1.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savin W, Dalmau SR, Dealmeida VC. Role of extracellular matrix-mediated interactions in thymocyte migration. Dev Immunol. 2000;7:279–291. doi: 10.1155/2000/60247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 5.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 6.Anderson ME, Siahaan TJ. Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides. 2003;24:487–501. doi: 10.1016/s0196-9781(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 7.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 8.Bos JL, de Bruyn K, Enserink J, et al. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003;31:83–86. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- 9.Shimonaka M, Katagiri K, Nakayama T, et al. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Schwartz BR, Tupper J, Lin N, Winn RK, Harlan JM. The GTPase Rap1 regulates phorbol 12-myristate 13-acetate-stimulated but not ligand-induced beta 1 integrin-dependent leukocyte adhesion. J Biol Chem. 2002;277:40893–40900. doi: 10.1074/jbc.M206208200. [DOI] [PubMed] [Google Scholar]
- 11.Duchniewicz M, Zemojtel T, Kolanczyk M, Grossman S, Scheele JS, Zwartkruis FJ. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Biol Cell. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bruyn KM, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA-1- and VLA-4-mediated cell adhesion. J Biol Chem. 2002;277:29468–29476. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- 13.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Lim CJ, Watanabe N, et al. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Kinashi T, Katagiri K. Regulation of immune cell adhesion and migration by regulator of adhesion and cell polarization enriched in lymphoid tissues. Immunology. 2005;116:164–171. doi: 10.1111/j.1365-2567.2005.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170:141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Stone JC. Regulation of Ras in lymphocytes: get a GRP. Biochem Soc Trans. 2006;34:858–861. doi: 10.1042/BST0340858. [DOI] [PubMed] [Google Scholar]
- 20.Crittenden JR, Bergmeier W, Zhang Y, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 21.Cullere X, Shaw SK, Anderson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 22.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 23.Shamri R, Grabovsky V, Gauguet JM, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 24.Katagiri K, Shimonaka M, Kinashi T. Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-gamma1. J Biol Chem. 2004;279:11875–11881. doi: 10.1074/jbc.M310717200. [DOI] [PubMed] [Google Scholar]
- 25.Kurachi H, Wada Y, Tsukamoto N, et al. Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. Segregate expression profiles from a rap1GAP gene product. J Biol Chem. 1997;272:28081–28088. doi: 10.1074/jbc.272.44.28081. [DOI] [PubMed] [Google Scholar]
- 26.Tsukamoto N, Hattori M, Yang H, Bos JL, Minato N. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J Biol Chem. 1999;274:18463–18469. doi: 10.1074/jbc.274.26.18463. [DOI] [PubMed] [Google Scholar]
- 27.Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson MK, Andrew D, Rosen H, et al. Antibody against the Leu-CAM beta-chain (CD18) promotes both LFA-1- and CR3-dependent adhesion events. J Immunol. 1992;148:1080–1085. [PubMed] [Google Scholar]
- 29.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J Exp Med. 2001;193:1149–1158. doi: 10.1084/jem.193.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose D. Soluble VCAM-1 binding to alpha4 integrins is cell-type specific and activation dependent and is disrupted during apoptosis in T cells. Blood. 2000;95:602–609. [PubMed] [Google Scholar]
- 31.Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 32.Hyduk SJ, Chan JR, Duffy ST, et al. Phospholipase C, calcium, and calmodulin are critical for alpha4beta1 integrin affinity up-regulation and monocyte arrest triggered by chemoattractants. Blood. 2007;109:176–184. doi: 10.1182/blood-2006-01-029199. [DOI] [PubMed] [Google Scholar]
- 33.Simonson WT, Franco SJ, Huttenlocher A. Talin1 regulates TCR-mediated LFA-1 function. J Immunol. 2006;177:7707–7714. doi: 10.4049/jimmunol.177.11.7707. [DOI] [PubMed] [Google Scholar]
- 34.Pasvolsky R, Feigelson SW, Kilic SS, et al. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–1582. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergmeier W, Goerge T, Wang HW, et al. Mice lacking the signaling molecule CalDAG-GEFI represent a model for leukocyte adhesion deficiency type III. J Clin Invest. 2007;117:1699–1707. doi: 10.1172/JCI30575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuijpers TW, van Bruggen R, Kamerbeek N, et al. Natural history and early diagnosis of LAD-1/variant syndrome. Blood. 2006;109:3529–3537. doi: 10.1182/blood-2006-05-021402. [DOI] [PubMed] [Google Scholar]
- 37.Kawasaki H, Springett GM, Toki S, et al. A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc Natl Acad Sci U S A. 1998;95:13278–13283. doi: 10.1073/pnas.95.22.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Mobley JL, Dwir O, et al. High affinity very late antigen-4 subsets expressed on T cells are mandatory for spontaneous adhesion strengthening but not for rolling on VCAM-1 in shear flow. J Immunol. 1999;162:1084–1095. [PubMed] [Google Scholar]
- 40.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–258. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 41.Berlin C, Bargatze RF, Campbell JJ, et al. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 42.Bazzoni G, Hemler ME. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci. 1998;23:30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 43.Grabovsky V, Feigelson S, Chen C, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Bernal D, Wright N, Sotillo-Mallo E, et al. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1. Mol Biol Cell. 2005;16(7):3223–3235. doi: 10.1091/mbc.E04-12-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vielkind S, Gallagher-Gambarelli M, Gomez M, Hinton HJ, Cantrell DA. Integrin regulation by RhoA in thymocytes. J Immunol. 2005;175:350–357. doi: 10.4049/jimmunol.175.1.350. [DOI] [PubMed] [Google Scholar]
- 46.Cinamon G, Grabovsky V, Winter E, et al. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leuk Biol. 2001;69:860–866. [PubMed] [Google Scholar]
- 47.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. Embo J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.